Abstract
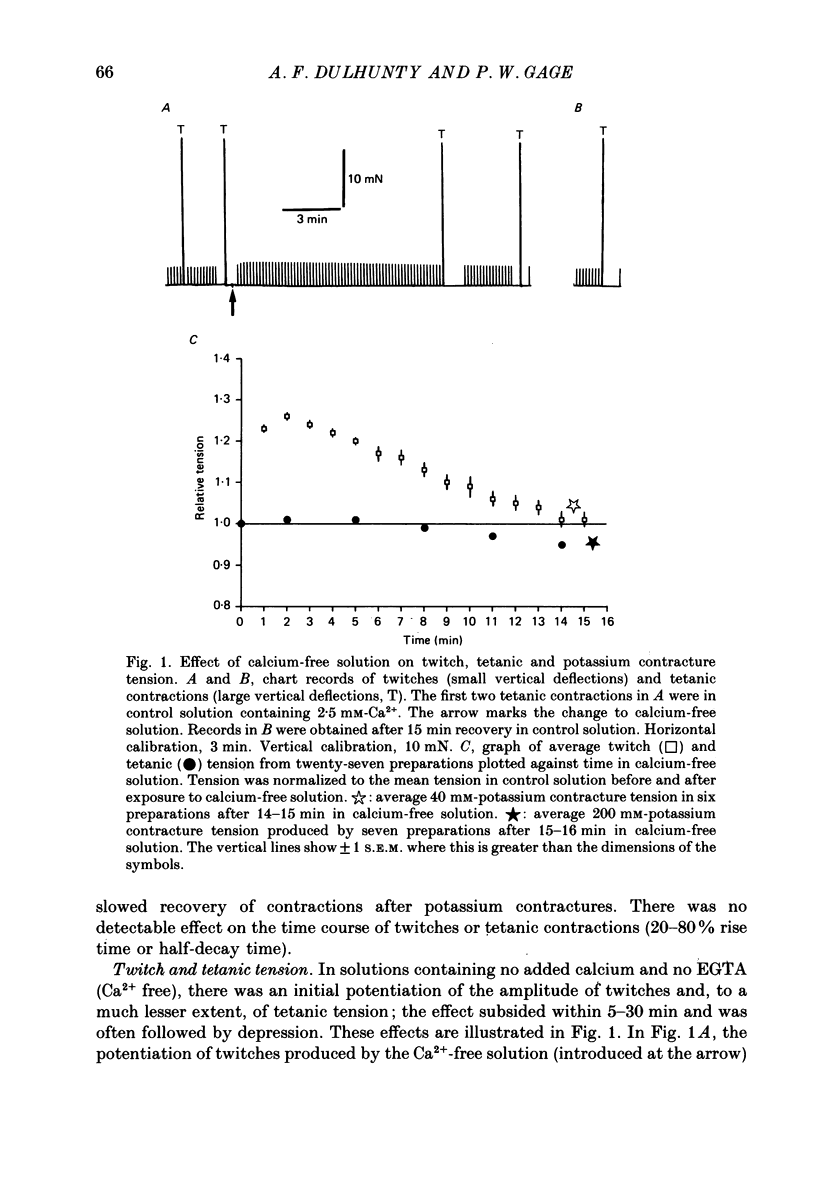
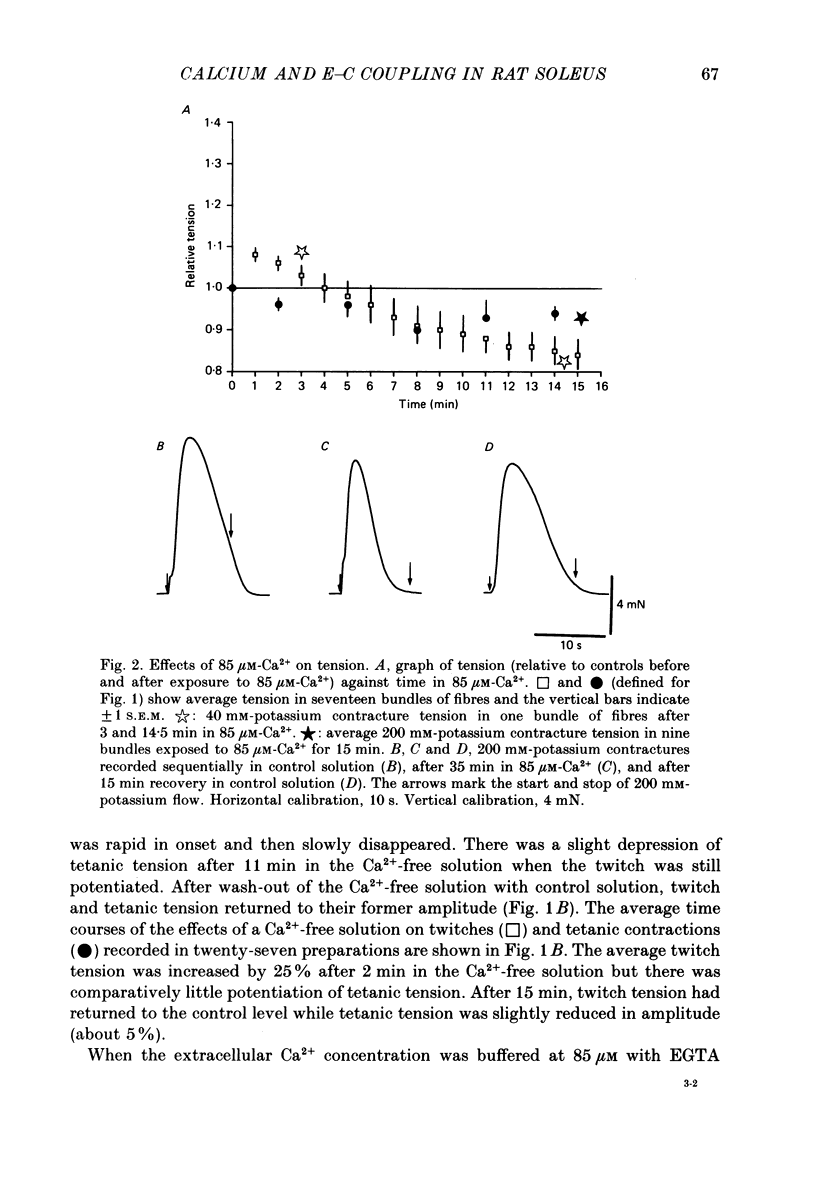
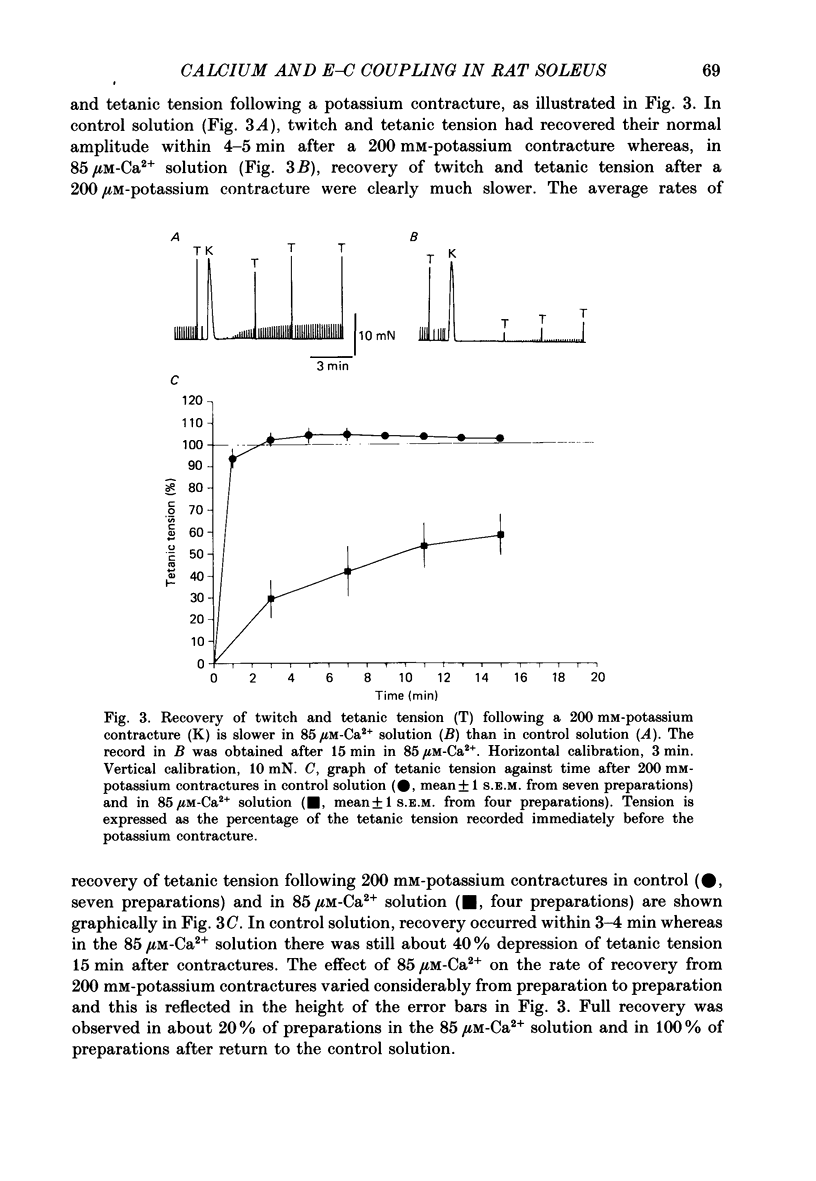
1. Twitches, tetanic contractions and potassium contractures were recorded isometrically from small bundles of rat soleus muscle fibres. 2. Solutions with reduced calcium concentrations (low-calcium solutions), whether buffered with EGTA (85 and 3 microM-Ca2+) or not (15 microM-Ca2+), caused an initial potentiation of contraction followed by depression. 3. The decay of potassium contractures (200 mM-potassium) was more rapid than normal in low-calcium solutions. 4. Recovery from the inactivation produced by a 200 mM-potassium contracture was slowed in low-calcium solutions but full recovery was seen within 10-15 min after return to a solution containing 2.5 mM-Ca2+. 5. Nifedipine (50 microM) in solutions containing 2.5 mM-Ca2+ potentiated contraction whereas, in low-calcium solutions, contraction was depressed and the depression was more pronounced the lower the Ca2+ concentration. 