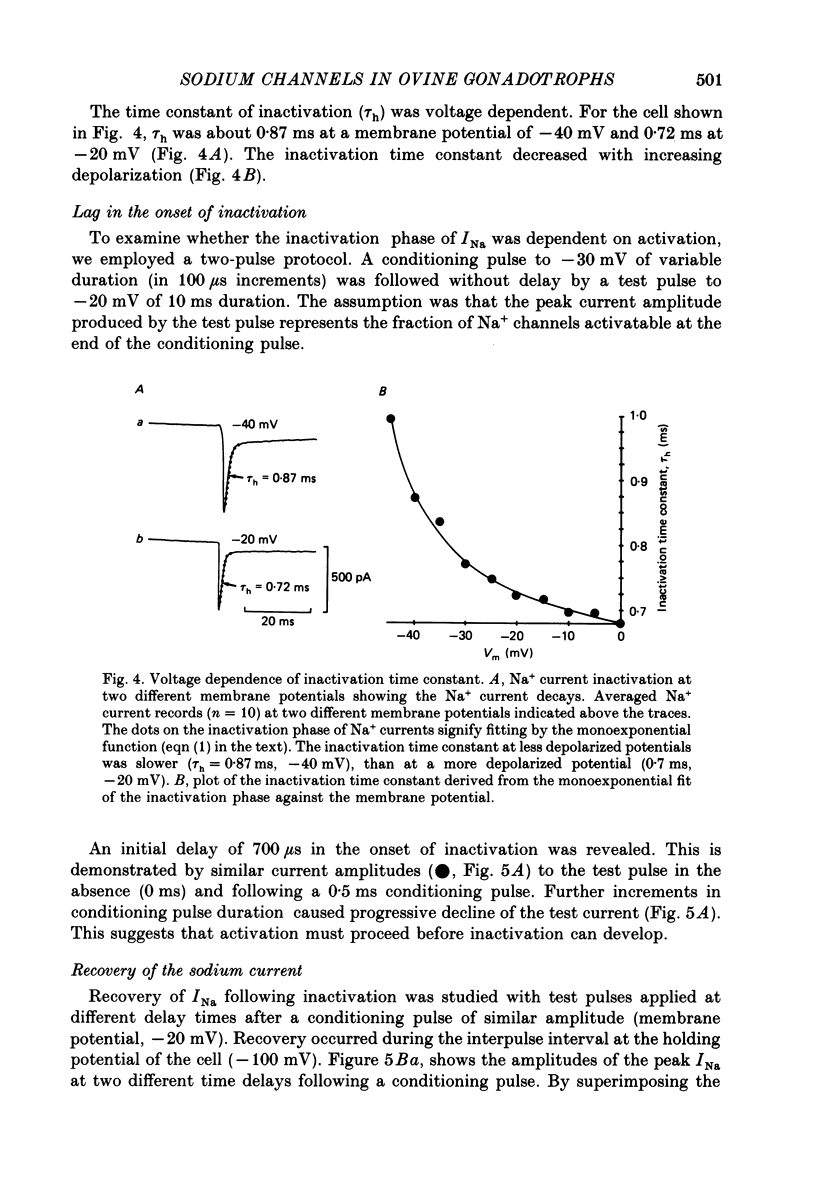
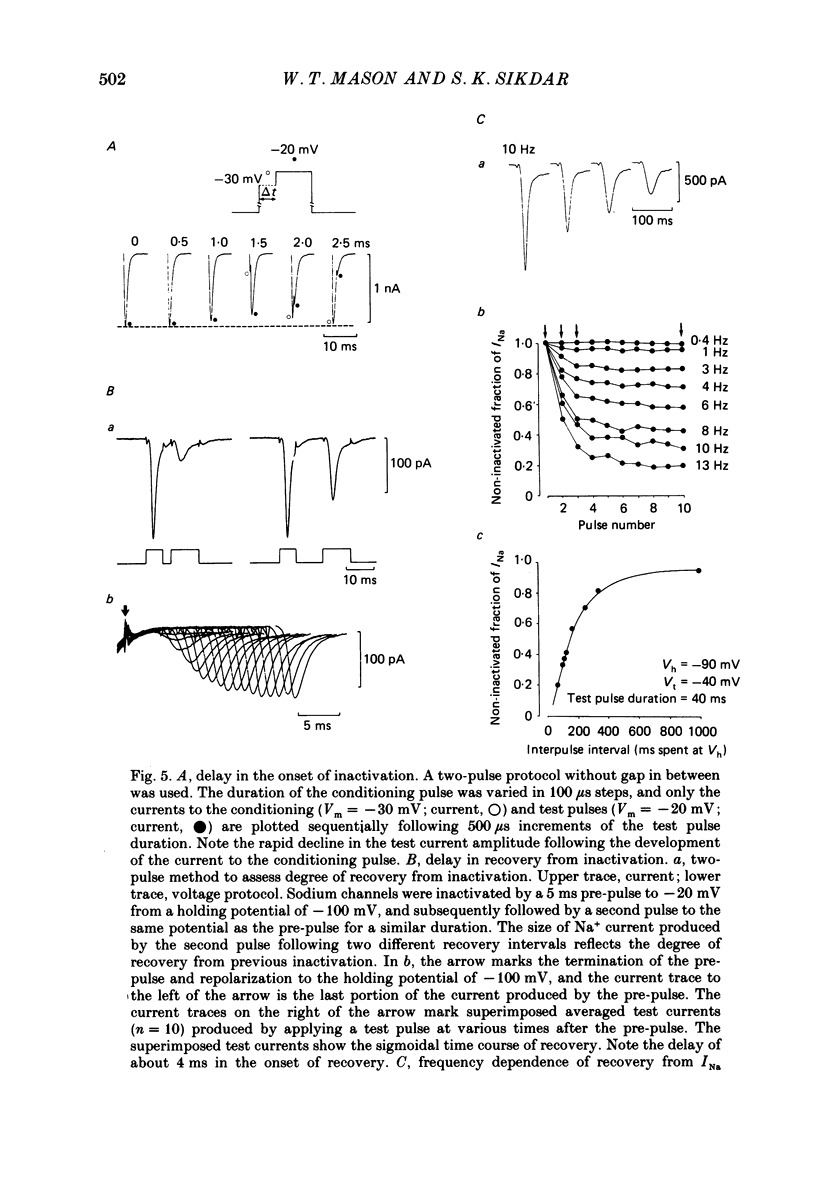
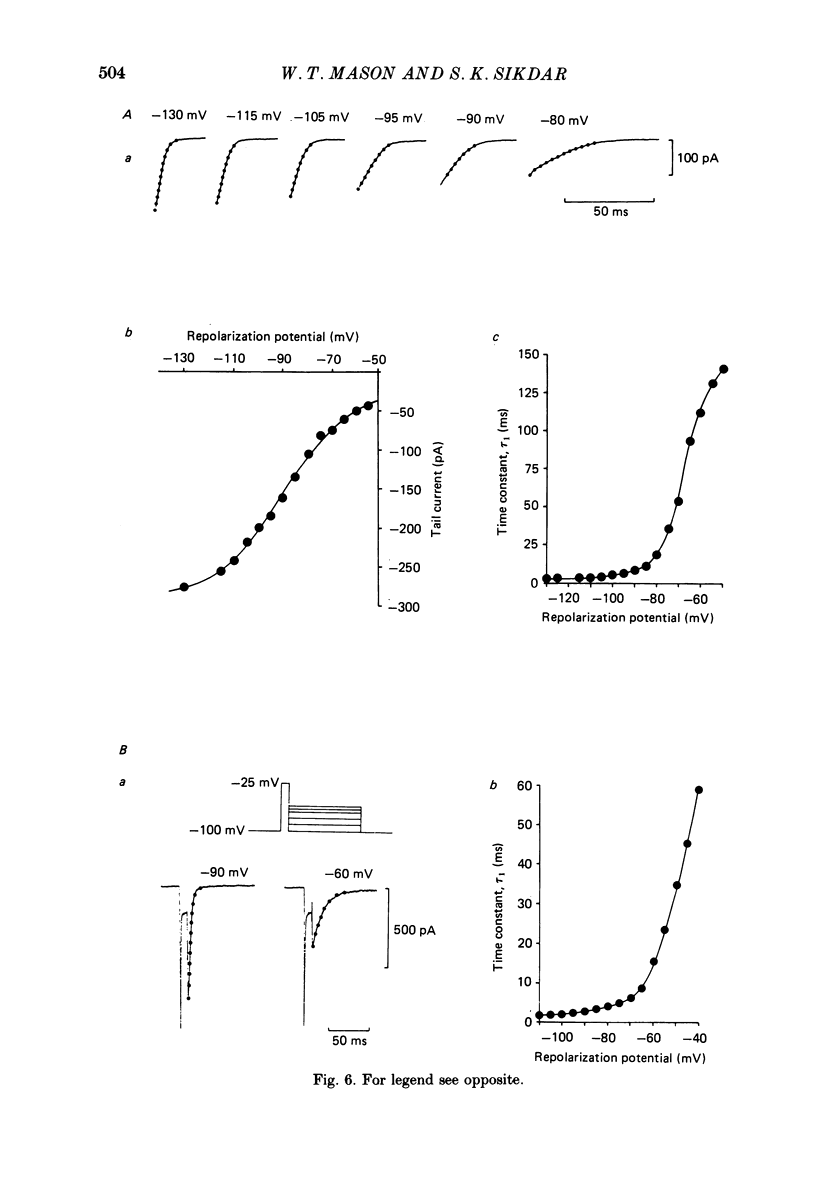
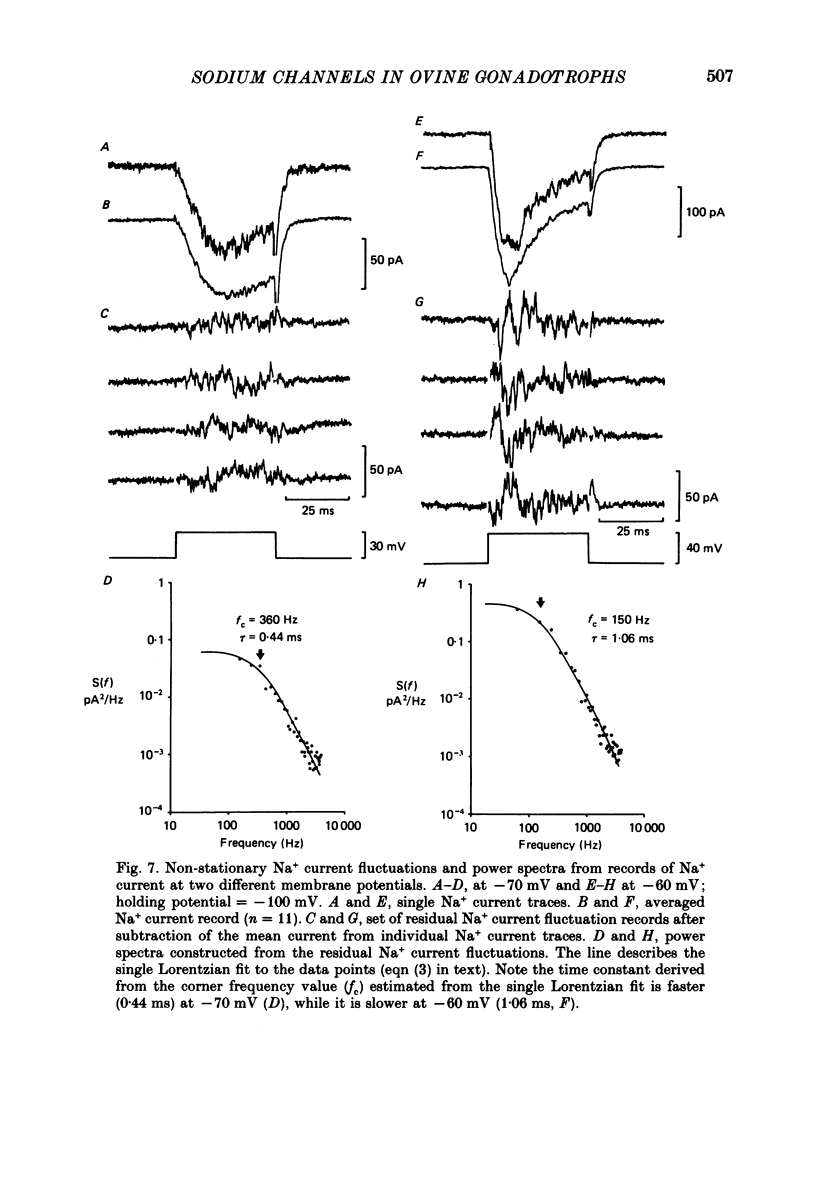
Abstract
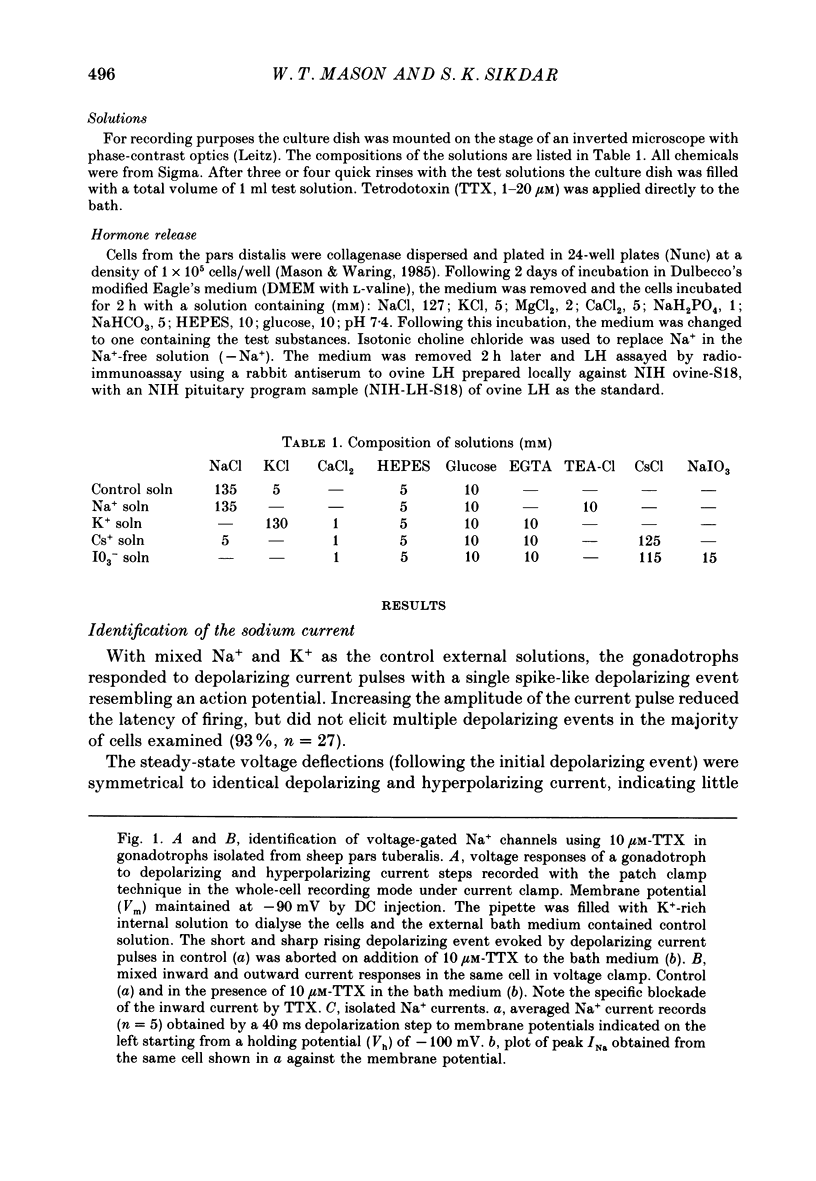
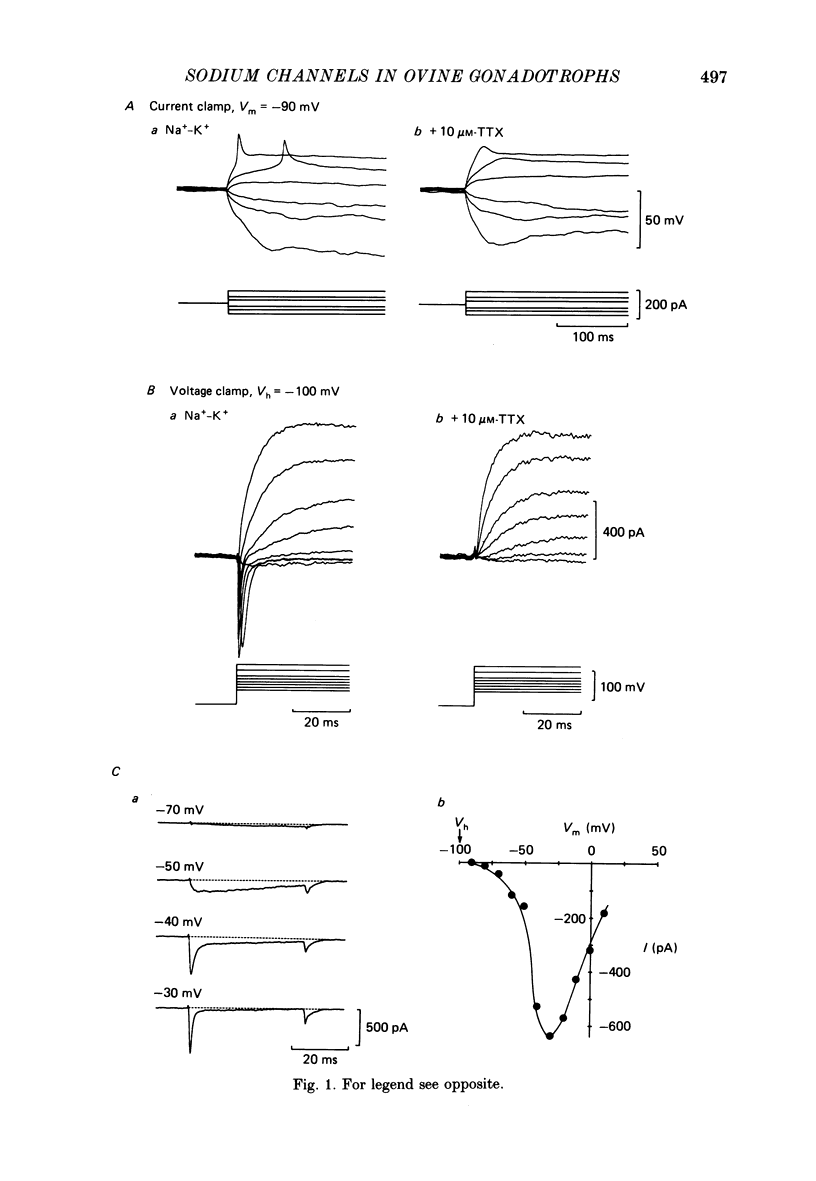
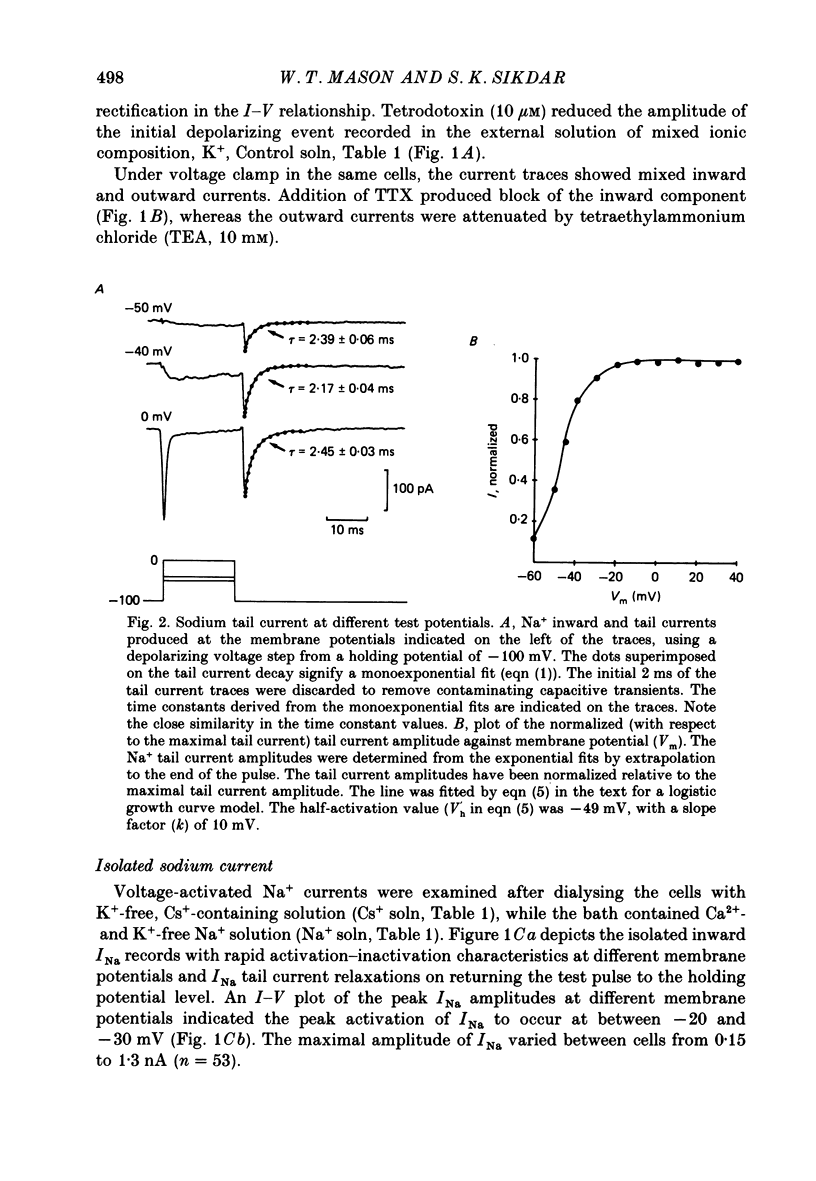
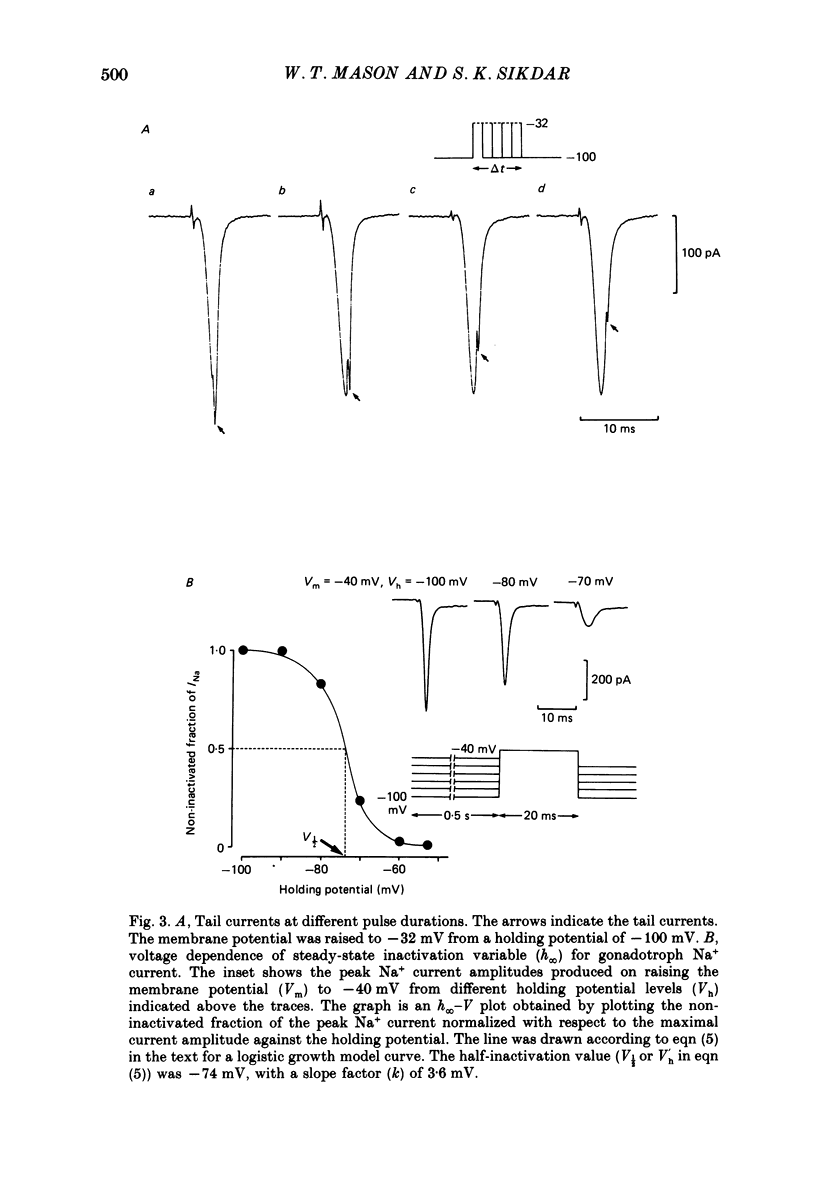
1. The properties of whole-cell Na+ currents (INa) were studied in immunocytochemically identified ovine gonadotrophs using the patch clamp technique. 2. Voltage recording under current clamp revealed that gonadotrophs did not fire spontaneously, and fired only a single action potential in response to a depolarizing current clamp step. 3. Under voltage clamp, INa was found to be sensitive to tetrodotoxin (TTX) and had an activation threshold of about -75 mV, with peak current occurring at -20 to -30 mV. 4. Using a two-pulse protocol a delay in the onset of inactivation was observed, suggesting that inactivation is dependent on and preceded by the activation phenomenon. 5. Kinetics of recovery from inactivation of the Na+ channels were studied with test pulses applied at various times after a depolarizing pre-pulse. Recovery from inactivation showed an initial delay, in contrast to the predictions of the Hodgkin-Huxley equations. 