Abstract
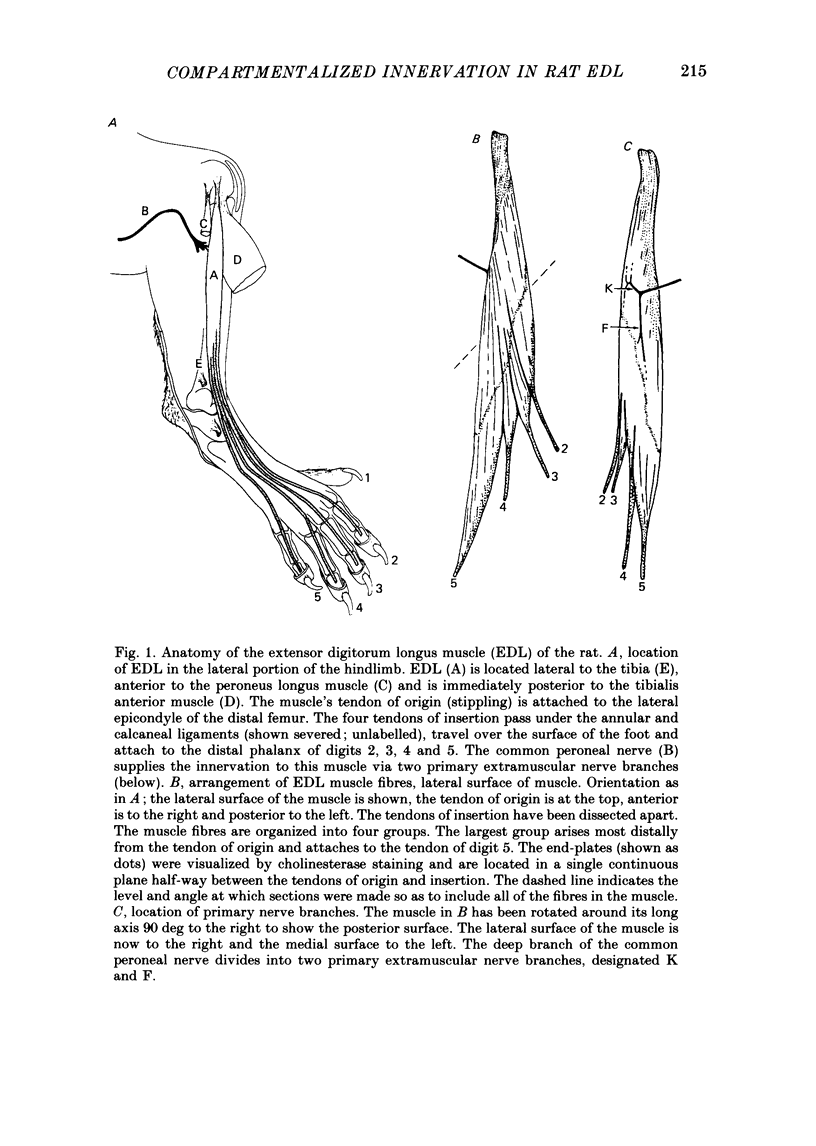
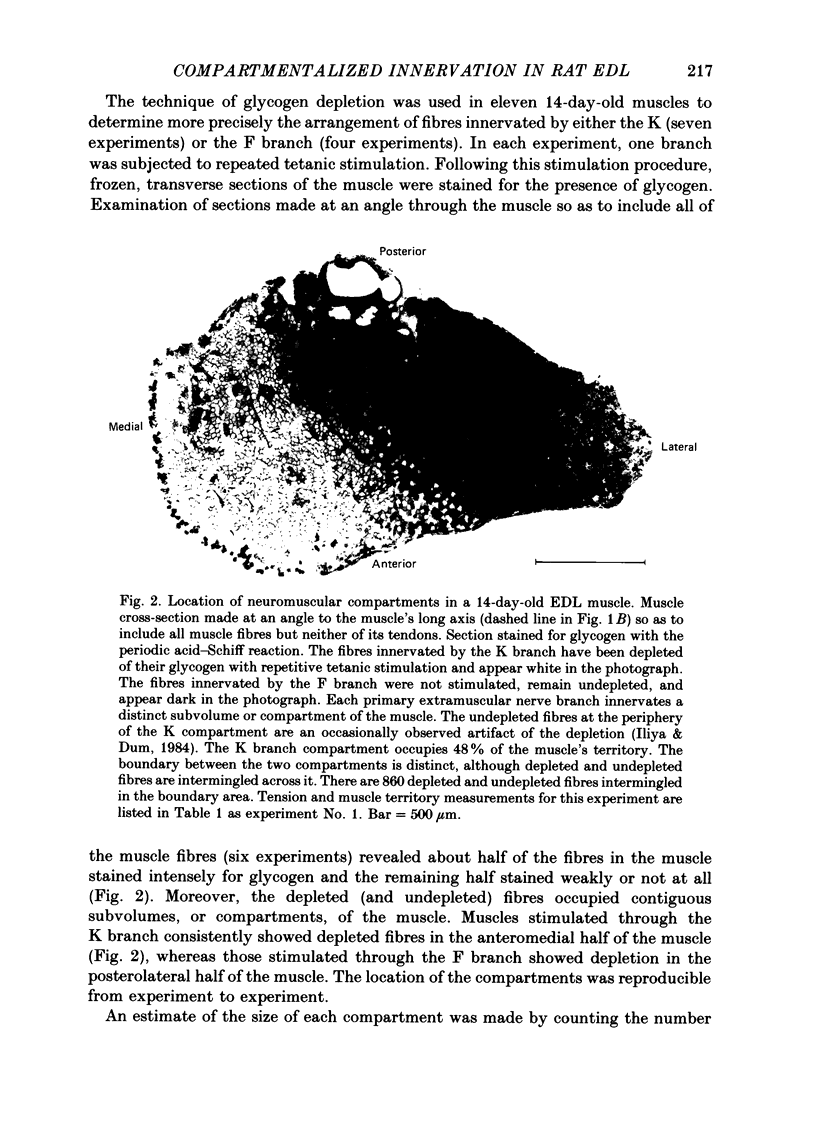
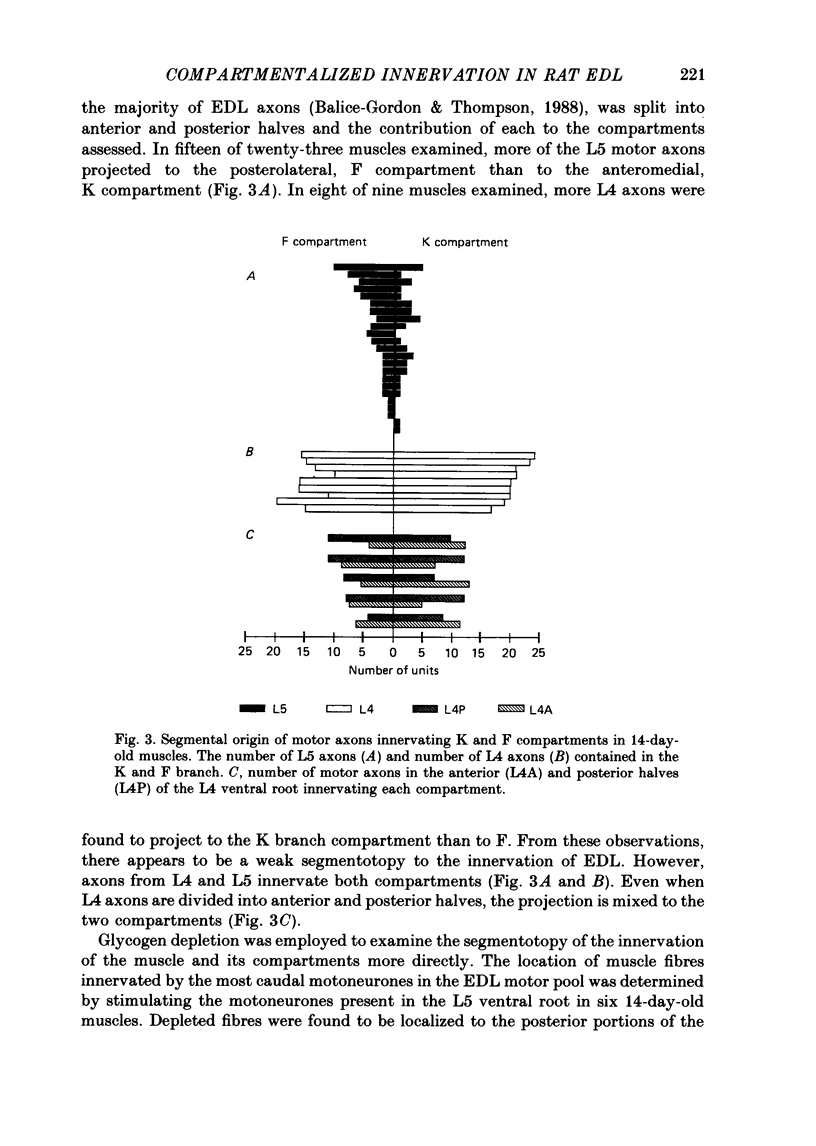
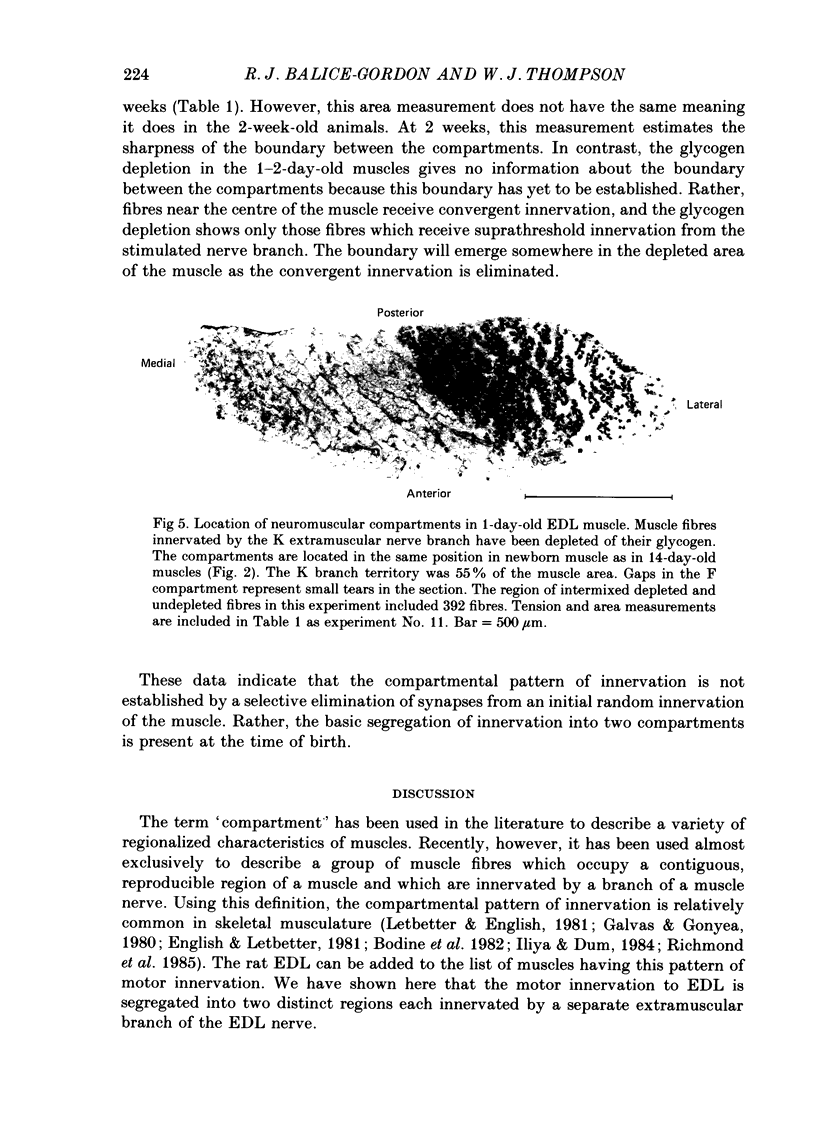
1. We have examined the innervation of the rat extensor digitorum longus (EDL) muscle by the two extramuscular branches formed from the bifurcation of its muscle nerve. Observations of muscle contractions, recordings of end-plate potentials, and glycogen depletion of young adult muscles show that each branch innervates a separate region or 'compartment' in the muscle. The branch entering the muscle nearer the knee (the K branch) innervates fibres in the anteromedial half of the muscle whereas the branch entering closer to the foot (the F branch) innervates fibres located posterolaterally. Individual EDL motoneurones project either into the K or the F branch and therefore innervate fibres located in one compartment. The boundary between the compartments is usually sharply delineated. No obvious anatomical feature exists within the muscle which would explain the division of the muscle into two distinct regions. 2. The presence of a segmentotopic projection from the spinal cord to the muscle was investigated to evaluate its possible contribution to the compartmental pattern. The most posterior neurones of the EDL motor pool were found to project more frequently to the posterolateral F compartment; similarly, the most anterior neurones most frequently project to the anteromedial K compartment. However, each compartment is innervated by both anteriorly and posteriorly located motoneurones. The segmentotopic projection is too weak to explain the presence of neuromuscular compartments. 