6. As with low-calcium solutions, potassium contractures decayed more rapidly in solutions containing nifedipine. Nifedipine slowed still further the rate of recovery from inactivation in low-calcium solutions. 7. (-) Bay K 8644 (50 microM) depressed contraction, increased the rate of decay of potassium contractures and slowed recovery from inactivation, like nifedipine. The racemate of Bay K 8644 was less effective. 8. In explanation of these and other observations, it is proposed that there is a dihydropyridine-binding molecule in the walls of the transverse tubular system that normally exists predominantly in a 'precursor' form at the resting membrane potential and is converted by membrane depolarization to an 'activator' form essential for excitation-contraction coupling. Conversion of the precursor to activator involves both conformational change and dissociation of calcium. Prolonged depolarization converts activator to an inactivated form by inducing further conformational change and dissociation of calcium. Recovery from inactivation requires reverse conformational changes and rebinding of calcium. The dihydropyridines affect contraction by reducing the affinity of the molecule for calcium.
Full text
PDF


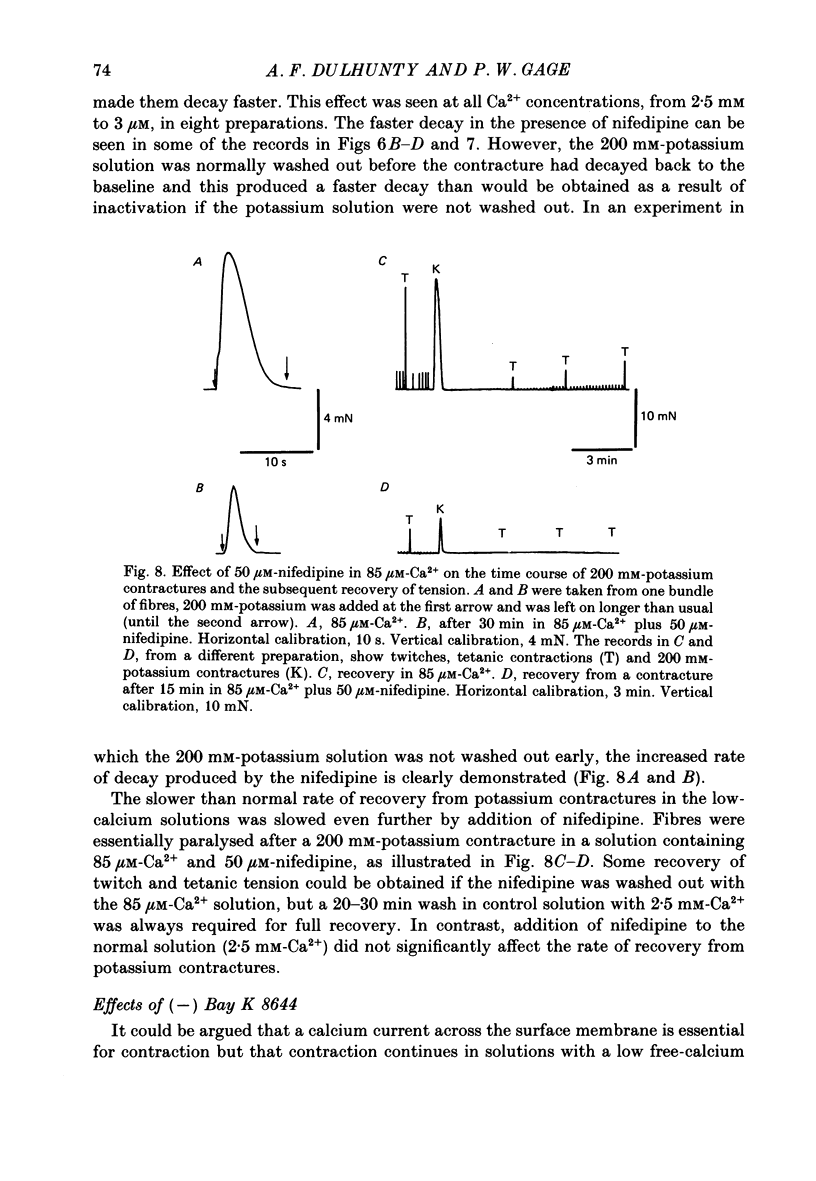
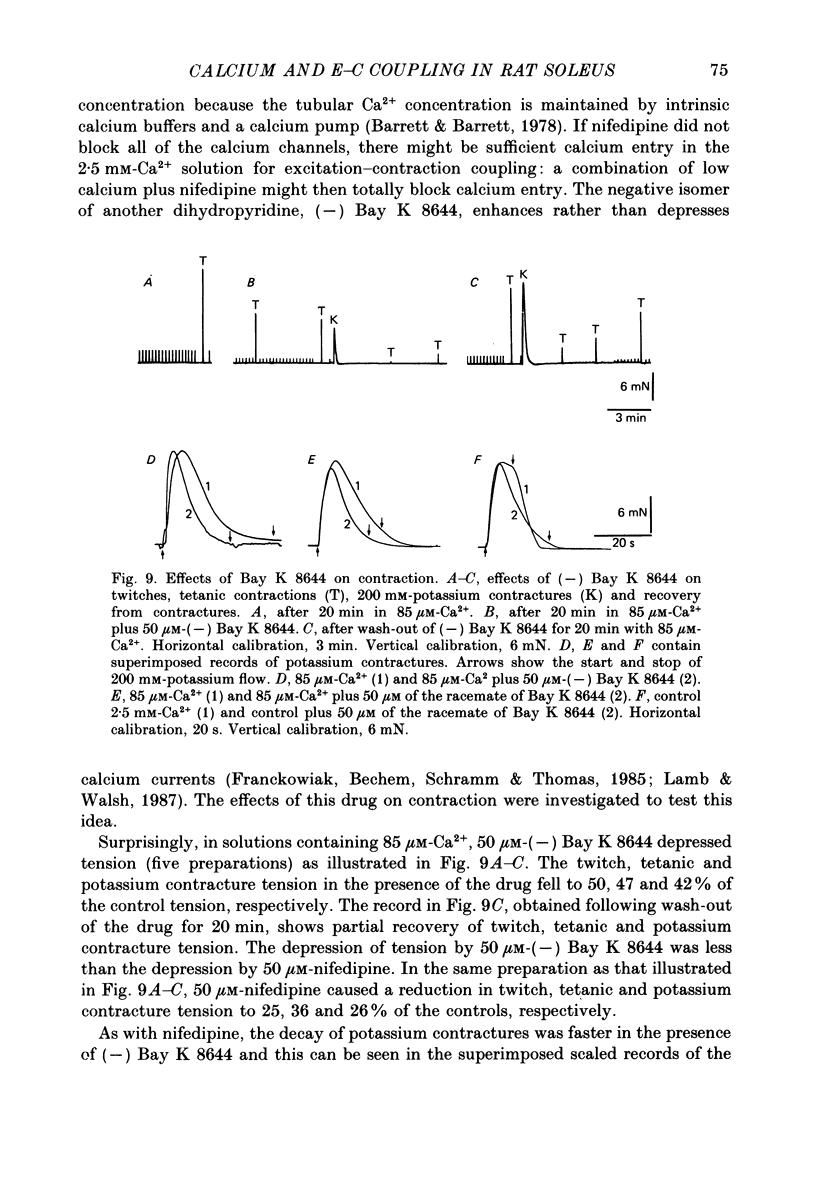





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. M., Horowicz P. Twitches in the presence of ethylene glycol bis( -aminoethyl ether)-N,N'-tetracetic acid. Biochim Biophys Acta. 1972 Jun 23;267(3):605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Lopez-Barneo J. External calcium ions are required for potassium channel gating in squid neurons. Science. 1987 May 8;236(4802):712–714. doi: 10.1126/science.2437654. [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Moisescu D. G. Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils. J Physiol. 1977 Sep;270(3):627–652. doi: 10.1113/jphysiol.1977.sp011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Sakar A. J., Cota G., Gamboa-Aldeco R., Garcia J., Huerta M., Muñiz J., Stefani E. Skeletal muscle Ca2+ channels. J Muscle Res Cell Motil. 1986 Aug;7(4):291–298. doi: 10.1007/BF01753649. [DOI] [PubMed] [Google Scholar]
- Barrett N., Barrett E. F. Excitation-contraction coupling in skeletal muscle: blockade by high extracellular concentrations of calcium buffers. Science. 1978 Jun 16;200(4347):1270–1272. doi: 10.1126/science.96524. [DOI] [PubMed] [Google Scholar]