6. Recovery from inactivation was examined by using a repetitive pulse protocol, showing approximately 1 s is required for the channels to achieve a 95% recovery. 7. The steady-state inactivation (h infinity -V) curve was sigmoidal and fitted by a logistic growth curve model. The half-inactivation value of the Na+ current occurred at a membrane potential of -70 +/- 8 mV. 8. Noise power spectra derived from fluctuations of INa could be fitted with a single Lorentzian function, and the time constant value was slower at more depolarizing potentials. 9. The single-Na+-channel conductance was estimated from fluctuation analysis under conditions of reduced Na+ current amplitude by depolarizing pre-pulses. The single-channel conductance derived by the above method (approximately equal to 11 pS) corresponded to the single-channel conductance derived from single-channel current measurements using the outside-out version of the patch clamp technique (approximately equal to 13 pS). 10. Inactivation of INa was slowed by including 15 mM-iodate in the pipette. Ensemble fluctuation analysis of INa under these conditions was carried out using the steady state portion of the inactivation phase of the modified INa records, revealing a process best fitted by a double Lorentzian power spectrum, consistent with inactivation kinetics involving both a fast and a slow process. The time constant values correlated well with those obtained from a double-exponential fit to the decaying inactivation phase of the iodate-modified INa.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Palti Y. The effects of external potassium and long duration voltage conditioning on the amplitude of sodium currents in the giant axon of the squid, Loligo pealei. J Gen Physiol. 1969 Nov;54(5):589–606. doi: 10.1085/jgp.54.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Marshall M. W. Sodium currents in mammalian muscle. J Physiol. 1977 Jun;268(1):223–250. doi: 10.1113/jphysiol.1977.sp011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado L. I., Hancke J. L., Rodriguez S., Rodriguez E. M. Changes in the luteinizing hormone content of the rat pars tuberalis during the estrus cycle and after lesions in the preoptic area. Neuroendocrinology. 1982;35(3):178–185. doi: 10.1159/000123378. [DOI] [PubMed] [Google Scholar]
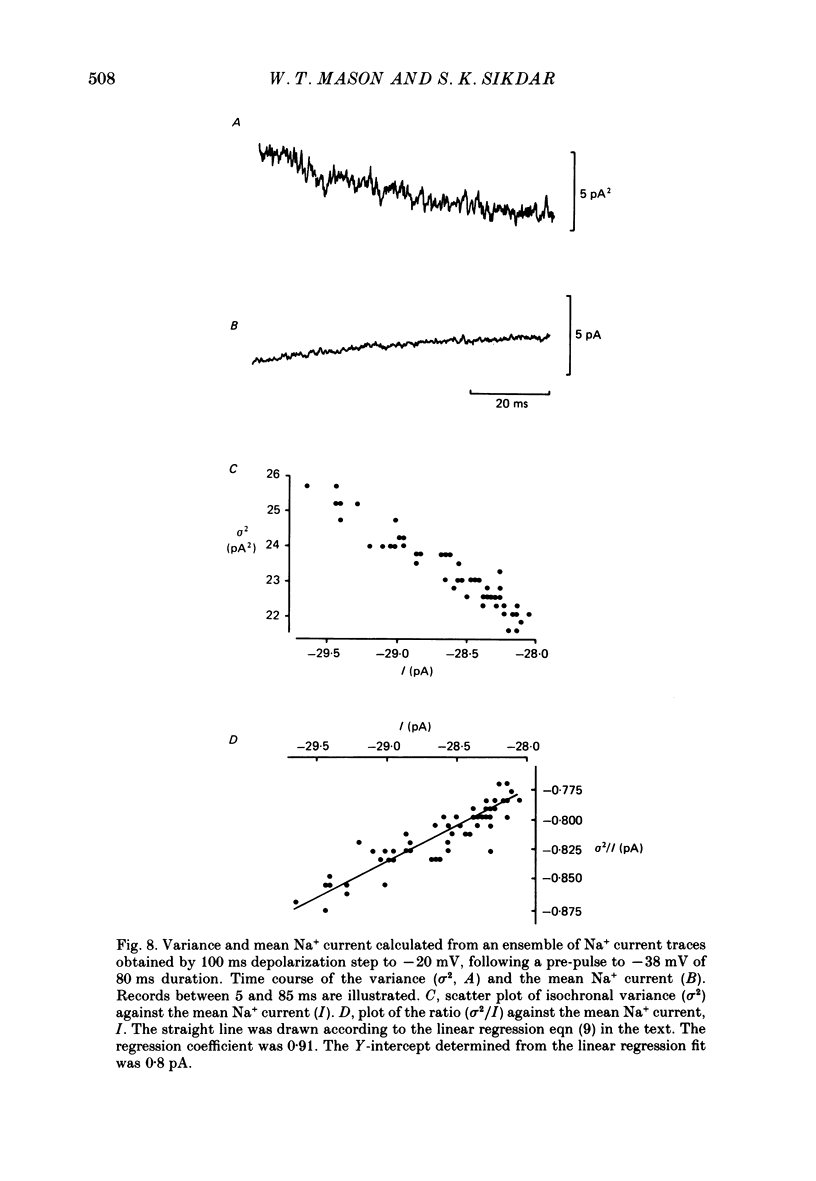
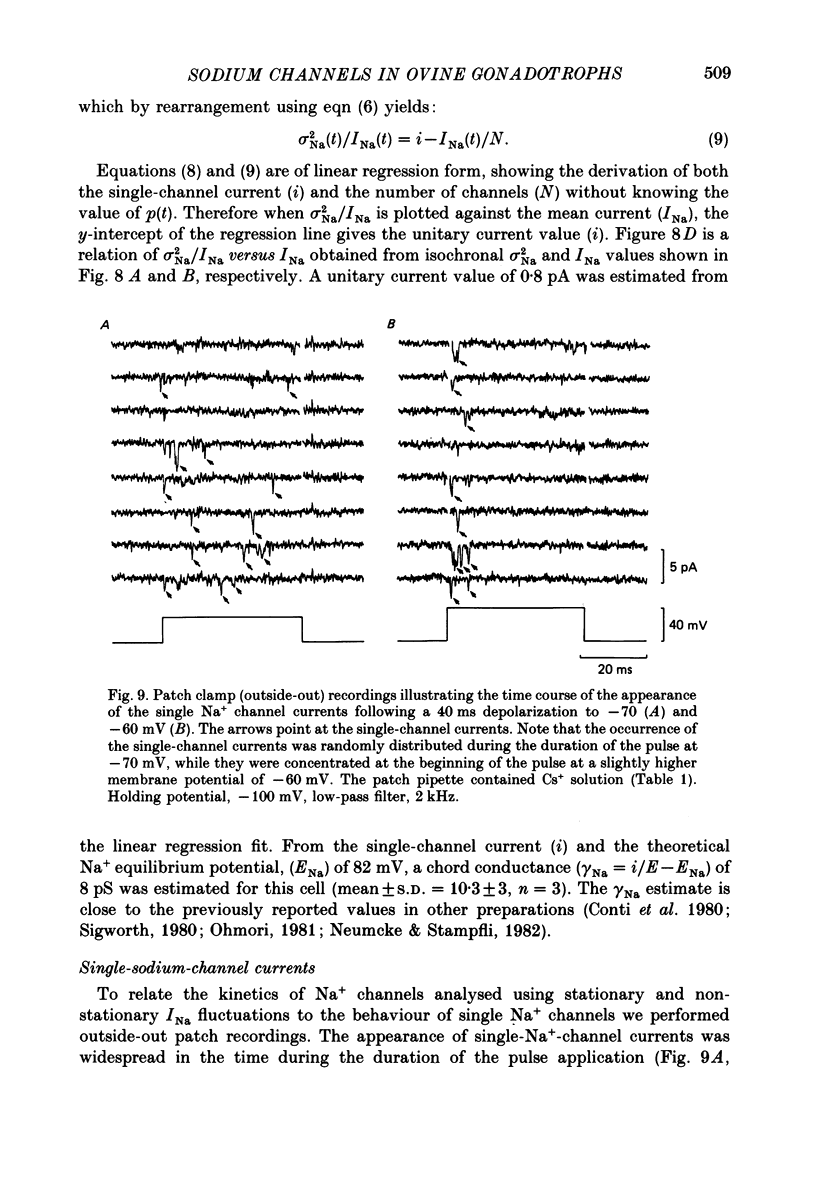
- Attwell D., Cohen I., Eisner D., Ohba M., Ojeda C. The steady state TTX-sensitive ("window") sodium current in cardiac Purkinje fibres. Pflugers Arch. 1979 Mar 16;379(2):137–142. doi: 10.1007/BF00586939. [DOI] [PubMed] [Google Scholar]