3. The post-natal period of synapse elimination appears to play at best a minor role in setting up the compartmentalized innervation. Glycogen depletion and intracellular recording in 1-2-day-old muscles show that each nerve branch innervates fibres in the same region of the muscle as in the adult. Most of the fibres in each compartment are polyneuronally innervated by axons in their own particular nerve branch, although fibres located near the boundary between the two compartments are innervated by axons from both nerve branches. This convergent innervation from the two branches disappears in concert with the elimination of polyneuronal innervation throughout the muscle. A random elimination of these convergent inputs appears adequate to explain the final compartmental pattern. 4. Our findings suggest that the compartmental pattern is primarily the consequence of te segregation of EDL motoneurones into two nerve branches which are directed into separate regions of the muscle.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balice-Gordon R. J., Thompson W. J. Synaptic rearrangements and alterations in motor unit properties in neonatal rat extensor digitorum longus muscle. J Physiol. 1988 Apr;398:191–210. doi: 10.1113/jphysiol.1988.sp017038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D. Changing perspectives on the functional organization of the segmental motor system. Can J Physiol Pharmacol. 1986 Apr;64(4):495–498. doi: 10.1139/y86-080. [DOI] [PubMed] [Google Scholar]
- Bodine S. C., Roy R. R., Meadows D. A., Zernicke R. F., Sacks R. D., Fournier M., Edgerton V. R. Architectural, histochemical, and contractile characteristics of a unique biarticular muscle: the cat semitendinosus. J Neurophysiol. 1982 Jul;48(1):192–201. doi: 10.1152/jn.1982.48.1.192. [DOI] [PubMed] [Google Scholar]
- Botterman B. R., Hamm T. M., Reinking R. M., Stuart D. G. Localization of monosynaptic Ia excitatory post-synaptic potentials in the motor nucleus of the cat biceps femoris muscle. J Physiol. 1983 May;338:355–377. doi: 10.1113/jphysiol.1983.sp014677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Booth C. M. Postnatal development of the adult pattern of motor axon distribution in rat muscle. Nature. 