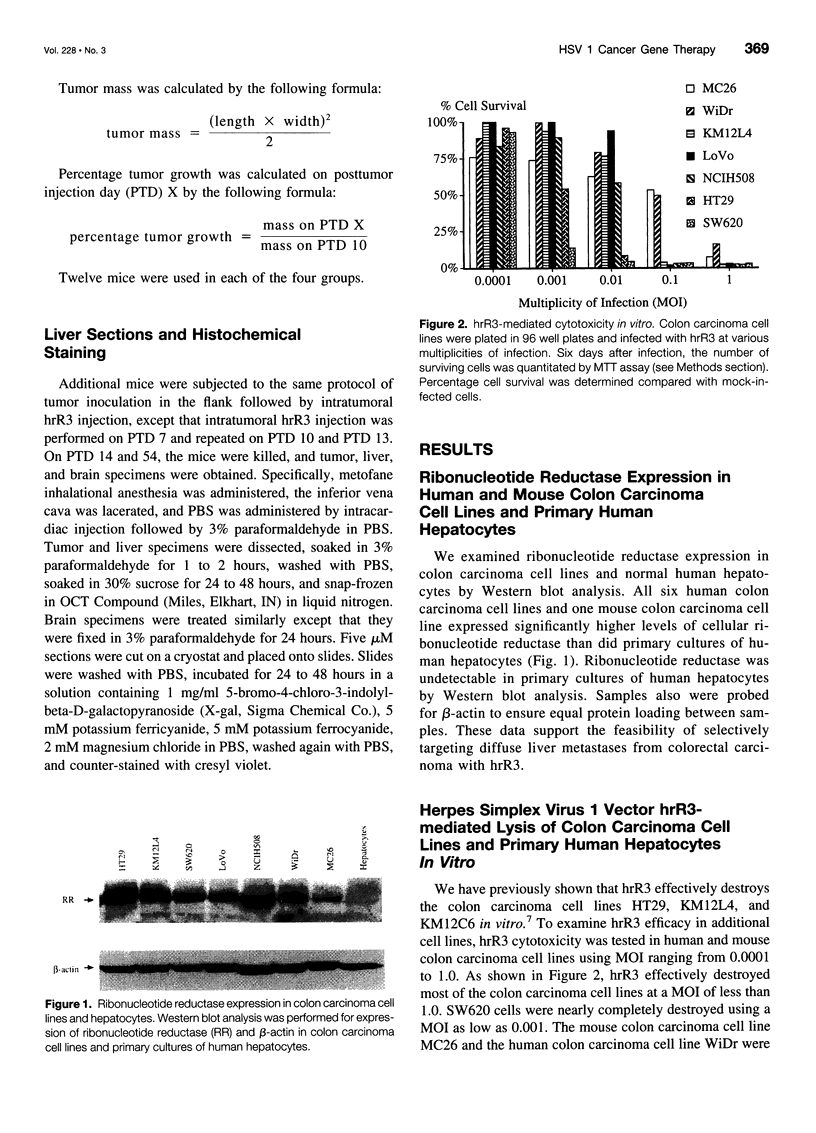
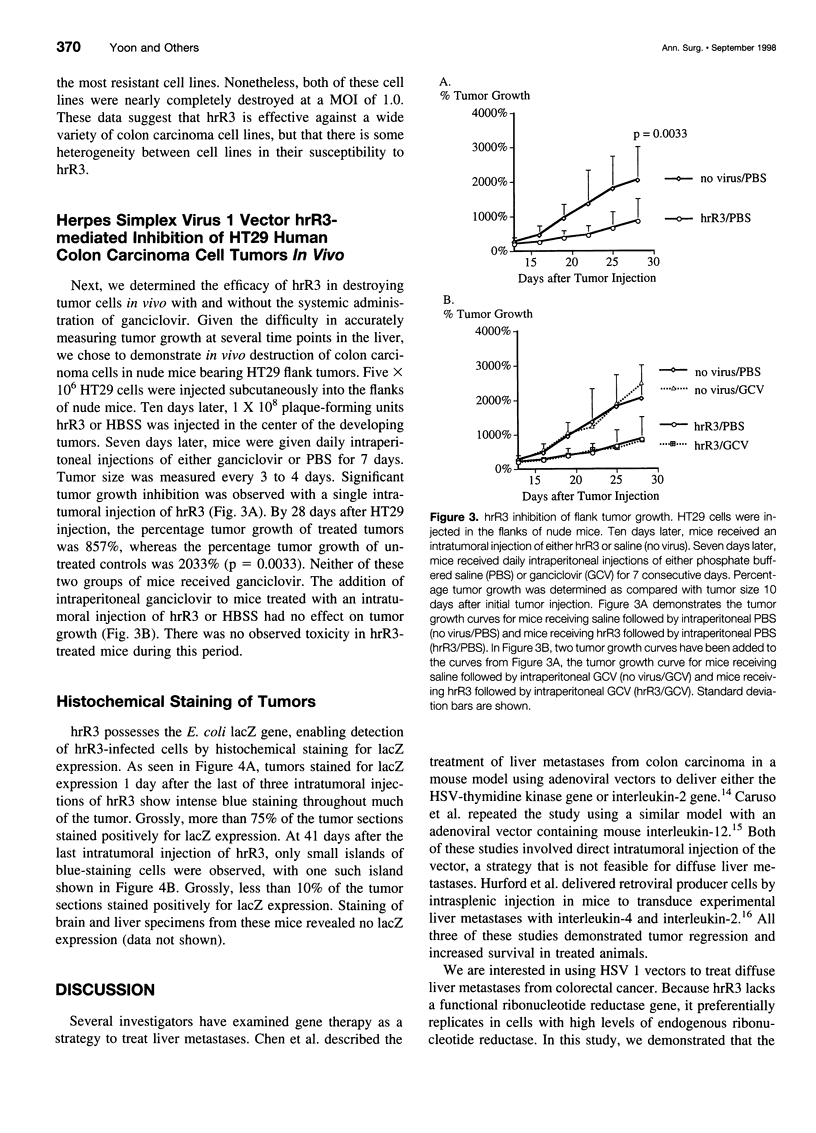
Abstract
OBJECTIVE: The authors investigate the efficacy of hrR3, a viral vector derived from herpes simplex virus type 1 (HSV 1), in destroying colon carcinoma cells in vitro and in vivo. The effect of adding the prodrug ganciclovir in combination with hrR3 infection also is assessed. SUMMARY BACKGROUND DATA: Most cancer gene therapy strategies use viral vectors that are incapable of replication. The HSV 1 vector hrR3 is capable of replication, and its replication is cytotoxic to cells. hrR3 also possesses the HSV-thymidine kinase gene, which converts ganciclovir into a toxic metabolite. Thus, the addition of ganciclovir to hrR3-infected cells may enhance the ability of hrR3 to destroy tumor cells. To increase specificity for tumor cells, hrR3 has a mutated ribonucleotide reductase gene and replicates selectively in cells with high levels of endogenous rbonucleotide reductase. Actively dividing cells such as tumor cells have high levels of endogenous ribonucleotide reductase for synthesis of DNA precursors. The authors are interested in the use of HSV 1 vectors to treat liver metastases from colorectal cancer. METHODS: Ribonucleotide reductase expression in several colon carcinoma cell lines and in primary cultures of human hepatocytes was determined by Western blot analysis. hrR3-mediated cytotoxicity in the colon carcinoma cell lines was determined using an in vitro assay. The human colon carcinoma cell line HT29 was injected into the flanks of nude mice followed by intratumoral injection of hrR3. Tumor growth rate was assessed with and without the addition of intraperitoneal ganciclovir. RESULTS: Ribonucleotide reductase levels in colon carcinoma cell lines are much higher than in primary cultures of human hepatocytes. hrR3 efficiently destroys colon carcinoma cell lines in vitro. A single intratumoral injection of hrR3 into HT29 flank tumors significantly reduces tumor growth rate, and the administration of ganciclovir has no additive effect. CONCLUSIONS: The inherent cytotoxicity of hrR3 replication effectively destroys colon carcinoma cells in vitro and in vivo. This cytotoxicity is not enhanced in vivo by the addition of ganciclovir. In the future, more efficacious and selective HSV 1 vectors may be useful in the treatment of cancer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carroll N. M., Chase M., Chiocca E. A., Tanabe K. K. The effect of ganciclovir on herpes simplex virus-mediated oncolysis. J Surg Res. 1997 May;69(2):413–417. doi: 10.1006/jsre.1997.5089. [DOI] [PubMed] [Google Scholar]