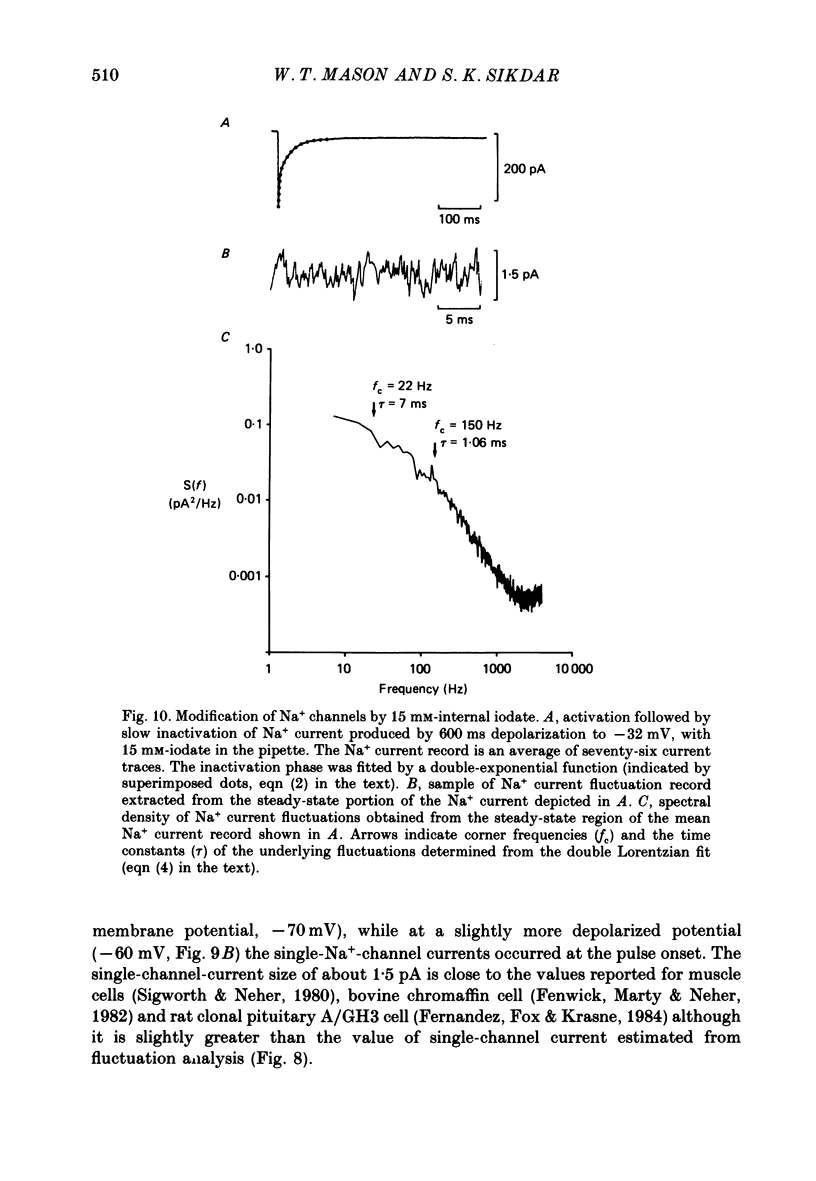
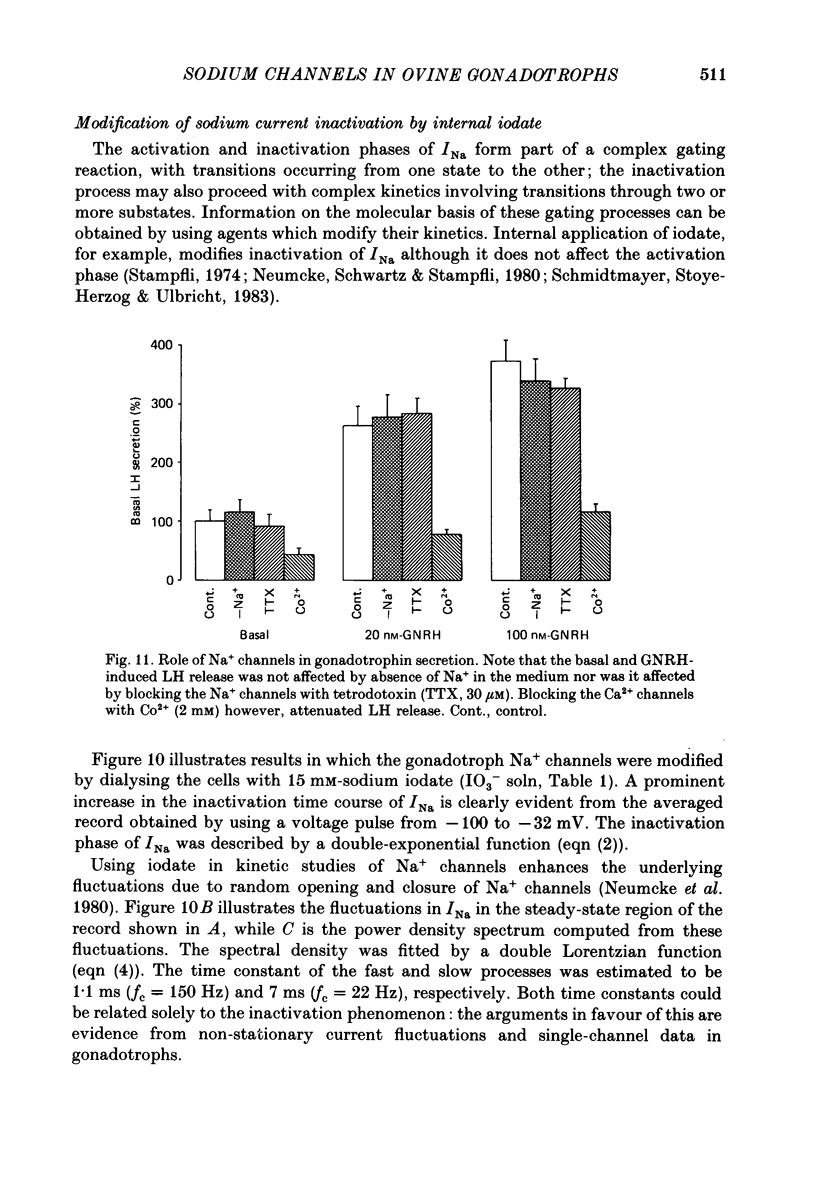
- Bean B. P. Sodium channel inactivation in the crayfish giant axon. Must channels open before inactivating? Biophys J. 1981 Sep;35(3):595–614. doi: 10.1016/S0006-3495(81)84815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs G. V., Hyde C., Naor Z., Catt K. Heterogeneous luteinizing hormone and follicle-stimulating hormone storage patterns in subtypes of gonadotropes separated by centrifugal elutriation. Endocrinology. 1983 Dec;113(6):2120–2128. doi: 10.1210/endo-113-6-2120. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M., Rogart R. B., Stagg D. A quantitative description of membrane currents in rabbit myelinated nerve. J Physiol. 1979 Jul;292:149–166. doi: 10.1113/jphysiol.1979.sp012843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett P., Ingram C. D., Mason W. T. Sodium and potassium currents involved in action potential propagation in normal bovine lactotrophs. J Physiol. 1987 Nov;392:273–299. doi: 10.1113/jphysiol.1987.sp016780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn P. M., Rogers D. C. Gonadotropin release from pituitary cultures following activation of endogenous ion channels. Endocrinology. 1980 Dec;107(6):2133–2134. doi: 10.1210/endo-107-6-2133. [DOI] [PubMed] [Google Scholar]