- Bolaños P., Caputo C., Velaz L. Effects of calcium, barium and lanthanum on depolarization-contraction coupling in skeletal muscle fibres of Rana pipiens. J Physiol. 1986 Jan;370:39–60. doi: 10.1113/jphysiol.1986.sp015921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Gimenez M. Effects of external calcium deprivation on single muscle fibers. J Gen Physiol. 1967 Oct;50(9):2177–2195. doi: 10.1085/jgp.50.9.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. Nickel substitution for calcium and the time course of potassium contractures of single muscle fibres. J Muscle Res Cell Motil. 1981 Jun;2(2):167–182. doi: 10.1007/BF00711867. [DOI] [PubMed] [Google Scholar]
- Close R., Hoh J. F. The after-effects of repetitive stimulation on the isometric twitch contraction of rat fast skeletal muscle. J Physiol. 1968 Jul;197(2):461–477. doi: 10.1113/jphysiol.1968.sp008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota G., Stefani E. Effects of external calcium reduction on the kinetics of potassium contractures in frog twitch muscle fibres. J Physiol. 1981 Aug;317:303–316. doi: 10.1113/jphysiol.1981.sp013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Excitation-contraction coupling and charge movement in denervated rat extensor digitorum longus and soleus muscles. J Physiol. 1985 Jan;358:75–89. doi: 10.1113/jphysiol.1985.sp015541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDMAN K. A., GRIEVE D. W. ON THE ROLE OF CALCIUM IN THE EXCITATION-CONTRACTION PROCESS OF FROG SARTORIUS MUSCLE. J Physiol. 1964 Jan;170:138–152. doi: 10.1113/jphysiol.1964.sp007319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., McCarthy R. T., Milton R. L. Paralysis of frog skeletal muscle fibres by the calcium antagonist D-600. J Physiol. 1983 Aug;341:495–505. doi: 10.1113/jphysiol.1983.sp014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franckowiak G., Bechem M., Schramm M., Thomas G. The optical isomers of the 1,4-dihydropyridine BAY K 8644 show opposite effects on Ca channels. Eur J Pharmacol. 1985 Aug 15;114(2):223–226. doi: 10.1016/0014-2999(85)90631-4. [DOI] [PubMed] [Google Scholar]
