Abstract
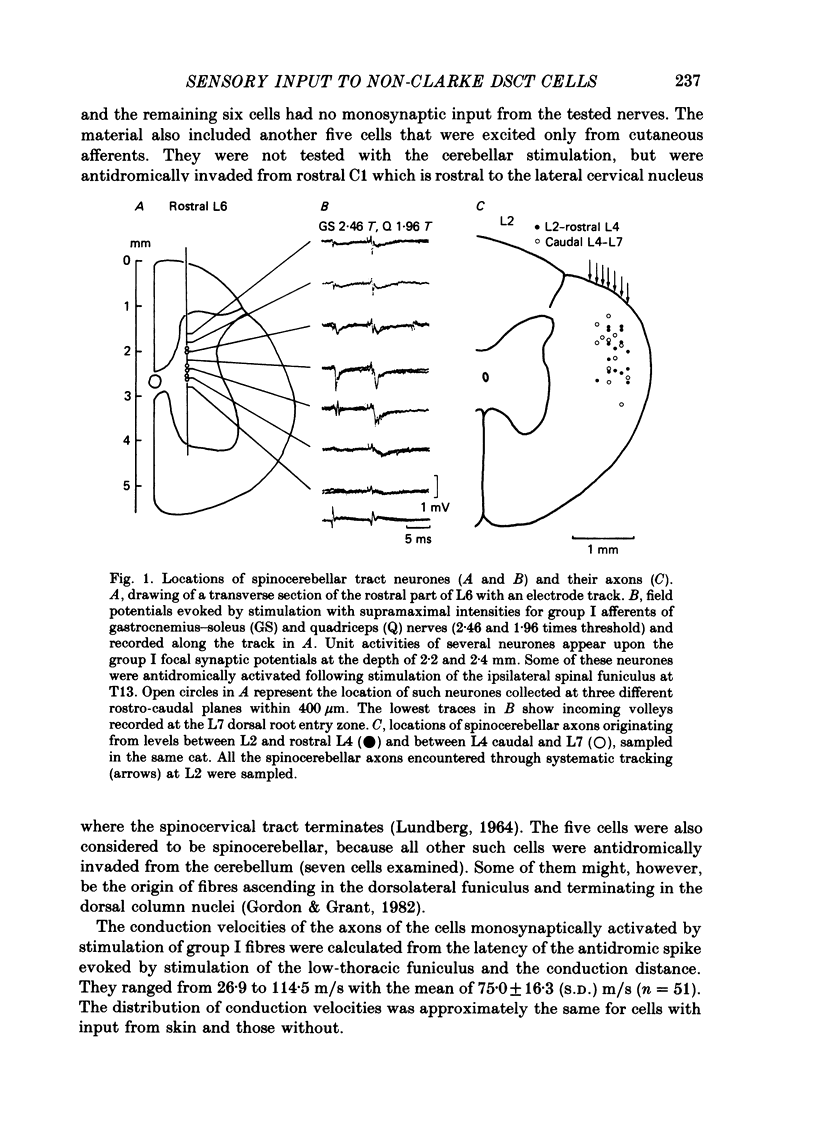
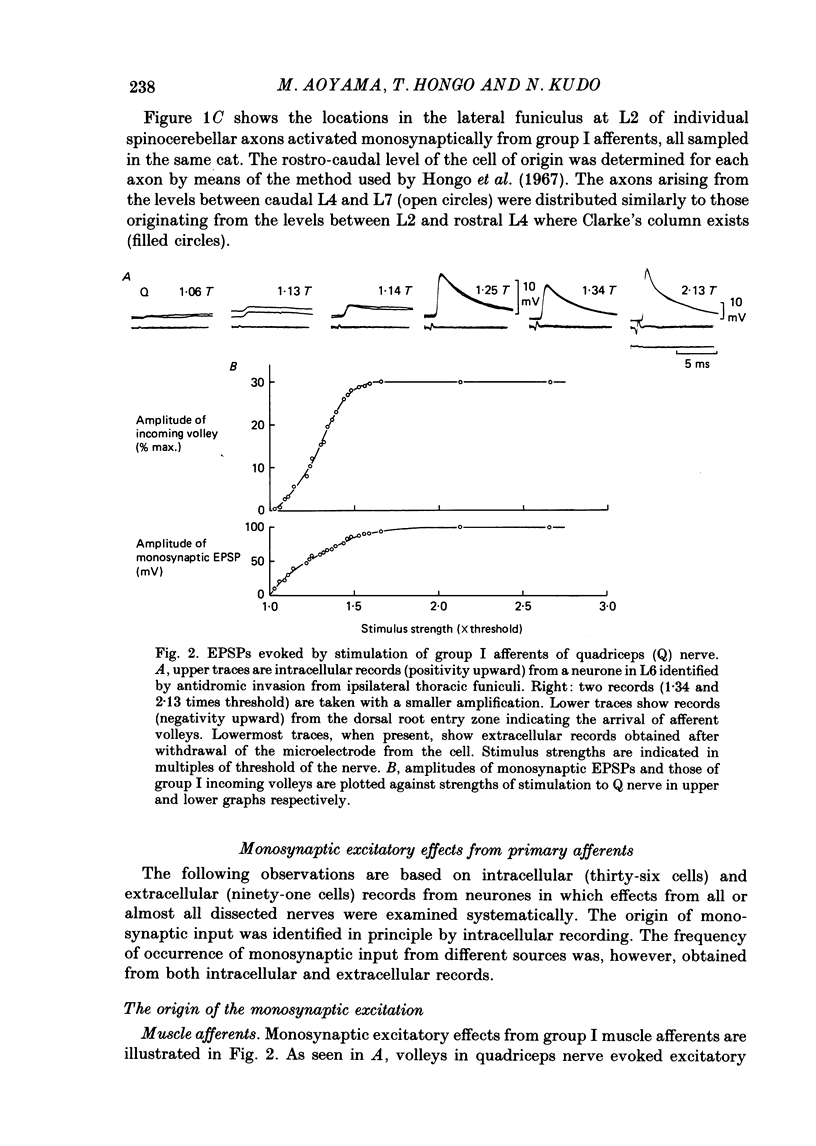
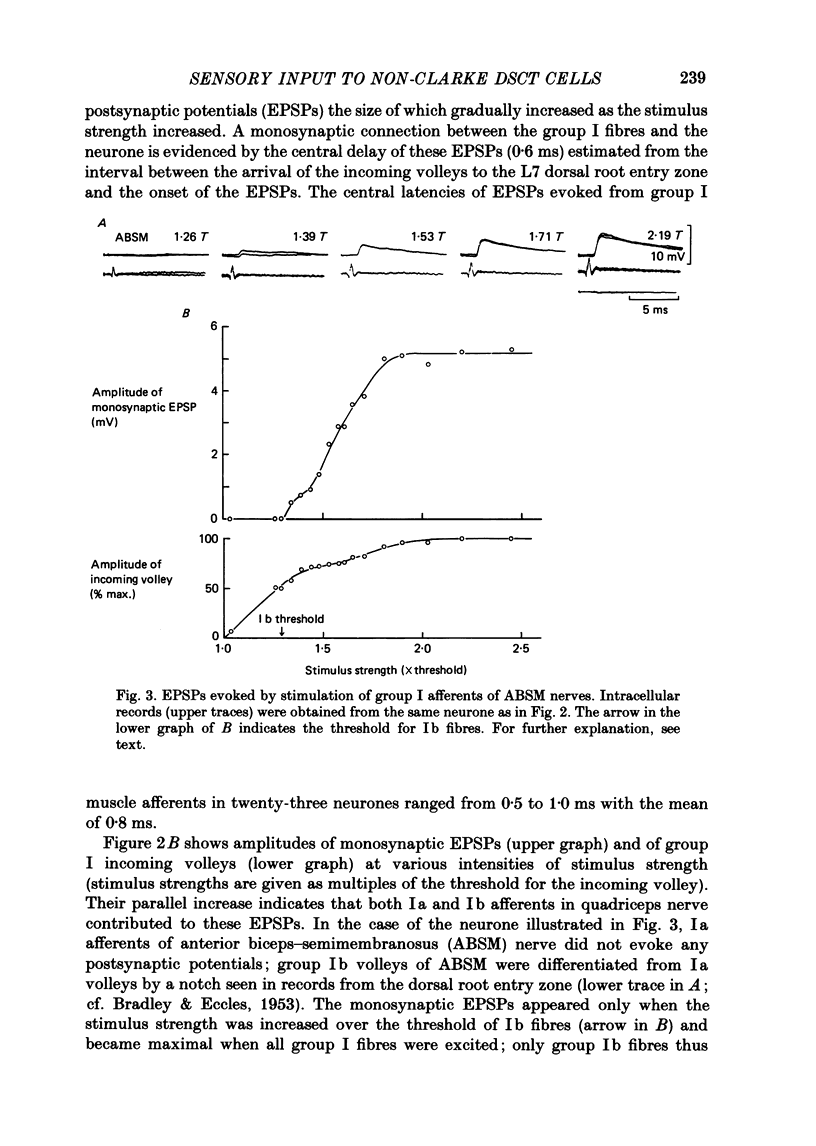
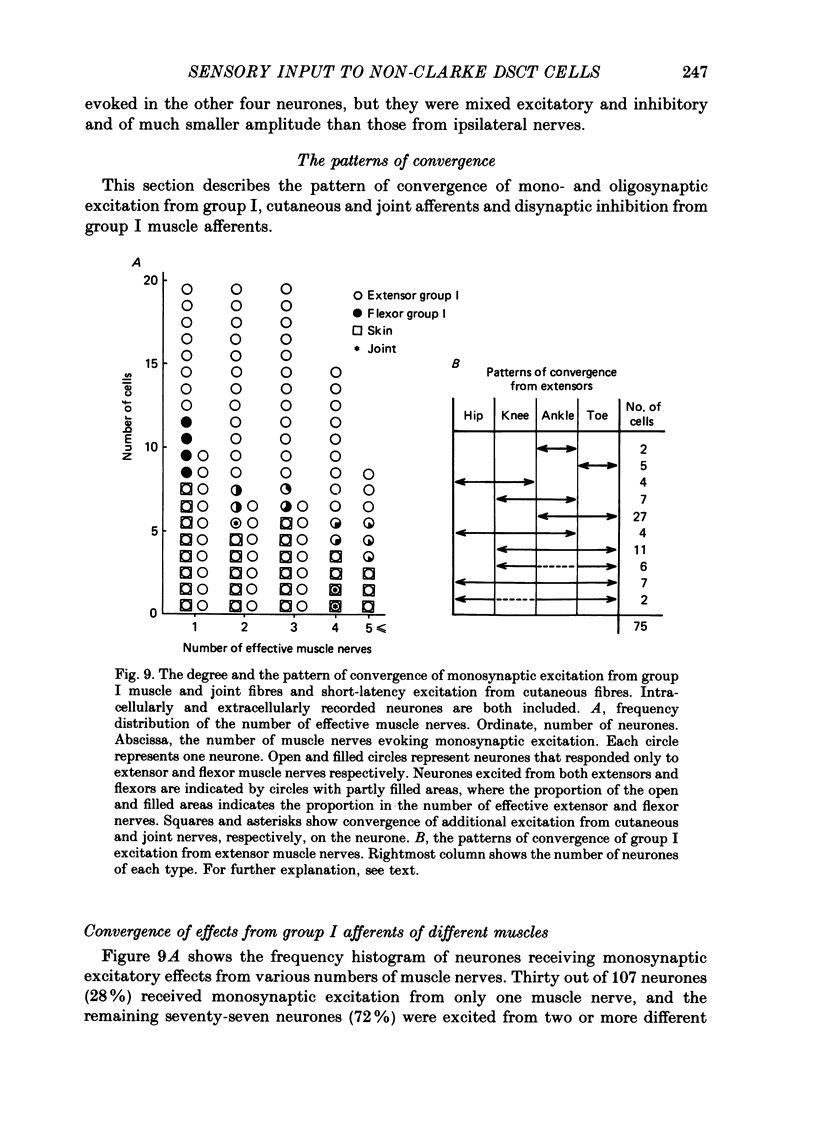
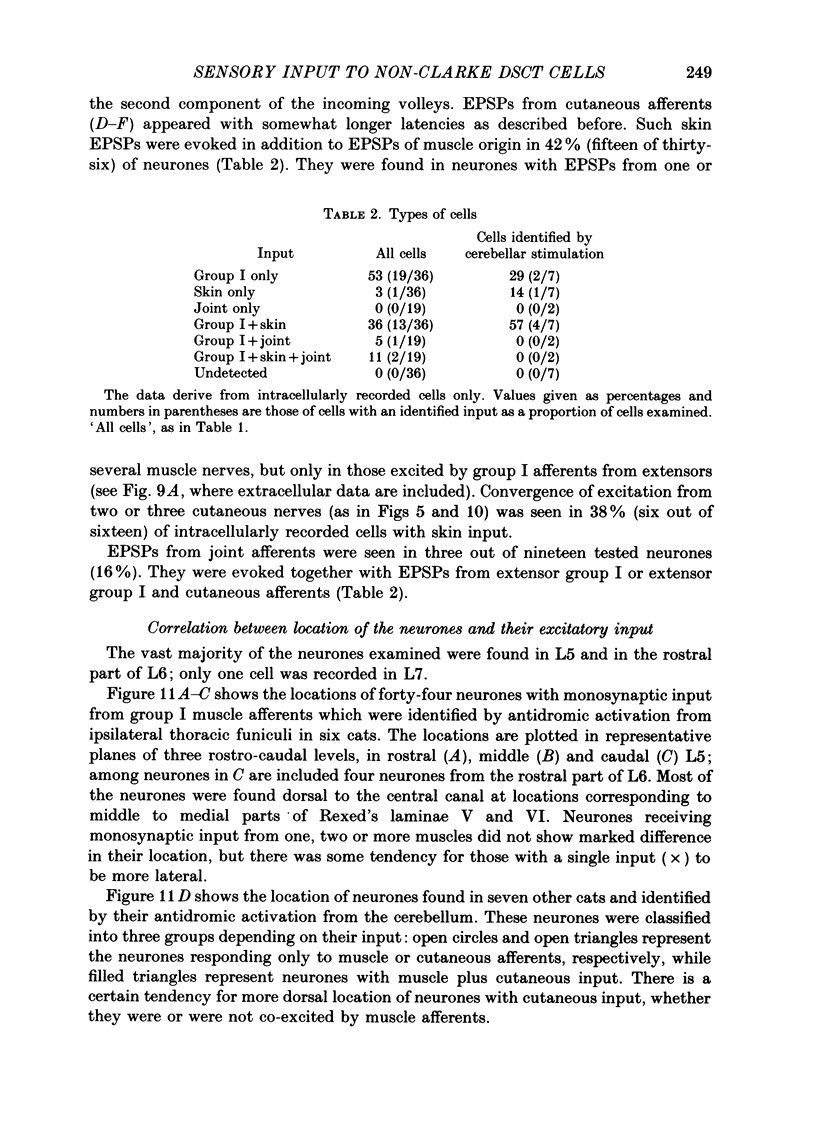
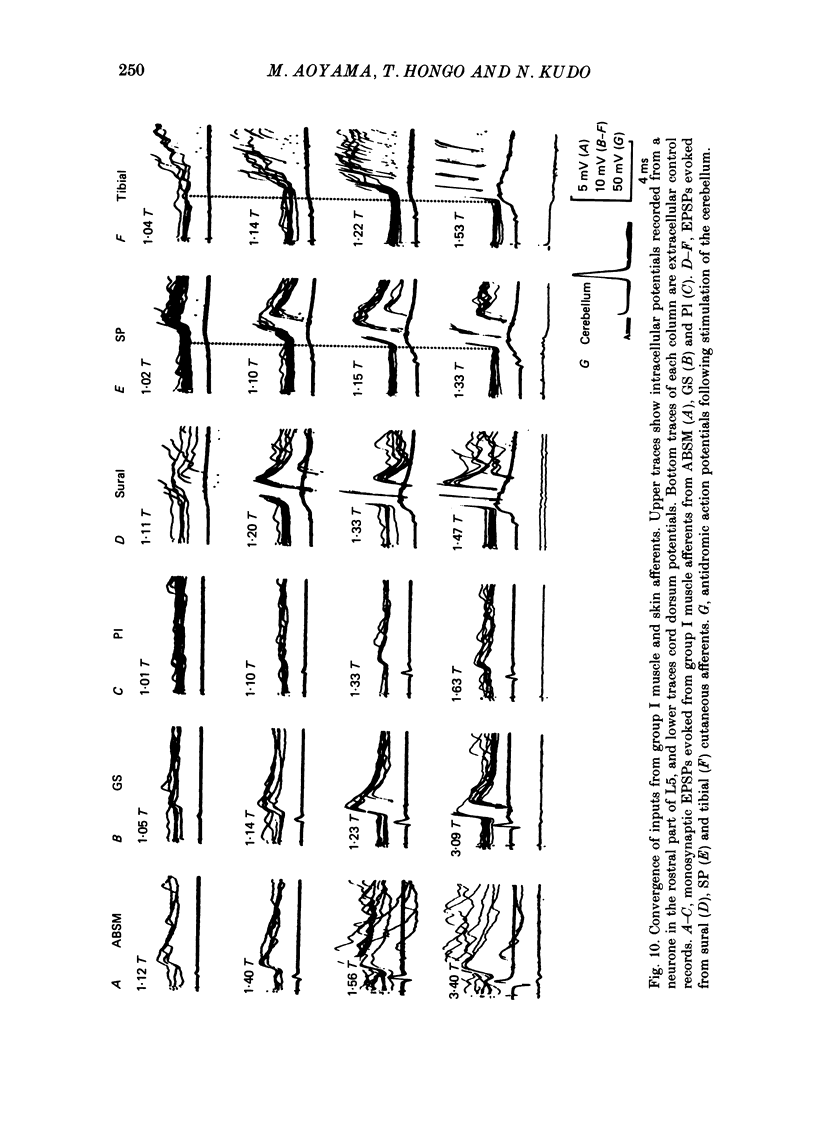
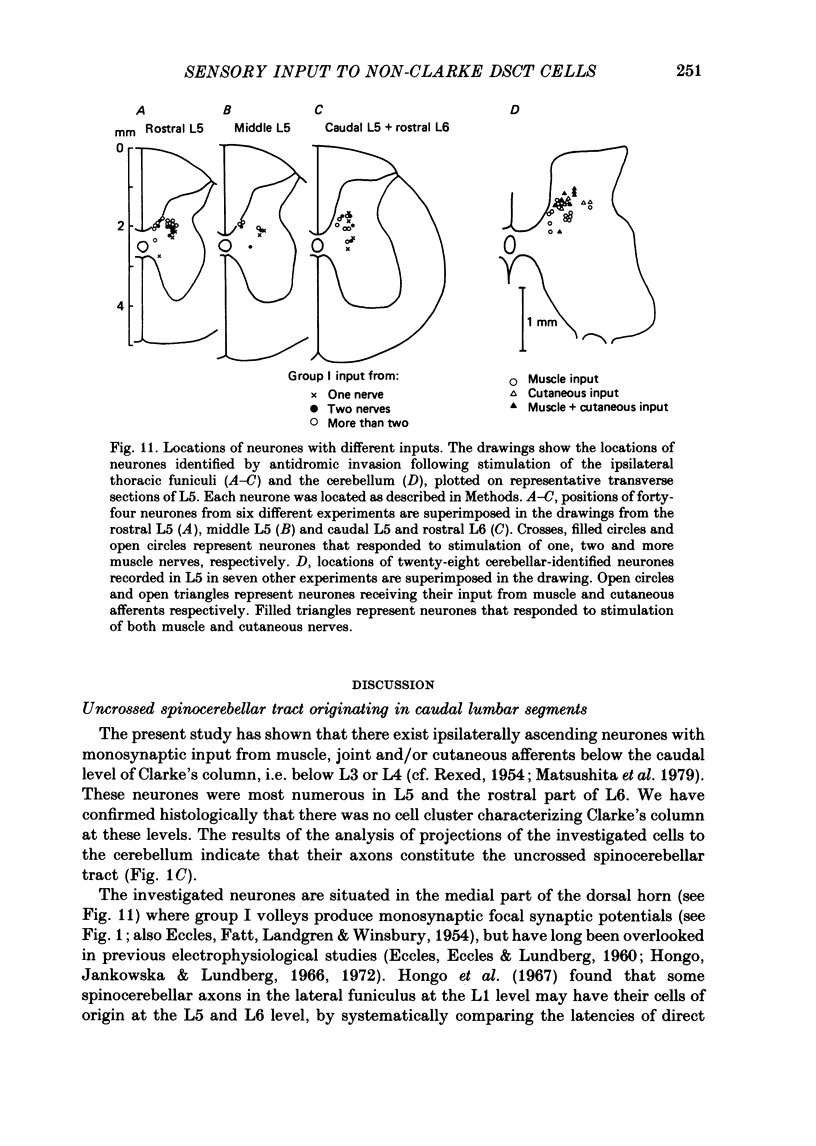
1. Sensory inputs to and locations of uncrossed spinocerebellar tract neurones in the lower lumbar cord were studied in chloralose-anaesthetized cats. 2. Neurones with axons ascending in the ipsilateral thoracic funiculi and projecting to the cerebellum were found mainly dorsal to the central canal (laminae V and VI) in the L5-L6 segments, i.e. at levels caudal to Clarke's column. Axons considered to originate from these cells were located in the dorsal half of the lateral funiculus at the level of L2, intermingled with axons of the dorsal spinocerebellar tract originating at the levels of Clarke's column. 3. Synaptic actions of primary afferents on neurones with antidromic invasion following stimuli applied to ipsilateral thoracic funiculi or to the cerebellum were investigated using intracellular or extracellular recording in the caudal lumbar segments. 4. Monosynaptic excitatory effects were evoked by electrical stimulation of group I muscle afferents of the hindlimb ipsilateral to the cell body. The majority of neurones received monosynaptic excitation from two or more muscles, predominantly extensors. They were frequently co-excited by group Ia muscle spindle and group Ib tendon organ afferents. 