- Carroll N. M., Chiocca E. A., Takahashi K., Tanabe K. K. Enhancement of gene therapy specificity for diffuse colon carcinoma liver metastases with recombinant herpes simplex virus. Ann Surg. 1996 Sep;224(3):323–330. doi: 10.1097/00000658-199609000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso M., Pham-Nguyen K., Kwong Y. L., Xu B., Kosai K. I., Finegold M., Woo S. L., Chen S. H. Adenovirus-mediated interleukin-12 gene therapy for metastatic colon carcinoma. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11302–11306. doi: 10.1073/pnas.93.21.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Chen X. H., Wang Y., Kosai K., Finegold M. J., Rich S. S., Woo S. L. Combination gene therapy for liver metastasis of colon carcinoma in vivo. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2577–2581. doi: 10.1073/pnas.92.7.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couffinhal T., Kearney M., Sullivan A., Silver M., Tsurumi Y., Isner J. M. Histochemical staining following LacZ gene transfer underestimates transfection efficiency. Hum Gene Ther. 1997 May 20;8(8):929–934. doi: 10.1089/hum.1997.8.8-929. [DOI] [PubMed] [Google Scholar]
- Crumpacker C. S. Ganciclovir. N Engl J Med. 1996 Sep 5;335(10):721–729. doi: 10.1056/NEJM199609053351007. [DOI] [PubMed] [Google Scholar]
- Fong Y., Blumgart L. H., Cohen A. M. Surgical treatment of colorectal metastases to the liver. CA Cancer J Clin. 1995 Jan-Feb;45(1):50–62. doi: 10.3322/canjclin.45.1.50. [DOI] [PubMed] [Google Scholar]
- Freeman S. M., Abboud C. N., Whartenby K. A., Packman C. H., Koeplin D. S., Moolten F. L., Abraham G. N. The "bystander effect": tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993 Nov 1;53(21):5274–5283. [PubMed] [Google Scholar]
- Freeman S. M., Ramesh R., Marrogi A. J. Immune system in suicide-gene therapy. Lancet. 1997 Jan 4;349(9044):2–3. doi: 10.1016/S0140-6736(97)22001-5. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Weller S. K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988 Jan;62(1):196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber B. E., Austin E. A., Good S. S., Knick V. C., Tibbels S., Richards C. A. In vivo antitumor activity of 5-fluorocytosine on human colorectal carcinoma cells genetically modified to express cytosine deaminase. Cancer Res. 1993 Oct 1;53(19):4619–4626. [PubMed] [Google Scholar]