1983 Aug 25;304(5928):741–742. doi: 10.1038/304741a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley G. A., Heaton J. A quantitative study of cholinesterase in myoneural junctions from rat and guinea-pig extraocular muscles. J Physiol. 1968 Dec;199(3):743–749. doi: 10.1113/jphysiol.1968.sp008676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron W. E., Binder M. D., Botterman B. R., Reinking R. M., Stuart D. G. "Sensory partitioning" of cat medial gastrocnemius muscle by its muscle spindles and tendon organs. J Neurophysiol. 1981 Jul;46(1):32–47. doi: 10.1152/jn.1981.46.1.32. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Donselaar Y., Kernell D., Eerbeek O., Verhey B. A. Somatotopic relations between spinal motoneurones and muscle fibres of the cat's musculus peroneus longus. Brain Res. 1985 May 27;335(1):81–88. doi: 10.1016/0006-8993(85)90278-1. [DOI] [PubMed] [Google Scholar]
- English A. W. An electromyographic analysis of compartments in cat lateral gastrocnemius muscle during unrestrained locomotion. J Neurophysiol. 1984 Jul;52(1):114–125. doi: 10.1152/jn.1984.52.1.114. [DOI] [PubMed] [Google Scholar]
- English A. W., Letbetter W. D. A histochemical analysis of identified compartments of cat lateral gastrocnemius muscle. Anat Rec. 1982 Oct;204(2):123–130. doi: 10.1002/ar.1092040205. [DOI] [PubMed] [Google Scholar]
- English A. W., Letbetter W. D. Anatomy and innervation patterns of cat lateral gastrocnemius and plantaris muscles. Am J Anat. 1982 May;164(1):67–77. doi: 10.1002/aja.1001640107. [DOI] [PubMed] [Google Scholar]
- English A. W., Weeks O. I. Compartmentalization of single muscle units in cat lateral gastrocnemius. Exp Brain Res. 1984;56(2):361–368. doi: 10.1007/BF00236292. [DOI] [PubMed] [Google Scholar]
