Abstract
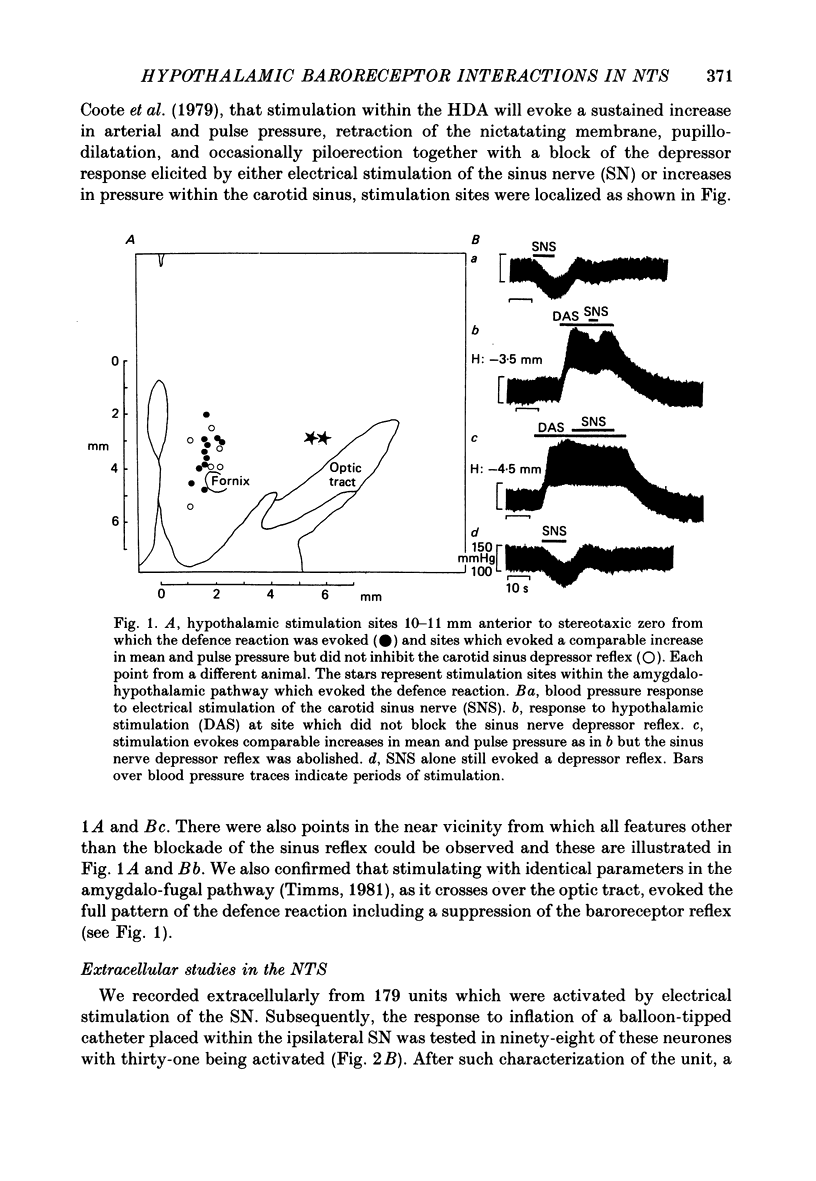
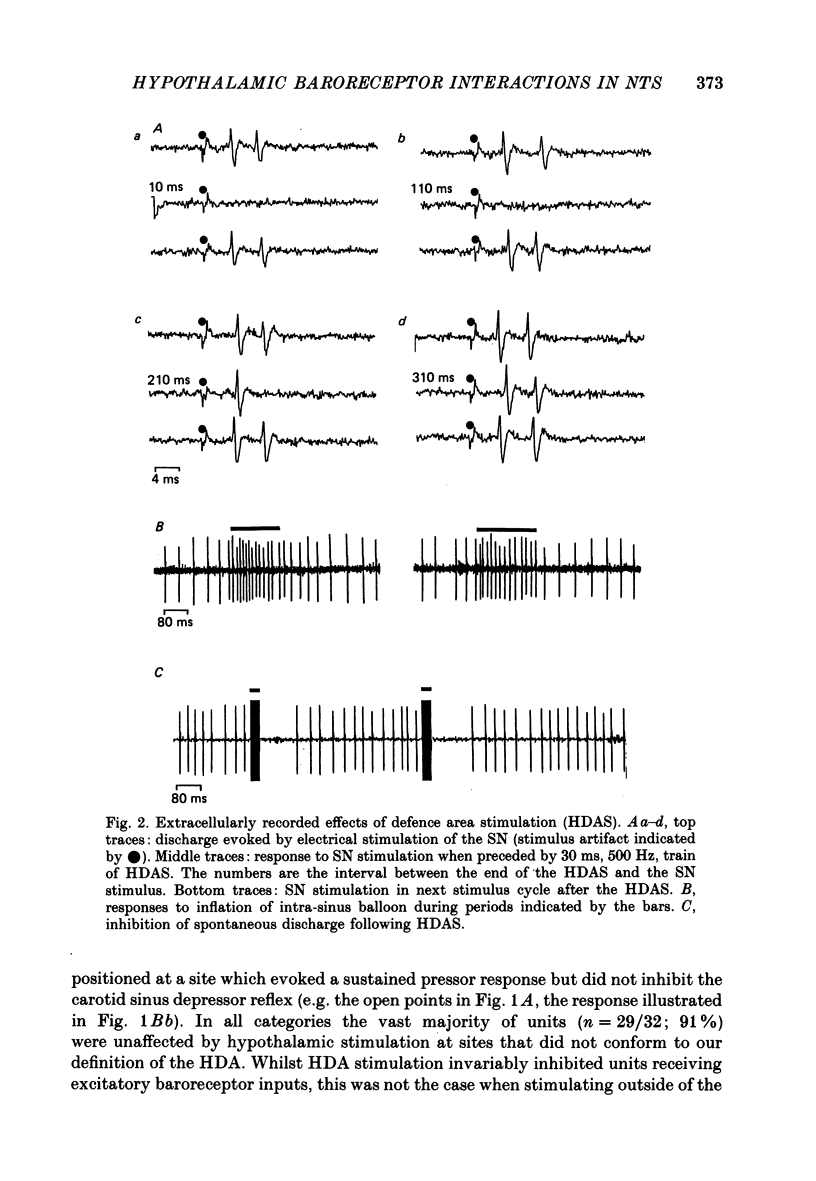
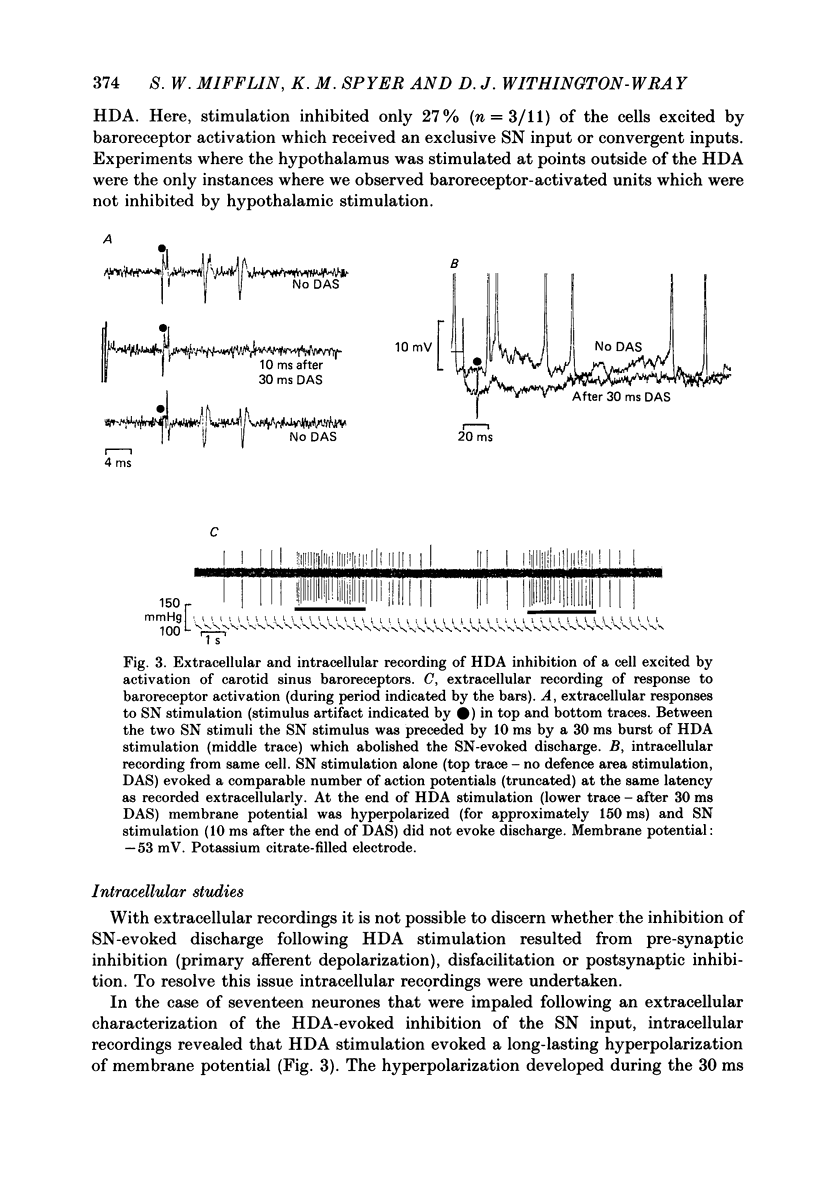
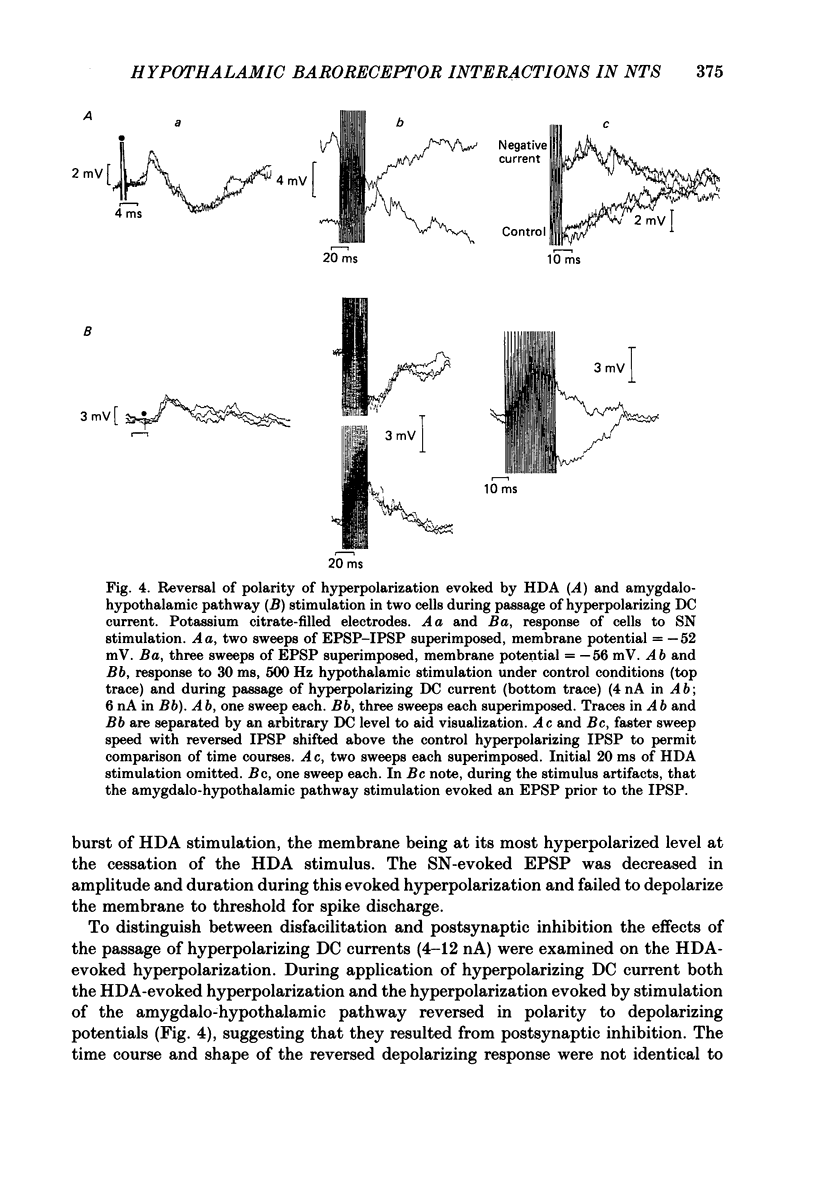
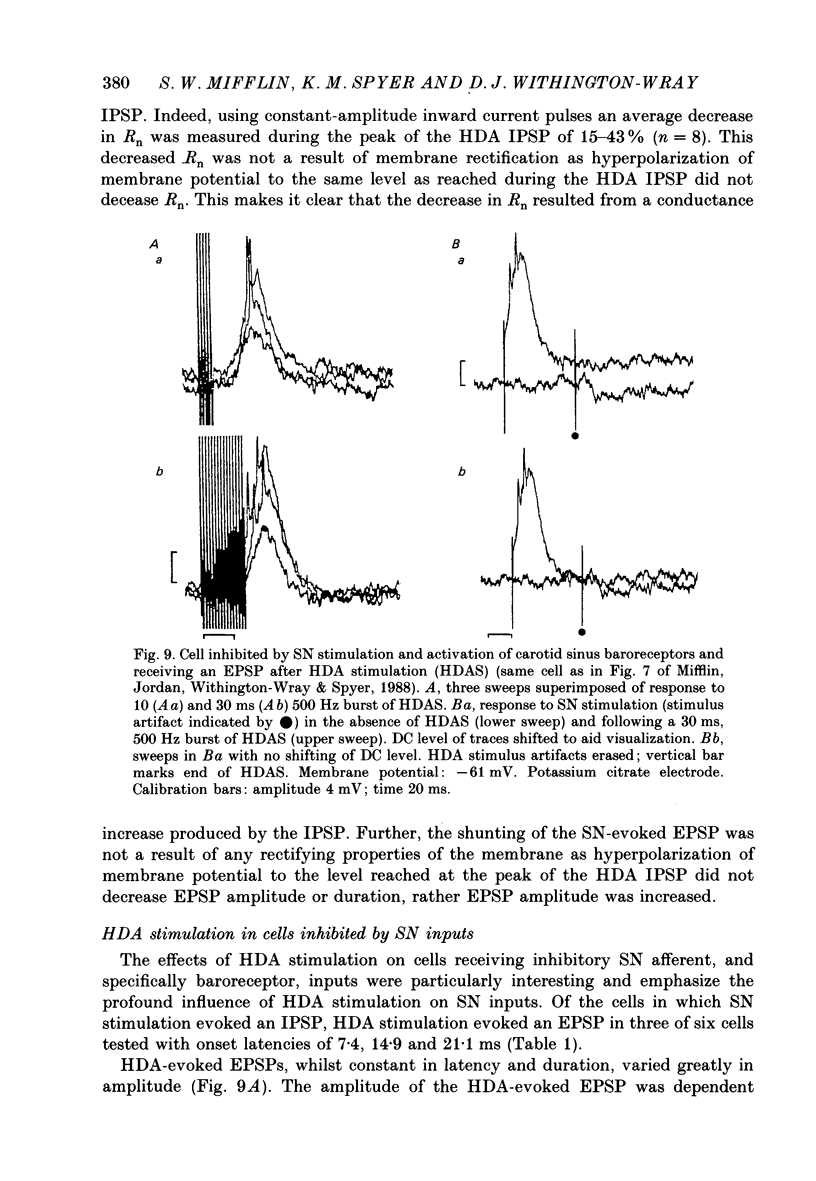
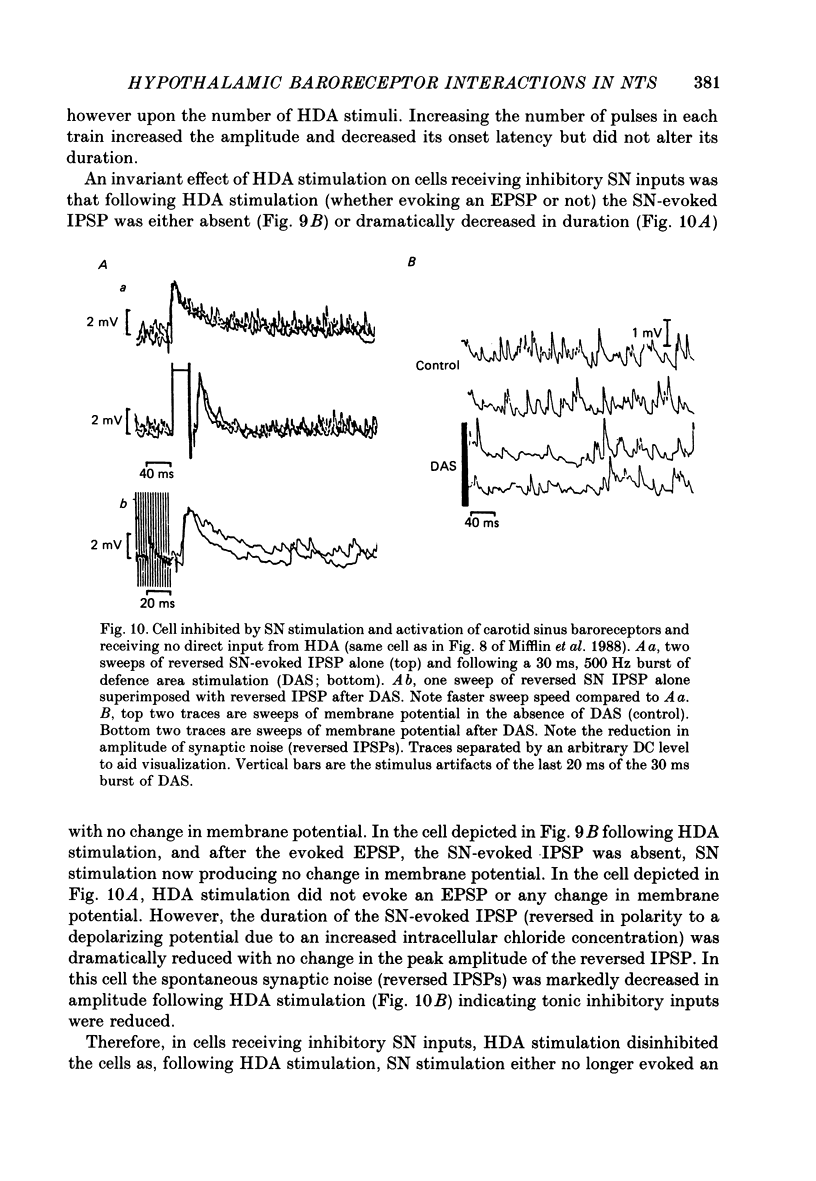
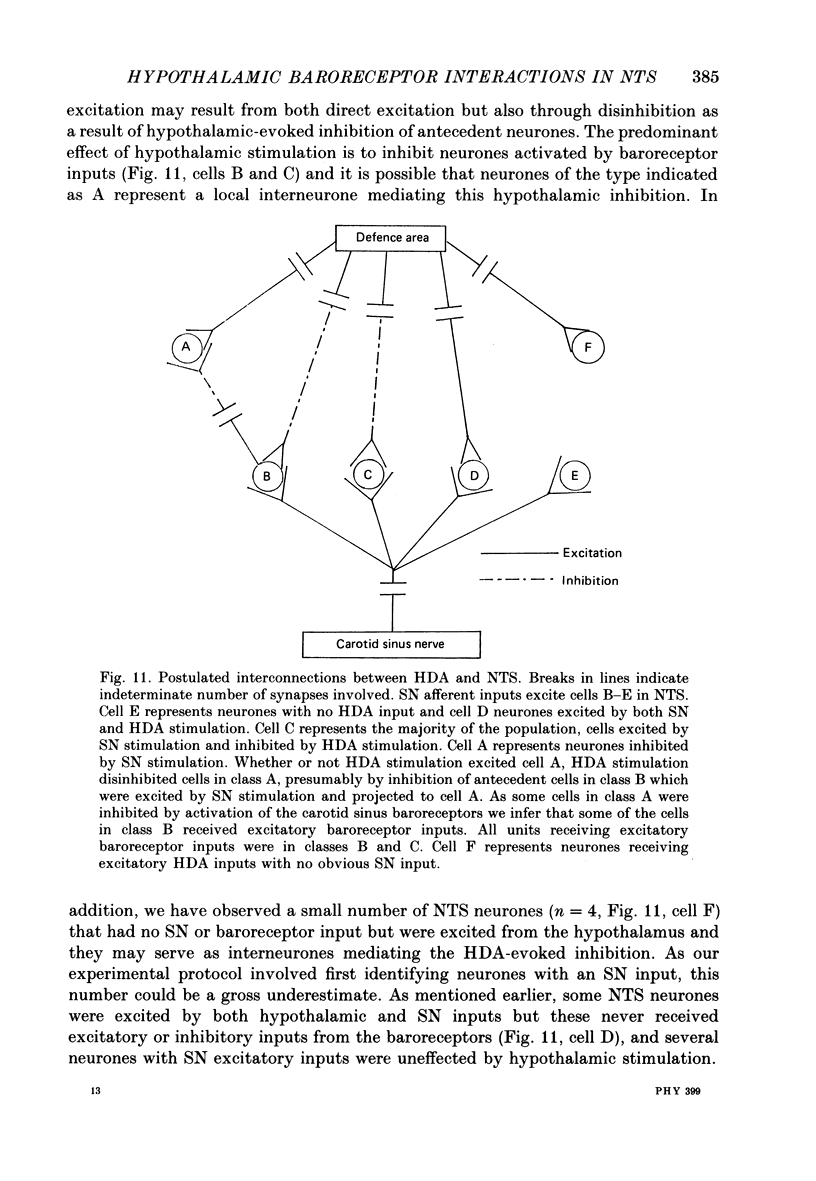
1. The effects of stimulation within the hypothalamic defence area (HDA) on the activity of neurones in the nucleus of the solitary tract (NTS) have been investigated in vivo. 2. HDA stimulation exerted marked influences on NTS neurones. Approximately two-thirds of units receiving a carotid sinus nerve (SN) input were inhibited by HDA stimulation. All units shown to receive an excitatory input from carotid sinus baroreceptors were inhibited by HDA stimulation. 