- Hurford R. K., Jr, Dranoff G., Mulligan R. C., Tepper R. I. Gene therapy of metastatic cancer by in vivo retroviral gene targeting. Nat Genet. 1995 Aug;10(4):430–435. doi: 10.1038/ng0895-430. [DOI] [PubMed] [Google Scholar]
- Hussein A. M. The potential applications of gene transfer in the treatment of patients with cancer: a concise review. Cancer Invest. 1996;14(4):343–352. doi: 10.3109/07357909609012162. [DOI] [PubMed] [Google Scholar]
- Kong H. L., Crystal R. G. Gene therapy strategies for tumor antiangiogenesis. J Natl Cancer Inst. 1998 Feb 18;90(4):273–286. doi: 10.1093/jnci/90.4.273. [DOI] [PubMed] [Google Scholar]
- Kramm C. M., Chase M., Herrlinger U., Jacobs A., Pechan P. A., Rainov N. G., Sena-Esteves M., Aghi M., Barnett F. H., Chiocca E. A. Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Hum Gene Ther. 1997 Nov 20;8(17):2057–2068. doi: 10.1089/hum.1997.8.17-2057. [DOI] [PubMed] [Google Scholar]
- Marcel T., Grausz J. D. The TMC Worldwide Gene Therapy Enrollment Report, end 1996. Hum Gene Ther. 1997 Apr 10;8(6):775–800. doi: 10.1089/hum.1997.8.6-775. [DOI] [PubMed] [Google Scholar]
- Millikan K. W., Staren E. D., Doolas A. Invasive therapy of metastatic colorectal cancer to the liver. Surg Clin North Am. 1997 Feb;77(1):27–48. doi: 10.1016/s0039-6109(05)70531-4. [DOI] [PubMed] [Google Scholar]
- Paillard F. Bystander effects in enzyme/prodrug gene therapy. Hum Gene Ther. 1997 Oct 10;8(15):1733–1735. doi: 10.1089/hum.1997.8.15-1733. [DOI] [PubMed] [Google Scholar]
- Panis Y., Rad A. R., Boyer O., Houssin D., Salzmann J. L., Klatzmann D. Gene therapy for liver tumors. Surg Oncol Clin N Am. 1996 Apr;5(2):461–473. [PubMed] [Google Scholar]
- Trinh Q. T., Austin E. A., Murray D. M., Knick V. C., Huber B. E. Enzyme/prodrug gene therapy: comparison of cytosine deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 1995 Nov 1;55(21):4808–4812. [PubMed] [Google Scholar]
- Weichselbaum R. R., Kufe D. Gene therapy of cancer. Lancet. 1997 May;349 (Suppl 2):SII10–SII12. doi: 10.1016/s0140-6736(97)90013-1. [DOI] [PubMed] [Google Scholar]