- Conti F., Neumcke B., Nonner W., Stämpfli R. Conductance fluctuations from the inactivation process of sodium channels in myelinated nerve fibres. J Physiol. 1980 Nov;308:217–239. doi: 10.1113/jphysiol.1980.sp013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denef C., Swennen L., Andries M. Separated anterior pituitary cells and their response to hypophysiotropic hormones. Int Rev Cytol. 1982;76:225–244. doi: 10.1016/s0074-7696(08)61792-1. [DOI] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Ionic currents in two strains of rat anterior pituitary tumor cells. J Gen Physiol. 1984 Mar;83(3):309–339. doi: 10.1085/jgp.83.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Fox A. P., Krasne S. Membrane patches and whole-cell membranes: a comparison of electrical properties in rat clonal pituitary (GH3) cells. J Physiol. 1984 Nov;356:565–585. doi: 10.1113/jphysiol.1984.sp015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Inactivation of the sodium current in Myxicola giant axons. Evidence for coupling to the activation process. J Gen Physiol. 1972 Jun;59(6):659–675. doi: 10.1085/jgp.59.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. S. Effect of castration and steroid replacement on immunoreactive luteinizing hormone cells in the pars tuberalis of the rat. Endocrinology. 1978 Aug;103(2):583–588. doi: 10.1210/endo-103-2-583. [DOI] [PubMed] [Google Scholar]
