Abstract
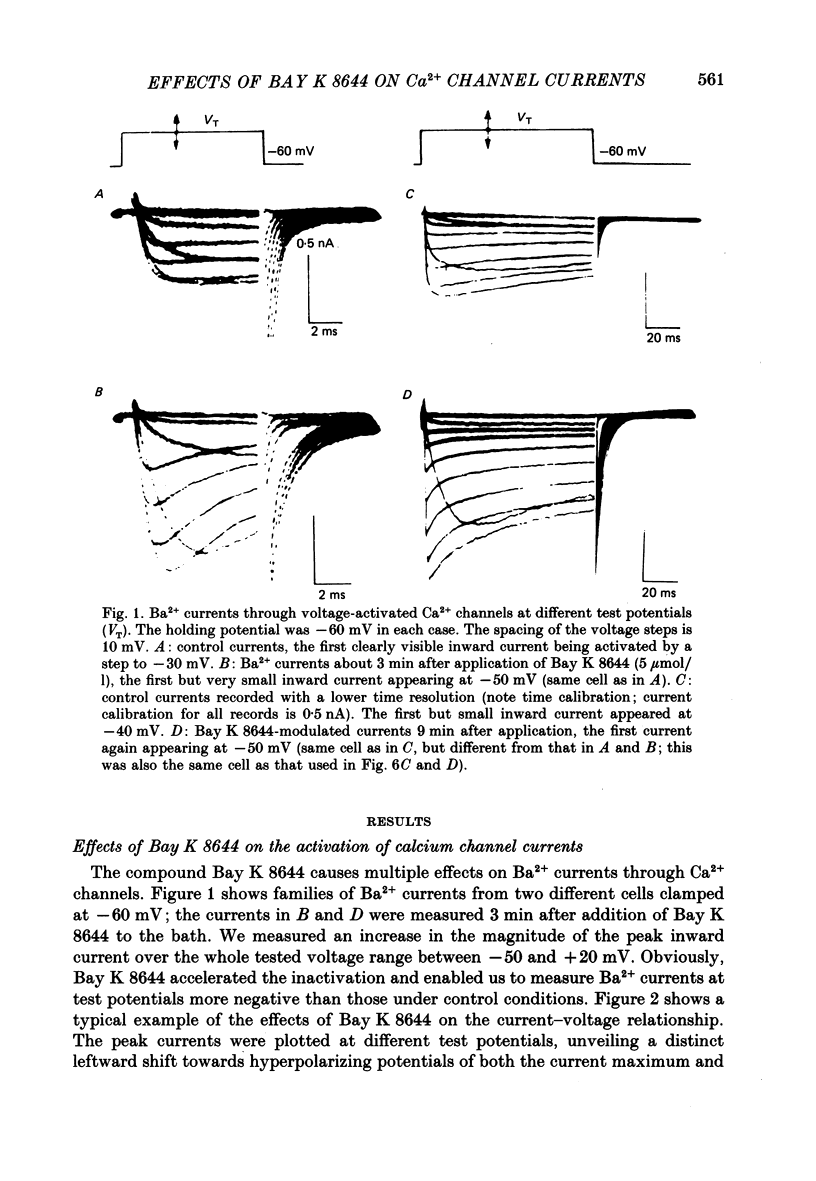
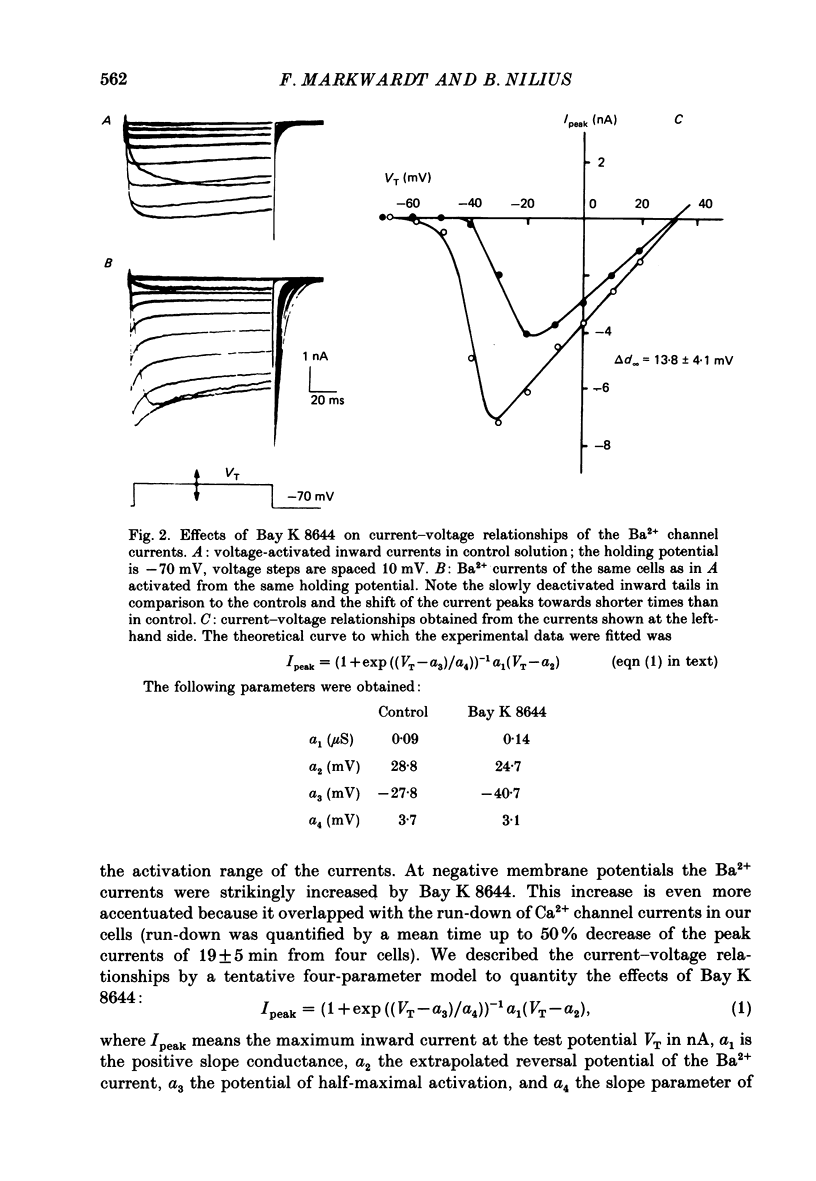
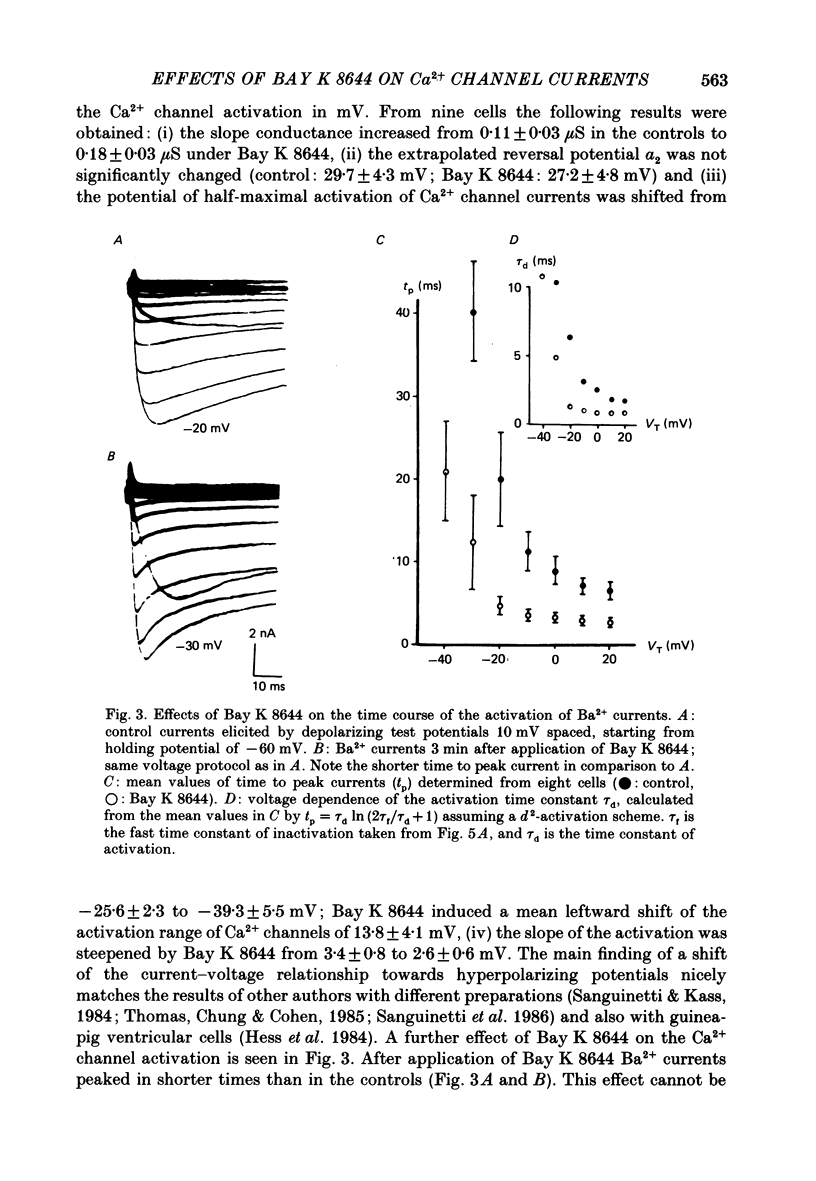
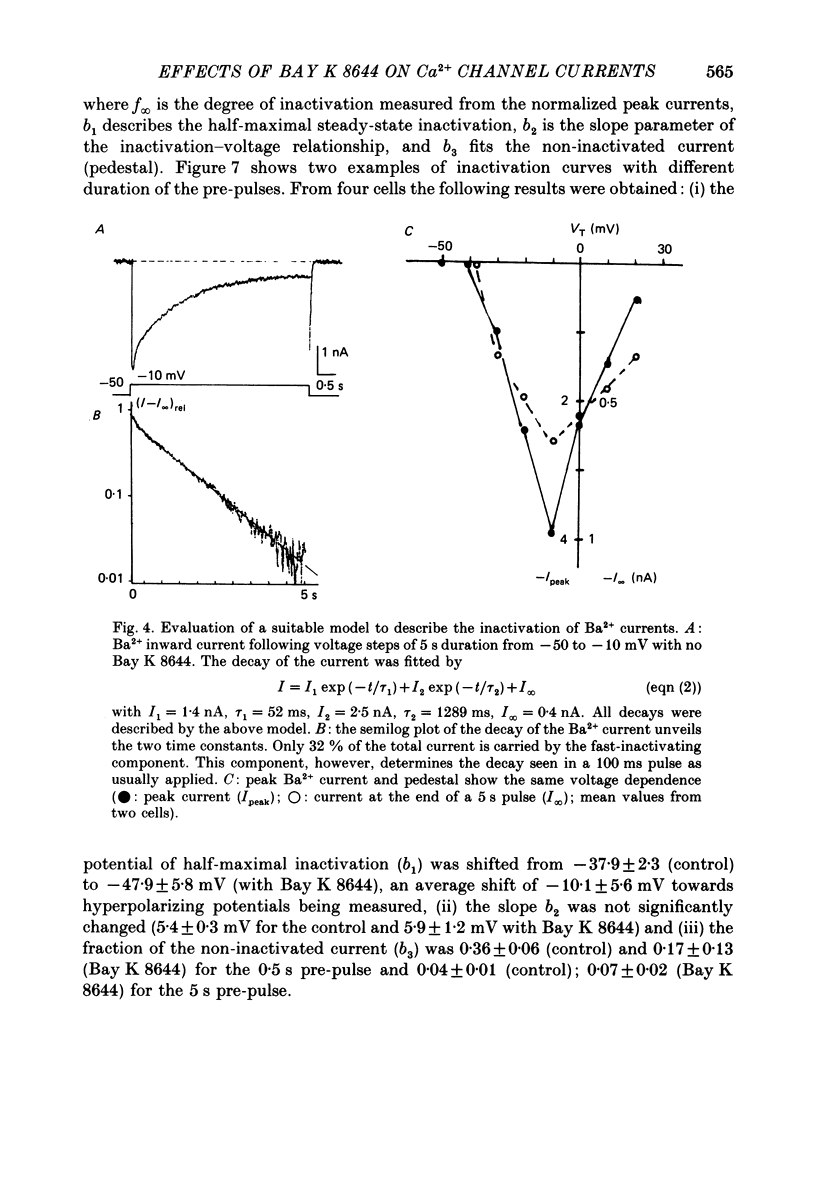
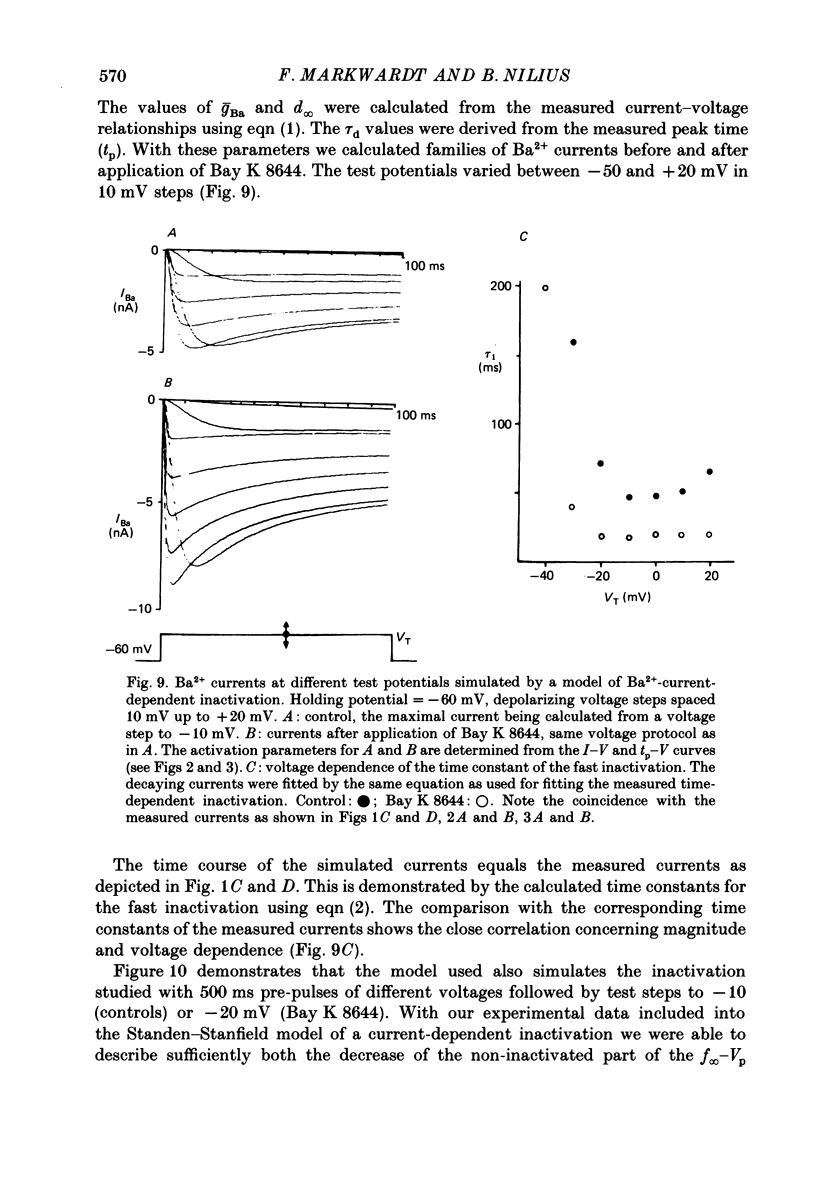
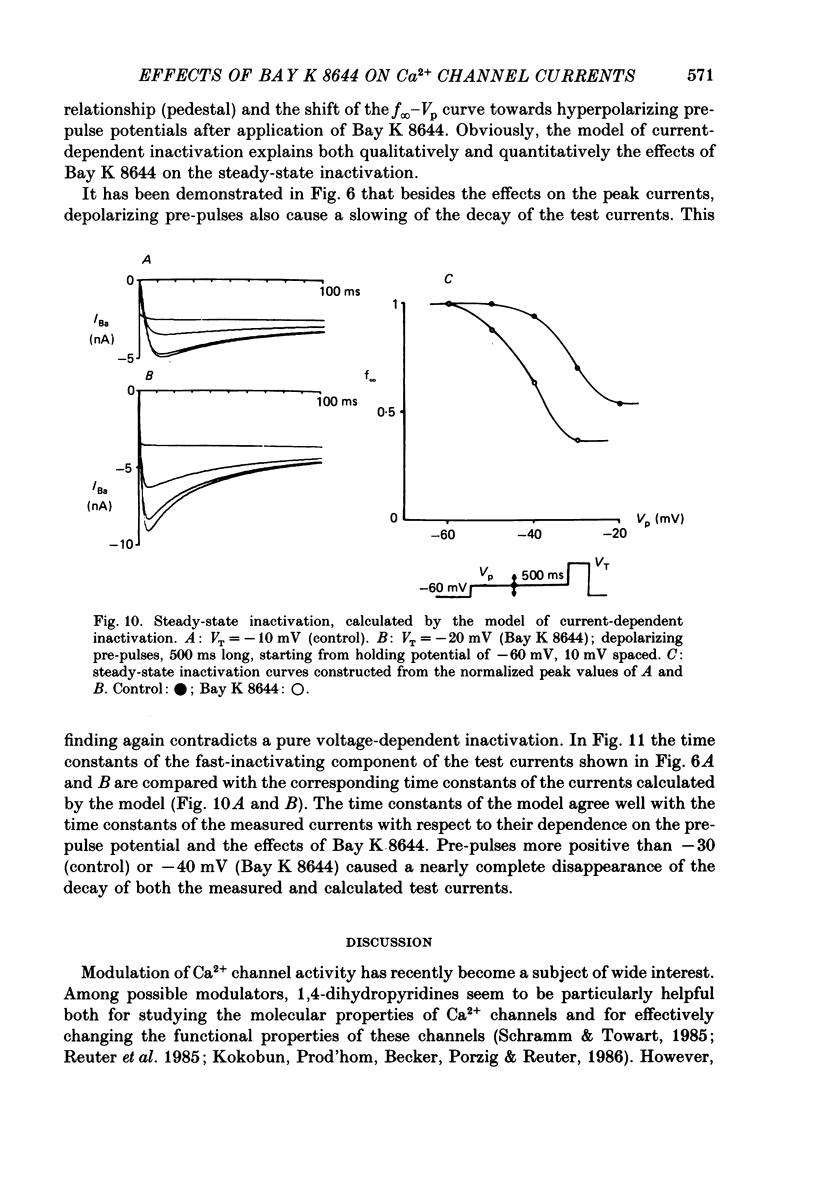
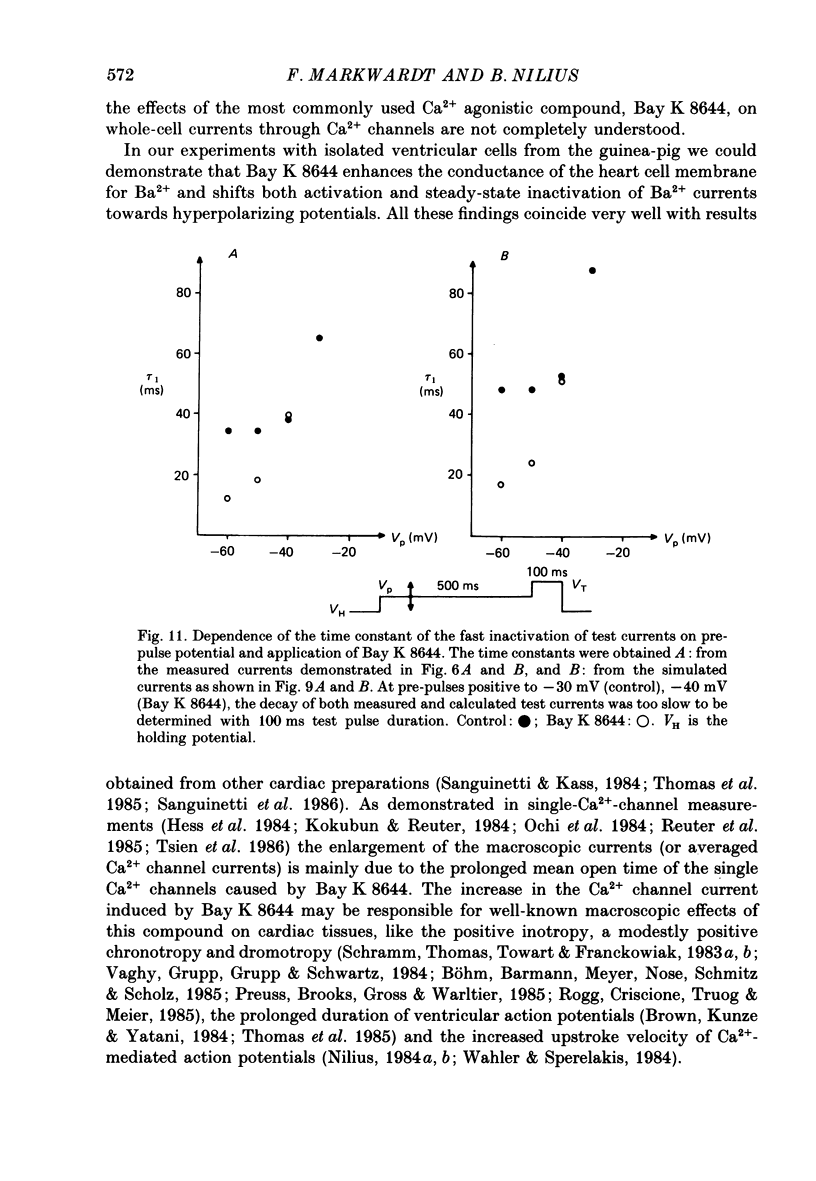
1. A single glass micropipette voltage clamp technique with intracellular dialysis was used to study Ba2+ currents in isolated ventricular cells from guinea-pig hearts. Effects of the 1,4-dihydropyridine Bay K 8644 on whole-cell currents were evaluated at 37 degrees C. 2. Bay K 8644 increased the Ba2+ peak currents at test potentials between -50 and +20 mV and shifted the current-voltage relationships towards hyperpolarizing potentials (leftward shift for Ca2+ channel activation, 13.8 +/- 4.1 mV; n = 9; Bay K 8644, 5 mumol/l). 