- Gallant E. M., Goettl V. M. Effects of calcium antagonists on mechanical responses of mammalian skeletal muscles. Eur J Pharmacol. 1985 Nov 5;117(2):259–265. doi: 10.1016/0014-2999(85)90611-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Valle-Aguilera R., Lathrop D. A., Garcia M. C. Slow inward calcium currents have no obvious role in muscle excitation-contraction coupling. Nature. 1982 Jul 15;298(5871):292–294. doi: 10.1038/298292a0. [DOI] [PubMed] [Google Scholar]
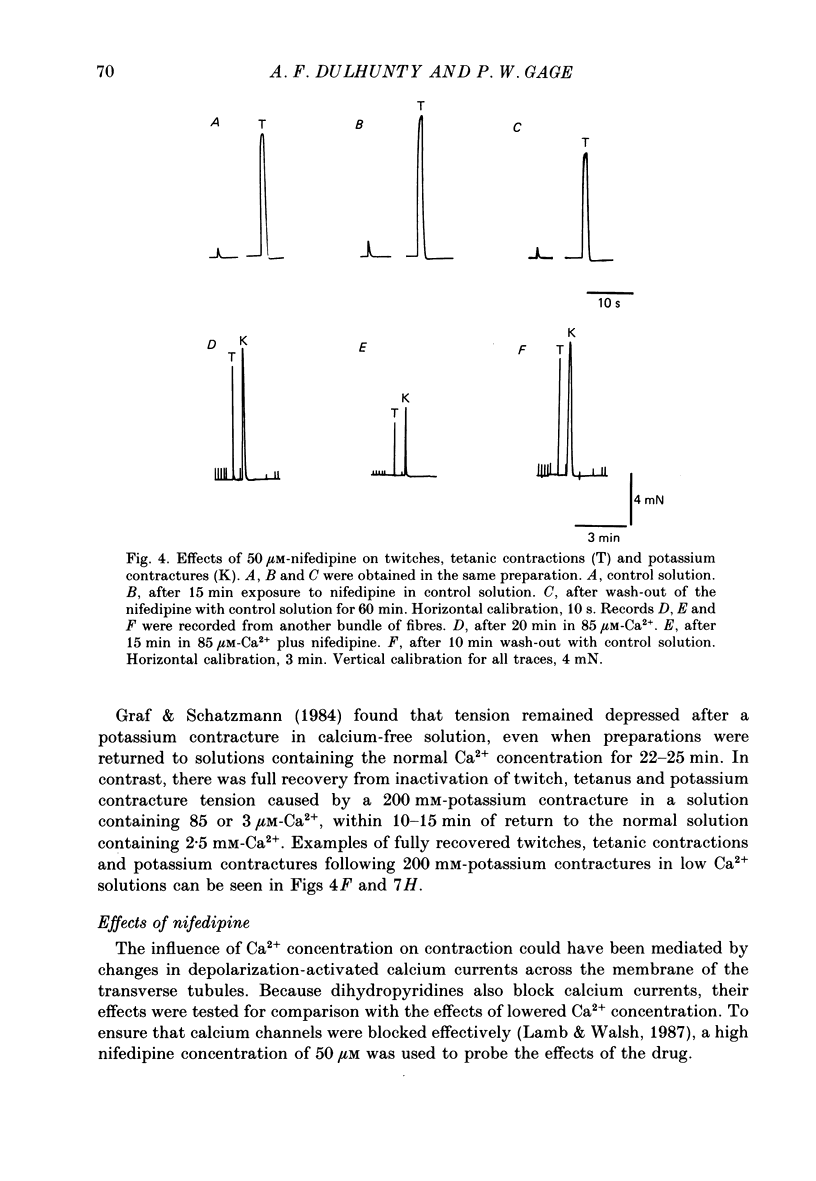
- Graf F., Schatzmann H. J. Some effects of removal of external calcium on pig striated muscle. J Physiol. 1984 Apr;349:1–13. doi: 10.1113/jphysiol.1984.sp015138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildefonse M., Jacquemond V., Rougier O., Renaud J. F., Fosset M., Lazdunski M. Excitation contraction coupling in skeletal muscle: evidence for a role of slow Ca2+ channels using Ca2+ channel activators and inhibitors in the dihydropyridine series. Biochem Biophys Res Commun. 1985 Jun 28;129(3):904–909. doi: 10.1016/0006-291x(85)91977-1. [DOI] [PubMed] [Google Scholar]
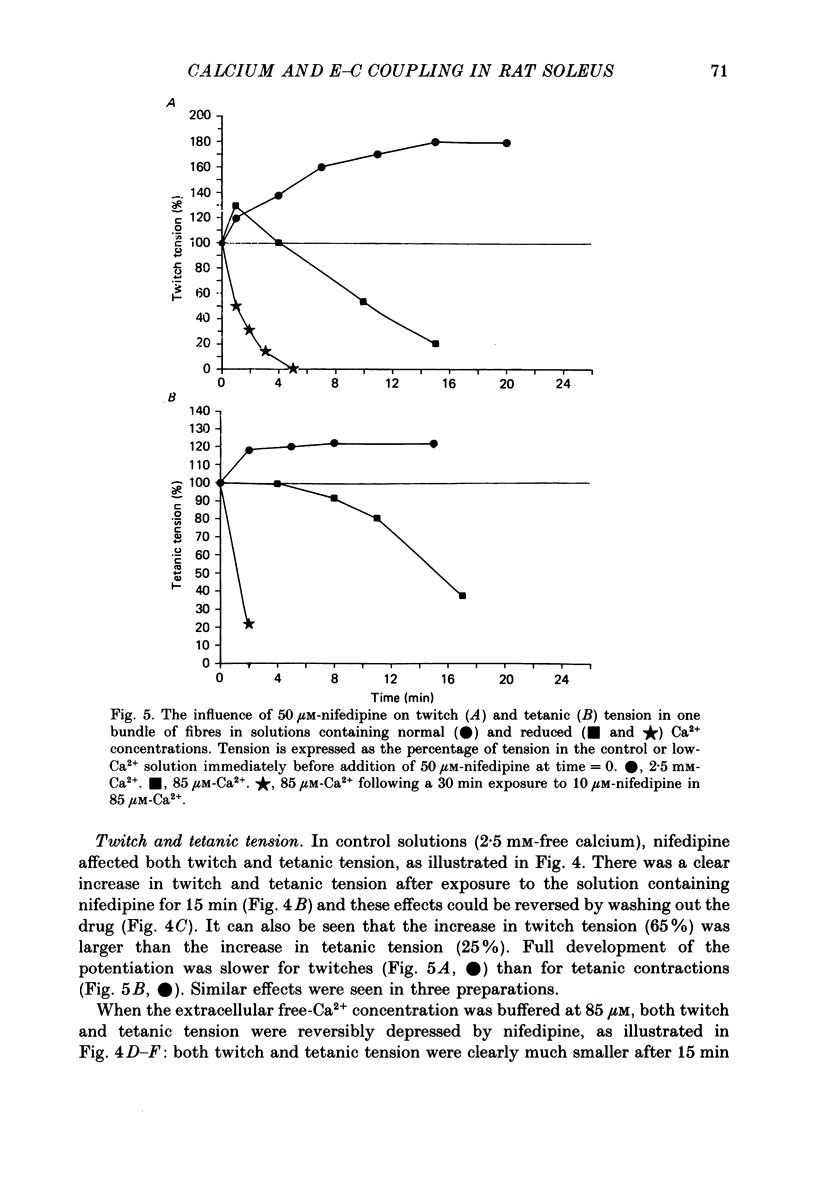