5. Volleys in cutaneous afferents produced excitation with short central latencies. In addition to the monosynaptic and disynaptic excitation from low-threshold cutaneous afferents, there were indications of monosynaptic effects from slightly slower conducting fibres. The majority of these neurones also received monosynaptic excitation from group I muscle afferents. Neurones with cutaneous input tended to be located more dorsally compared with those responding only to muscle afferents. 6. Volleys in joint afferents produced monosynaptic excitatory postsynaptic potentials (EPSPs) in the neurones with EPSPs from group I or group I and cutaneous afferents. 7. Some neurones were disynaptically inhibited from group I muscle afferents. Convergence of monosynaptic group I excitation and disynaptic group I inhibition occurred in varieties of patterns. 8. Polysynaptic excitation, inhibition or mixed effects of both were evoked from ipsilateral cutaneous afferents and high-threshold muscle and joint afferents, whereas effects from the controlateral afferents were feeble.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut-Petit D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):471–493. doi: 10.1007/BF00237349. [DOI] [PubMed] [Google Scholar]
- Aoyama M., Hongo T., Kudo N. An uncrossed ascending tract originating from below Clarke's column and conveying group I impulses from the hindlimb muscles in the cat. Brain Res. 1973 Nov 9;62(1):237–241. doi: 10.1016/0006-8993(73)90634-3. [DOI] [PubMed] [Google Scholar]
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E., Harrison P. J., Jankowska E., McCrea D. A., Skoog B. Post-synaptic potentials in a population of motoneurones following activity of single interneurones in the cat. J Physiol. 1983 Oct;343:341–359. doi: 10.1113/jphysiol.1983.sp014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol. 1981 Dec;321:31–47. doi: 10.1113/jphysiol.1981.sp013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ib afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1979 Nov;296:215–226. doi: 10.1113/jphysiol.1979.sp013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of spinocervical tract neurones revealed by intracellular injection of horseradish peroxidase. J Physiol. 1977 Sep;270(3):747–764. doi: 10.1113/jphysiol.1977.sp011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan R. N., Trevino D. L., Coulter J. D., Willis W. D. Location and somatotopic organization of the cells of origin of the spino-cervical tract. Exp Brain Res. 1973 Apr 30;17(2):177–189. doi: 10.1007/BF00235027. [DOI] [PubMed] [Google Scholar]
- Burke R., Lundberg A., Weight F. Spinal border cell origin of the ventral spinocerebellar tract. Exp Brain Res. 1971;12(3):283–294. doi: 10.1007/BF00237921. [DOI] [PubMed] [Google Scholar]