- Foehring R. C., Sypert G. W., Munson J. B. Anomolous path taken by branch of medial gastrocnemius nerve. Exp Neurol. 1986 May;92(2):440–444. doi: 10.1016/0014-4886(86)90096-8. [DOI] [PubMed] [Google Scholar]
- Galvas P. E., Gonyea W. J. Motor-end-plate and nerve distribution in a histochemically compartmentalized pennate muscle in the cat. Am J Anat. 1980 Oct;159(2):147–156. doi: 10.1002/aja.1001590203. [DOI] [PubMed] [Google Scholar]
- Gonyea W. J., Ericson G. C. Morphological and histochemical organization of the flexor carpi radialis muscle in the cat. Am J Anat. 1977 Mar;148(3):329–344. doi: 10.1002/aja.1001480304. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Herring S. W., Grimm A. F., Grimm B. R. Functional heterogeneity in a multipinnate muscle. Am J Anat. 1979 Apr;154(4):563–576. doi: 10.1002/aja.1001540410. [DOI] [PubMed] [Google Scholar]
- Iliya A. R., Dum R. P. Somatotopic relations between the motor nucleus and its innervated muscle fibers in the cat tibialis anterior. Exp Neurol. 1984 Nov;86(2):272–292. doi: 10.1016/0014-4886(84)90186-9. [DOI] [PubMed] [Google Scholar]
- Kozeka K., Ontell M. The three-dimensional cytoarchitecture of developing murine muscle spindles. Dev Biol. 1981 Oct 15;87(1):133–147. doi: 10.1016/0012-1606(81)90067-1. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Histochemical composition, contraction speed and fatiguability of rat soleus motor units. J Neurol Sci. 1973 Oct;20(2):177–198. doi: 10.1016/0022-510x(73)90029-4. [DOI] [PubMed] [Google Scholar]
- Lucas S. M., Binder M. D. Topographic factors in distribution of homonymous group Ia-afferent input to cat medial gastrocnemius motoneurons. J Neurophysiol. 1984 Jan;51(1):50–63. doi: 10.1152/jn.1984.51.1.50. [DOI] [PubMed] [Google Scholar]
- Lyons G. E., Haselgrove J., Kelly A. M., Rubinstein N. A. Myosin transitions in developing fast and slow muscles of the rat hindlimb. Differentiation. 1983;25(2):168–175. doi: 10.1111/j.1432-0436.1984.tb01352.x. [DOI] [PubMed] [Google Scholar]