3. The peak times of the Ba2+ currents were diminished over the voltage range tested between -40 and +20 mV after Bay K 8644 in parallel with a shortening of the time constant of activation that was estimated from fits of the recorded currents with a d2f model. 4. The decay of the Ba2+ currents was fitted with two exponentials including a pedestal. The compound Bay K 8644 accelerated the fast decay over the whole voltage range. The amplitude of the rapidly inactivated component of the Ba2+ currents was strikingly increased after application of Bay K 8644. 5. The steady-state inactivation using a 0.5 or 5 s pre-pulse was shifted towards hyperpolarizing potentials (leftward shift 10.3 +/- 5.2 mV; n = 4; Bay K 8644, 5 mumol/l). 6. The change in the time course of Bay K 8644-modified Ba2+ currents cannot be described solely by a decrease of the backward rate coefficient from an open to a closed state of the Ca2+ channel (Sanguinetti, Krafte & Kass, 1986). The described effects of Bay K 8644 on the inactivation can be both qualitatively and quantitatively described by a model of current-dependent inactivation (Standen & Stanfield, 1982), assuming a lower affinity of an internal binding site for Ba2+ than for Ca2+.
Full text
PDF


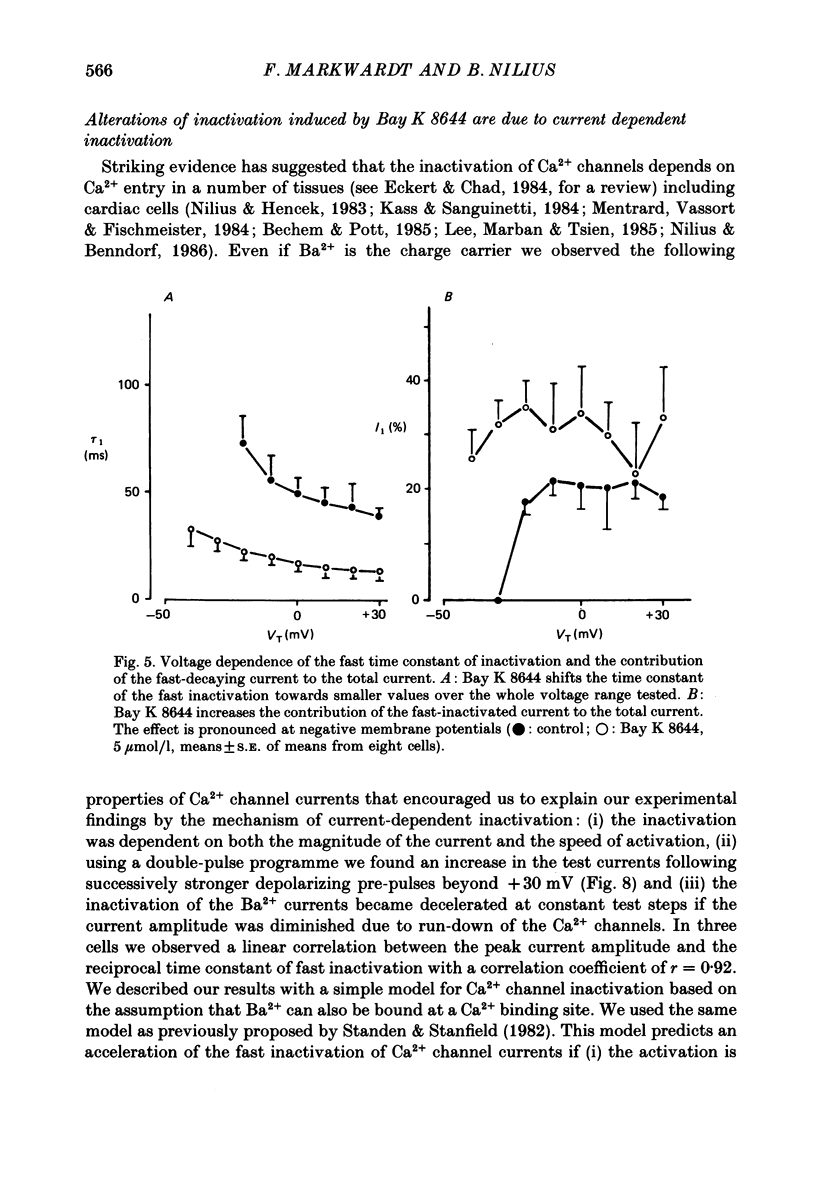
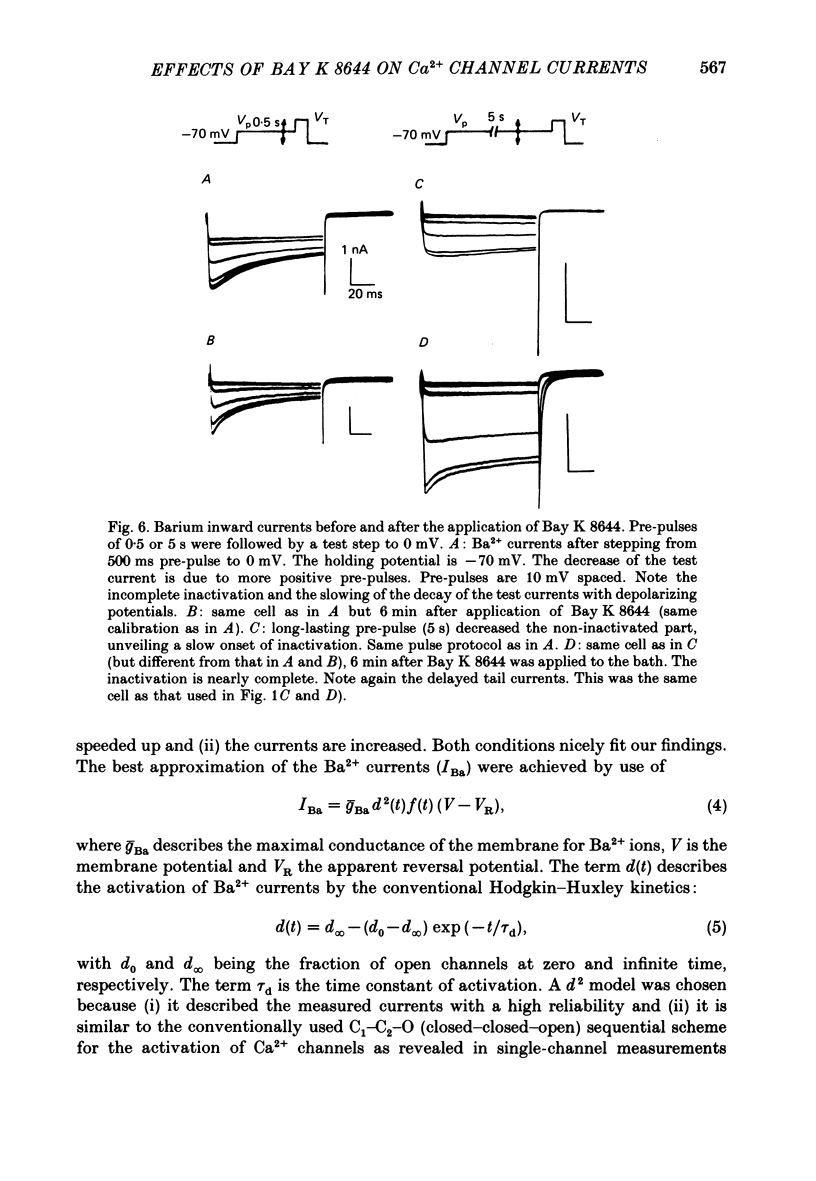
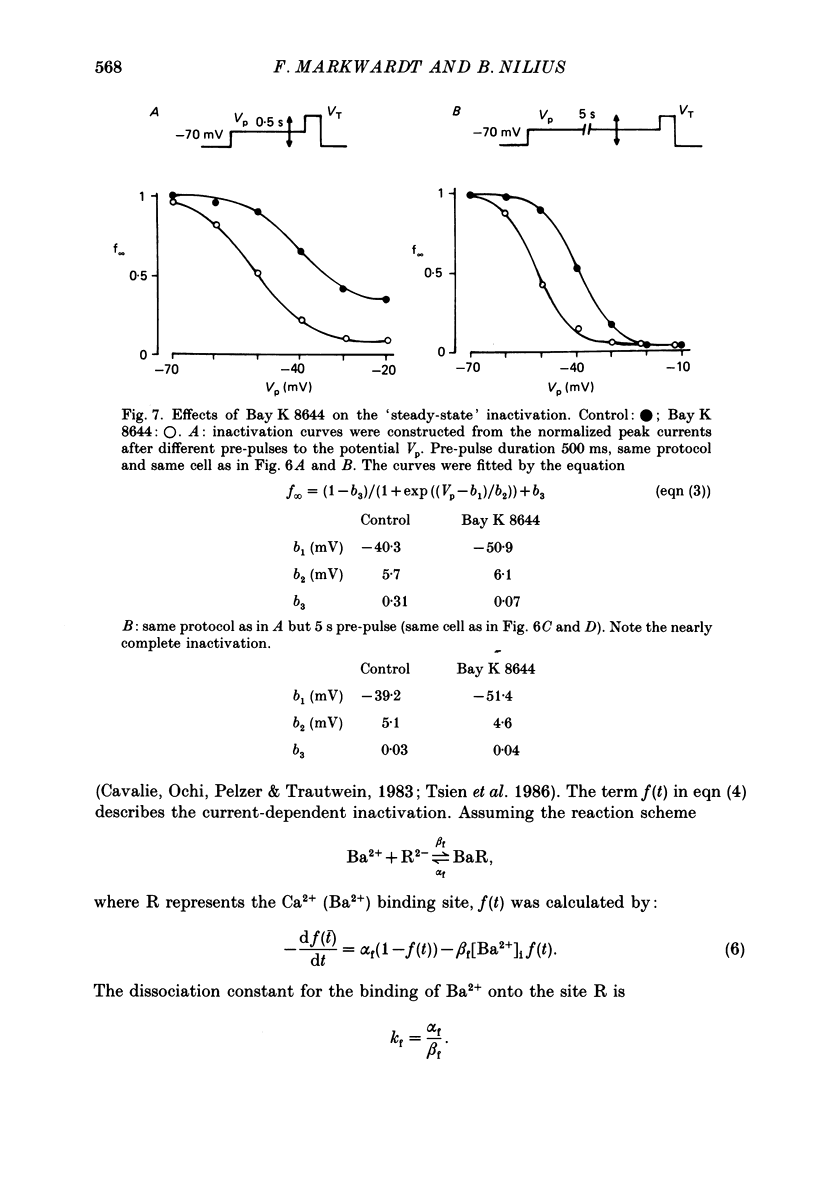
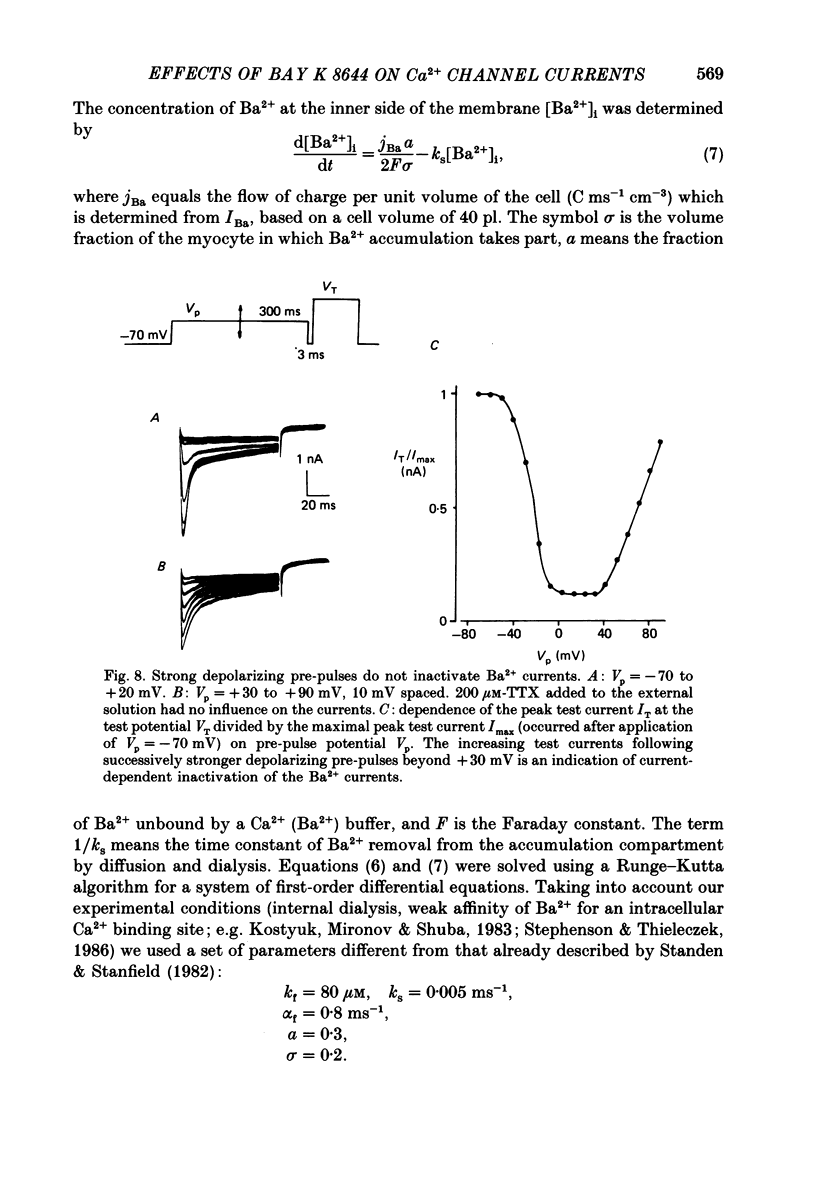



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechem M., Pott L. Removal of Ca current inactivation in dialysed guinea-pig atrial cardioballs by Ca chelators. Pflugers Arch. 1985 May;404(1):10–20. doi: 10.1007/BF00581485. [DOI] [PubMed] [Google Scholar]
- Benndorf K., Boldt W., Nilius B. Sodium current in single myocardial mouse cells. Pflugers Arch. 1985 May;404(2):190–196. doi: 10.1007/BF00585418. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. The agonist effect of dihydropyridines on Ca channels. Nature. 1984 Oct 11;311(5986):570–572. doi: 10.1038/311570a0. [DOI] [PubMed] [Google Scholar]