- Czarkowska J., Jankowska E., Sybirska E. Common interneurones in reflex pathways from group 1a and 1b afferents of knee flexors and extensors in the cat. J Physiol. 1981 Jan;310:367–380. doi: 10.1113/jphysiol.1981.sp013555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Types of neurone in and around the intermediate nucleus of the lumbosacral cord. J Physiol. 1960 Nov;154:89–114. doi: 10.1113/jphysiol.1960.sp006566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., LANDGREN S., WINSBURY G. J. Spinal cord potentials generated by volleys in the large muscle afferents. J Physiol. 1954 Sep 28;125(3):590–606. doi: 10.1113/jphysiol.1954.sp005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., HUBBARD J. I., OSCARSSON O. Intracellular recording from cells of the ventral spinocerebellar tract. J Physiol. 1961 Oct;158:486–516. doi: 10.1113/jphysiol.1961.sp006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., OSCARSSON O., WILLIS W. D. Synaptic action of group I and II afferent fibres of muscle on the cells of the dorsal spinocerebellar tract. J Physiol. 1961 Oct;158:517–543. doi: 10.1113/jphysiol.1961.sp006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G., Grant G. Dorsolateral spinal afferents to some medullary sensory nuclei. An anatomical study in the cat. Exp Brain Res. 1982;46(1):12–23. doi: 10.1007/BF00238093. [DOI] [PubMed] [Google Scholar]