- Kotsias B. A., Muchnik S., Obejero Paz C. A. Co2+, low Ca2+, and verapamil reduce mechanical activity in rat skeletal muscles. Am J Physiol. 1986 Jan;250(1 Pt 1):C40–C46. doi: 10.1152/ajpcell.1986.250.1.C40. [DOI] [PubMed] [Google Scholar]
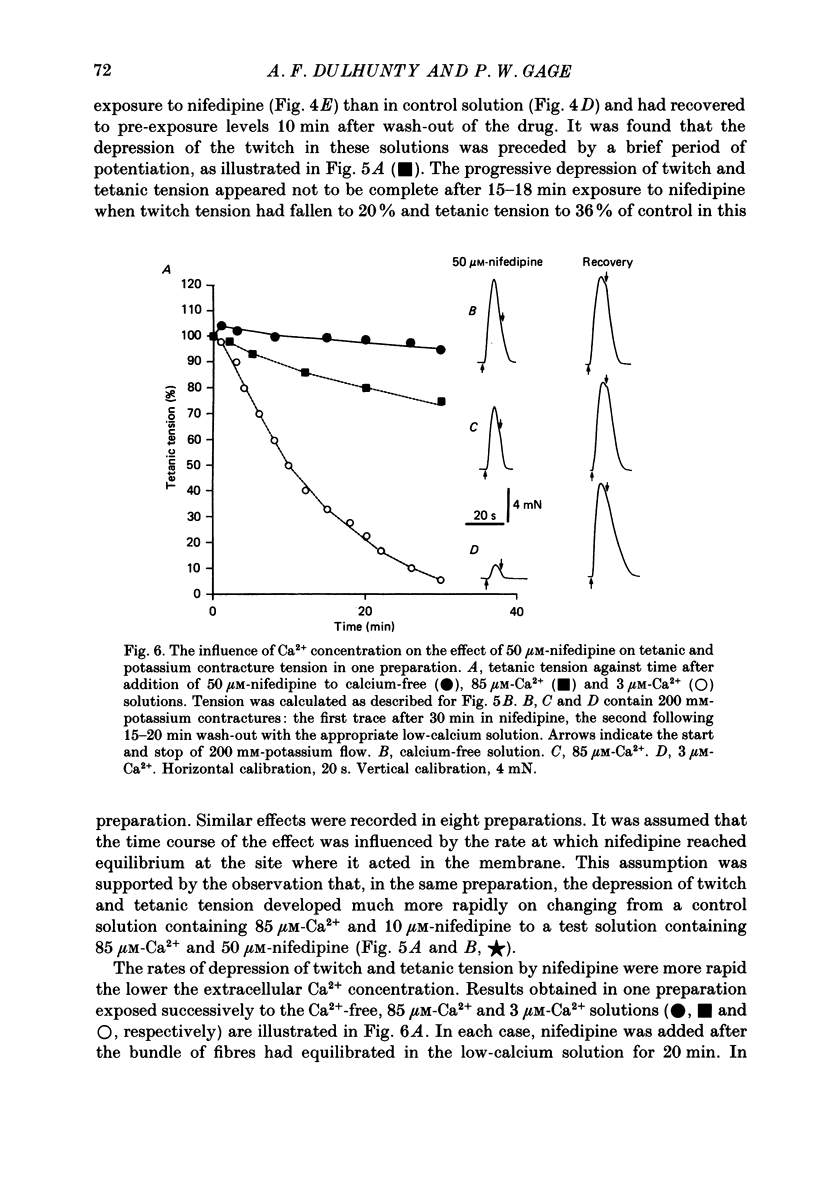
- Lamb G. D. Asymmetric charge movement in contracting muscle fibres in the rabbit. J Physiol. 1986 Jul;376:63–83. doi: 10.1113/jphysiol.1986.sp016142. [DOI] [PMC free article] [PubMed] [Google Scholar]
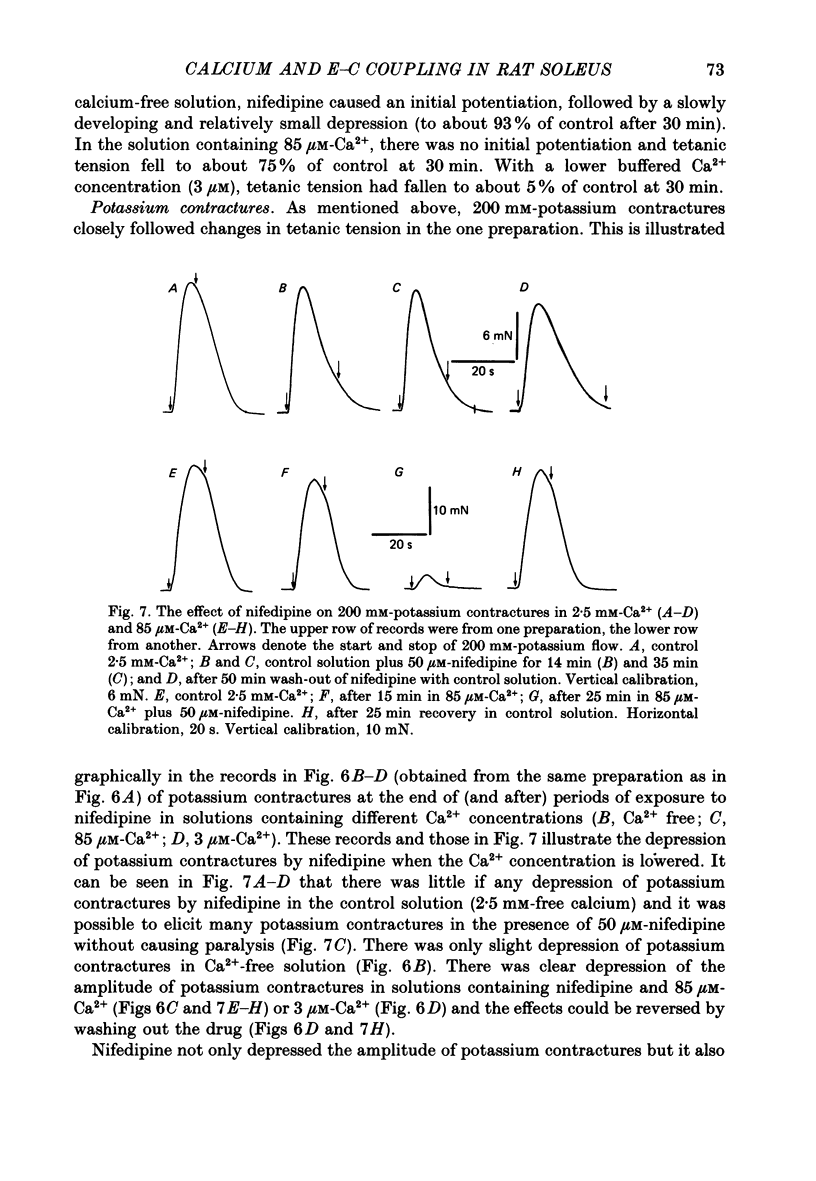
- Lamb G. D., Walsh T. Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J Physiol. 1987 Dec;393:595–617. doi: 10.1113/jphysiol.1987.sp016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Gottschalk G., Berwe D. The effect of calcium and Ca antagonists upon excitation-contraction coupling. Can J Physiol Pharmacol. 1987 Apr;65(4):717–723. doi: 10.1139/y87-118. [DOI] [PubMed] [Google Scholar]
- Lüttgau H. C., Spiecker W. The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres of the frog. J Physiol. 1979 Nov;296:411–429. doi: 10.1113/jphysiol.1979.sp013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouf N. N., Meissner G. Localization of a Mg2+- or Ca2+-activated ("basic") ATPase in skeletal muscle. Exp Cell Res. 1979 Sep;122(2):233–250. doi: 10.1016/0014-4827(79)90301-x. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W. Calcium channels and intracellular calcium release are pharmacologically different in frog skeletal muscle. J Physiol. 1985 Apr;361:231–249. doi: 10.1113/jphysiol.1985.sp015643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Zhu P. H. Extracellular ions and excitation-contraction coupling in frog twitch muscle fibres. J Physiol. 1984 Jun;351:687–710. doi: 10.1113/jphysiol.1984.sp015271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- SANDOW A., KAHN A. J. The immediate effects of potassium on responses of skeletal muscle. J Cell Physiol. 1952 Aug;40(1):89–114. doi: 10.1002/jcp.1030400107. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Krafte D. S., Kass R. S. Voltage-dependent modulation of Ca channel current in heart cells by Bay K8644. J Gen Physiol. 1986 Sep;88(3):369–392. doi: 10.1085/jgp.88.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]