- Böhm M., Burmann H., Meyer W., Nose M., Schmitz W., Scholz H. Positive inotropic effect of Bay K 8644: cAMP-independence and lack of inhibitory effect of adenosine. Naunyn Schmiedebergs Arch Pharmacol. 1985 Jun;329(4):447–450. doi: 10.1007/BF00496380. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Ochi R., Pelzer D., Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflugers Arch. 1983 Sep;398(4):284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Pelzer D., Trautwein W. Fast and slow gating behaviour of single calcium channels in cardiac cells. Relation to activation and inactivation of calcium-channel current. Pflugers Arch. 1986 Mar;406(3):241–258. doi: 10.1007/BF00640910. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Kao R. L., Christman E. W., Luh S. L., Krauhs J. M., Tyers G. F., Williams E. H. The effects of insulin and anoxia on the metabolism of isolated mature rat cardiac myocytes. Arch Biochem Biophys. 1980 Sep;203(2):587–599. doi: 10.1016/0003-9861(80)90216-7. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubun S., Prod'hom B., Becker C., Porzig H., Reuter H. Studies on Ca channels in intact cardiac cells: voltage-dependent effects and cooperative interactions of dihydropyridine enantiomers. Mol Pharmacol. 1986 Dec;30(6):571–584. [PubMed] [Google Scholar]
- Kokubun S., Reuter H. Dihydropyridine derivatives prolong the open state of Ca channels in cultured cardiac cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4824–4827. doi: 10.1073/pnas.81.15.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984 Jan;83(1):105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B. Activation of Ca channels in heart muscle by a new dihydropyridine derivative. Gen Physiol Biophys. 1984 Oct;3(5):437–440. [PubMed] [Google Scholar]