- HOLMQVIST B., LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. V. Further experiments on convergence of excitatory and inhibitory actions. Acta Physiol Scand. 1956 Dec 29;38(1):76–90. doi: 10.1111/j.1748-1716.1957.tb00174.x. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I., OSCARSSON O. Localization of the cell bodies of the ventral spino-cerebellar tract in lumbar segments of the cat. J Comp Neurol. 1962 Apr;118:199–204. doi: 10.1002/cne.901180206. [DOI] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. An intracellular study of descending and non-cutaneous afferent input to spinocervical tract neurones in the cat. J Physiol. 1984 Nov;356:245–261. doi: 10.1113/jphysiol.1984.sp015462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Organization of input to the interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985 Apr;361:403–418. doi: 10.1113/jphysiol.1985.sp015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985 Apr;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the lumbosacral cord. Exp Brain Res. 1966;1(4):338–358. doi: 10.1007/BF00237706. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. IV. Effects on interneurones. Exp Brain Res. 1972;15(1):54–78. doi: 10.1007/BF00234958. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Ohno T., Sasaki S., Yamashita M., Yoshida K. Inhibition of dorsal spinocerebellar tract cells by interneurones in upper and lower lumbar segments in the cat. J Physiol. 1983 Sep;342:145–159. doi: 10.1113/jphysiol.1983.sp014844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Ohno T., Sasaki S., Yamashita M., Yoshida K. The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol. 1983 Sep;342:161–180. doi: 10.1113/jphysiol.1983.sp014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Okada Y., Sato M. Corticofugal influences on transmission to the dorsal spinocerebellar tract from hindlimb primary afferents. Exp Brain Res. 1967;3(2):135–149. doi: 10.1007/BF00233258. [DOI] [PubMed] [Google Scholar]
- Ishizuka N., Mannen H., Hongo T., Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol. 1979 Jul 15;186(2):189–211. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Johannisson T., Lipski J. Common interneurones in reflex pathways from group 1a and 1b afferents of ankle extensors in the cat. J Physiol. 1981 Jan;310:381–402. doi: 10.1113/jphysiol.1981.sp013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Zarzecki P. Segmental and supraspinal input to cells of origin of non-primary fibres in the feline dorsal columns. J Physiol. 1979 May;290(2):185–200. doi: 10.1113/jphysiol.1979.sp012767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K., Kubota S., Poppele R. E. A determination of excitability changes in dorsal spinocerebellar tract neurons from spike-train analysis. J Neurophysiol. 1977 May;40(3):626–646. doi: 10.1152/jn.1977.40.3.626. [DOI] [PubMed] [Google Scholar]