3. The specificity of the HDA stimulation was investigated by generalized hypothalamic stimulation. In these experiments the number of units activated by SN stimulation that were inhibited was reduced considerably. A much smaller percentage (27%) of baroreceptive units were inhibited from hypothalamic stimulation outside the defence area. 4. Intracellular recordings revealed that HDA stimulation evoked a long-lasting hyperpolarization of membrane potential that resulted from postsynaptic inhibition (rather than disfacilitation). The HDA-evoked IPSP 'shunted' the SN-evoked EPSP when the SN stimulus was timed to occur during the initial peak hyperpolarizing phase of the HDA-evoked IPSP. 5. HDA stimulation disinhibited cells receiving an inhibitory input from the carotid sinus baroreceptors. 6. The effects of HDA stimulation were not limited to cells receiving SN afferent information or to cells within the NTS. 7. Our results explain, at the intracellular level, the mechanism for the central suppression of the baroreceptor reflex that forms an integral part of the defence response in the cat.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair J. R., Manning J. W. Hypothalamic modulation of baroreceptor afferent unit activity. Am J Physiol. 1975 Nov;229(5):1357–1364. doi: 10.1152/ajplegacy.1975.229.5.1357. [DOI] [PubMed] [Google Scholar]
- Applegate C. D., Kapp B. S., Underwood M. D., McNall C. L. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav. 1983 Sep;31(3):353–360. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- Coote J. H., Hilton S. M., Perez-Gonzalez J. F. Inhibition of the baroreceptor reflex on stimulation in the brain stem defence centre. J Physiol. 1979 Mar;288:549–560. [PMC free article] [PubMed] [Google Scholar]
- Cox G. E., Jordan D., Moruzzi P., Schwaber J. S., Spyer K. M., Turner S. A. Amygdaloid influences on brain-stem neurones in the rabbit. J Physiol. 