- Nilius B., Benndorf K. Joint voltage- and calcium dependent inactivation of Ca channels in frog atrial myocardium. Biomed Biochim Acta. 1986;45(6):795–811. [PubMed] [Google Scholar]
- Nilius B. Stimulation of Ca-dependent action potentials in mammalian ventricular myocardium by a novel dihydropyridine. Biomed Biochim Acta. 1984;43(12):1385–1397. [PubMed] [Google Scholar]
- Preuss K. C., Brooks H. L., Gross G. J., Warltier D. C. Positive inotropic actions of the calcium channel stimulator, Bay k 8644, in awake, unsedated dogs. Basic Res Cardiol. 1985 May-Jun;80(3):326–332. doi: 10.1007/BF01907908. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Regulation of cardiac calcium channel current and contractile activity by the dihydropyridine Bay K 8644 is voltage-dependent. J Mol Cell Cardiol. 1984 Jul;16(7):667–670. doi: 10.1016/s0022-2828(84)80631-8. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Krafte D. S., Kass R. S. Voltage-dependent modulation of Ca channel current in heart cells by Bay K8644. J Gen Physiol. 1986 Sep;88(3):369–392. doi: 10.1085/jgp.88.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Activation of calcium channels by novel 1,4-dihydropyridines. A new mechanism for positive inotropics or smooth muscle stimulants. Arzneimittelforschung. 1983;33(9):1268–1272. [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Schramm M., Towart R. Modulation of calcium channel function by drugs. Life Sci. 1985 Nov 18;37(20):1843–1860. doi: 10.1016/0024-3205(85)90001-3. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A binding-site model for calcium channel inactivation that depends on calcium entry. Proc R Soc Lond B Biol Sci. 1982 Dec 22;217(1206):101–110. doi: 10.1098/rspb.1982.0097. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Thieleczek R. Activation of the contractile apparatus of skinned fibres of frog by the divalent cations barium, cadmium and nickel. J Physiol. 1986 Nov;380:75–92. doi: 10.1113/jphysiol.1986.sp016273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Chung M., Cohen C. J. A dihydropyridine (Bay k 8644) that enhances calcium currents in guinea pig and calf myocardial cells. A new type of positive inotropic agent. Circ Res. 1985 Jan;56(1):87–96. doi: 10.1161/01.res.56.1.87. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Vaghy P. L., Grupp I. L., Grupp G., Schwartz A. Effects of Bay k 8644, a dihydropyridine analog, on [3H]nitrendipine binding to canine cardiac sarcolemma and the relationship to a positive inotropic effect. Circ Res. 1984 Oct;55(4):549–553. doi: 10.1161/01.res.55.4.549. [DOI] [PubMed] [Google Scholar]
- Wahler G. M., Sperelakis N. New Ca2+ agonist (Bay K 8644) enhances and induces cardiac slow action potentials. Am J Physiol. 1984 Aug;247(2 Pt 2):H337–H340. doi: 10.1152/ajpheart.1984.247.2.H337. [DOI] [PubMed] [Google Scholar]