- Kuno M., Muñoz-Martinez E. J., Randić M. Sensory inputs to neurones in Clarke's column from muscle, cutaneous and joint receptors. J Physiol. 1973 Jan;228(2):327–342. doi: 10.1113/jphysiol.1973.sp010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG A. ASCENDING SPINAL HINDLIMB PATHWAYS IN THE CAT. Prog Brain Res. 1964;12:135–163. doi: 10.1016/s0079-6123(08)60621-4. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. VII. Identification of units by antidromic activation from the cerebellar cortex with recognition of five functional subdivisions. Acta Physiol Scand. 1960 Dec 30;50:356–374. doi: 10.1111/j.1748-1716.1960.tb00189.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Three ascending spinal pathways in the dorsal part of the lateral funiculus. Acta Physiol Scand. 1961 Jan;51:1–16. doi: 10.1111/j.1748-1716.1961.tb02108.x. [DOI] [PubMed] [Google Scholar]
- Lindström S., Takata M. Monosynaptic excitation of dorsal spinocerebellar tract neurones from low threshold joint afferents. Acta Physiol Scand. 1972 Mar;84(3):430–432. [PubMed] [Google Scholar]
- Lundberg A., Weight F. Functional organization of connexions to the ventral spinocerebellar tract. Exp Brain Res. 1971;12(3):295–316. doi: 10.1007/BF00237922. [DOI] [PubMed] [Google Scholar]