- Gross D. S., Turgeon J. L., Waring D. W. The ovine pars tuberalis: a naturally occurring source of partially purified gonadotropes which secrete luteinizing hormone in vitro. Endocrinology. 1984 Jun;114(6):2084–2091. doi: 10.1210/endo-114-6-2084. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y. Spontaneous calcium action potentials in a clonal pituitary cell line and their relationship to prolactin secretion. Nature. 1975 Dec 25;258(5537):741–742. doi: 10.1038/258741a0. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-I. Sodium currents. Neuroscience. 1981;6(12):2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Waring D. W. Electrophysiological recordings from gonadotrophs. Evidence for Ca2+ channels mediated by gonadotrophin-releasing hormone. Neuroendocrinology. 1985 Sep;41(3):258–268. doi: 10.1159/000124186. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Waring D. W. Patch clamp recordings of single ion channel activation by gonadotrophin-releasing hormone in ovine pituitary gonadotrophs. Neuroendocrinology. 1986;43(2):205–219. doi: 10.1159/000124529. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Na and Ca channels in a transformed line of anterior pituitary cells. J Gen Physiol. 1984 Mar;83(3):371–394. doi: 10.1085/jgp.83.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Ionic currents in cultured mouse neuroblastoma cells under voltage-clamp conditions. J Physiol. 1978 May;278:265–286. doi: 10.1113/jphysiol.1978.sp012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Schwarz W., Stämpfli R. Modification of sodium inactivation in myelinated nerve by Anemonia toxin II and iodate. Analysis of current fluctuations and current relaxations. Biochim Biophys Acta. 1980 Aug 4;600(2):456–466. doi: 10.1016/0005-2736(80)90448-4. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Stämpfli R. Sodium currents and sodium-current fluctuations in rat myelinated nerve fibres. J Physiol. 1982 Aug;329:163–184. doi: 10.1113/jphysiol.1982.sp014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Unitary current through sodium channel and anomalous rectifier channel estimated from transient current noise in the tunicate egg. J Physiol. 1981 Feb;311:289–305. doi: 10.1113/jphysiol.1981.sp013585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Bullock J. O., Pencek T. L. Characteristics of sodium tail currents in Myxicola axons. Comparison with membrane asymmetry currents. Biophys J. 1977 Jul;19(1):7–28. doi: 10.1016/S0006-3495(77)85559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L. Sodium currents in Myxicola axons. Nonexponential recovery from the inactive state. Biophys J. 1974 Feb;14(2):151–154. doi: 10.1016/S0006-3495(74)70006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtmayer J., Stoye-Herzog M., Ulbricht W. Combined action of intraaxonal iodate and external sea anemone toxin ATX II on sodium channel inactivation of frog nerve fibres. Pflugers Arch. 1983 Aug;398(3):204–209. doi: 10.1007/BF00657152. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980 Oct 2;287(5781):447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. The conductance of sodium channels under conditions of reduced current at the node of Ranvier. J Physiol. 1980 Oct;307:131–142. doi: 10.1113/jphysiol.1980.sp013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stämpfli R. Intraaxonal iodate inhibits sodium inactivation. Experientia. 1974 May 15;30(5):505–508. doi: 10.1007/BF01926319. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Action potentials occur in cells of the normal anterior pituitary gland and are stimulated by the hypophysiotropic peptide thyrotropin-releasing hormone. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4064–4067. doi: 10.1073/pnas.74.9.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]