1986 Dec;381:135–148. doi: 10.1113/jphysiol.1986.sp016318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton S. M., Redfern W. S. A search for brain stem cell groups integrating the defence reaction in the rat. J Physiol. 1986 Sep;378:213–228. doi: 10.1113/jphysiol.1986.sp016215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D., Mifflin S. W., Spyer K. M. Hypothalamic inhibition of neurones in the nucleus tractus solitarius of the cat is GABA mediated. J Physiol. 1988 May;399:389–404. doi: 10.1113/jphysiol.1988.sp017087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D., Spyer K. M. Studies on the excitability of sinus nerve afferent terminals. J Physiol. 1979 Dec;297(0):123–134. doi: 10.1113/jphysiol.1979.sp013031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen R. M., Spyer K. M. The location of cardiac vagal preganglionic motoneurones in the medulla of the cat. J Physiol. 1976 Jun;258(1):187–204. doi: 10.1113/jphysiol.1976.sp011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin S. W., Spyer K. M., Withington-Wray D. J. Baroreceptor inputs to the nucleus tractus solitarius in the cat: postsynaptic actions and the influence of respiration. J Physiol. 1988 May;399:349–367. doi: 10.1113/jphysiol.1988.sp017085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986 Apr;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIL E., REDWOOD C. R. M., SCHWEITZER A. Pressor responses to electrical stimulation of the carotid sinus nerve in cats. J Physiol. 1949 Sep;109(3-4):259–271. doi: 10.1113/jphysiol.1949.sp004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypert G. W., Munson J. B., Fleshman J. W. Effect of presynaptic inhibition on axonal potentials, terminal potentials, focal synaptic potentials, and EPSPs in cat spinal cord. J Neurophysiol. 1980 Oct;44(4):792–803. doi: 10.1152/jn.1980.44.4.792. [DOI] [PubMed] [Google Scholar]
- Timms R. J. A study of the amygdaloid defence reaction showing the value of Althesin anaesthesia in studies of the functions of the fore-brain in cats. Pflugers Arch. 1981 Jul;391(1):49–56. doi: 10.1007/BF00580694. [DOI] [PubMed] [Google Scholar]