- Pullen A. H. The distribution and relative sized of fibre types in the extensor digitorum longus and soleus muscles of the adult rat. J Anat. 1977 Apr;123(Pt 2):467–486. [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond F. J., MacGillis D. R., Scott D. A. Muscle-fiber compartmentalization in cat splenius muscles. J Neurophysiol. 1985 Apr;53(4):868–885. doi: 10.1152/jn.1985.53.4.868. [DOI] [PubMed] [Google Scholar]
- Swett J. E., Eldred E., Buchwald J. S. Somatotopic cord-to-muscle relations in efferent innervation of cat gastrocnemius. Am J Physiol. 1970 Sep;219(3):762–766. doi: 10.1152/ajplegacy.1970.219.3.762. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Sutton L. A., Riley D. A. Fibre type composition of single motor units during synapse elimination in neonatal rat soleus muscle. Nature. 1984 Jun 21;309(5970):709–711. doi: 10.1038/309709a0. [DOI] [PubMed] [Google Scholar]
- Thompson W., Jansen J. K. The extent of sprouting of remaining motor units in partly denervated immature and adult rat soleus muscle. Neuroscience. 1977;2(4):523–535. doi: 10.1016/0306-4522(77)90049-5. [DOI] [PubMed] [Google Scholar]
- Tosney K. W., Landmesser L. T. Specificity of early motoneuron growth cone outgrowth in the chick embryo. J Neurosci. 1985 Sep;5(9):2336–2344. doi: 10.1523/JNEUROSCI.05-09-02336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkle W. B., Entman M. L., Bornet E. P., Schwartz A. Morphological and biochemical correlates of skeletal muscle contractility in the cat. II. Physiological and biochemical studies. J Cell Physiol. 1978 Oct;97(1):121–135. doi: 10.1002/jcp.1040970111. [DOI] [PubMed] [Google Scholar]
- Weeks O. I., English A. W. Compartmentalization of the cat lateral gastrocnemius motor nucleus. J Comp Neurol. 1985 May 8;235(2):255–267. doi: 10.1002/cne.902350208. [DOI] [PubMed] [Google Scholar]