- Mann M. D. Axons of dorsal spinocerebellar tract which respond to activity in cutaneous receptors. J Neurophysiol. 1971 Nov;34(6):1035–1050. doi: 10.1152/jn.1971.34.6.1035. [DOI] [PubMed] [Google Scholar]
- Mann M. D. Clarke's column and the dorsal spinocerebellar tract: a review. Brain Behav Evol. 1973;7(1):34–83. doi: 10.1159/000124397. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Hosoya Y., Ikeda M. Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979 Mar 1;184(1):81–106. doi: 10.1002/cne.901840106. [DOI] [PubMed] [Google Scholar]
- OSCARSSON O. Primary afferent collaterals and spinal relays of the dorsal and ventral spino-cerebellar tracts. Acta Physiol Scand. 1957 Oct 10;40(2-3):222–231. doi: 10.1111/j.1748-1716.1957.tb01491.x. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Rastad J., Jankowska E., Westman J. Arborization of initial axon collaterals of spinocervical tract cells stained intracellularly with horseradish peroxidase. Brain Res. 1977 Oct 21;135(1):1–10. doi: 10.1016/0006-8993(77)91047-2. [DOI] [PubMed] [Google Scholar]
- Tapper D. N., Mann M. D., Brown P. B., Cogdell B. Cells of origin of the cutaneous subdivision of the dorsal spinocerebellar tract. Brain Res. 1975 Feb 21;85(1):59–63. doi: 10.1016/0006-8993(75)91005-7. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res. 1968;4(4):377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]


