Abstract
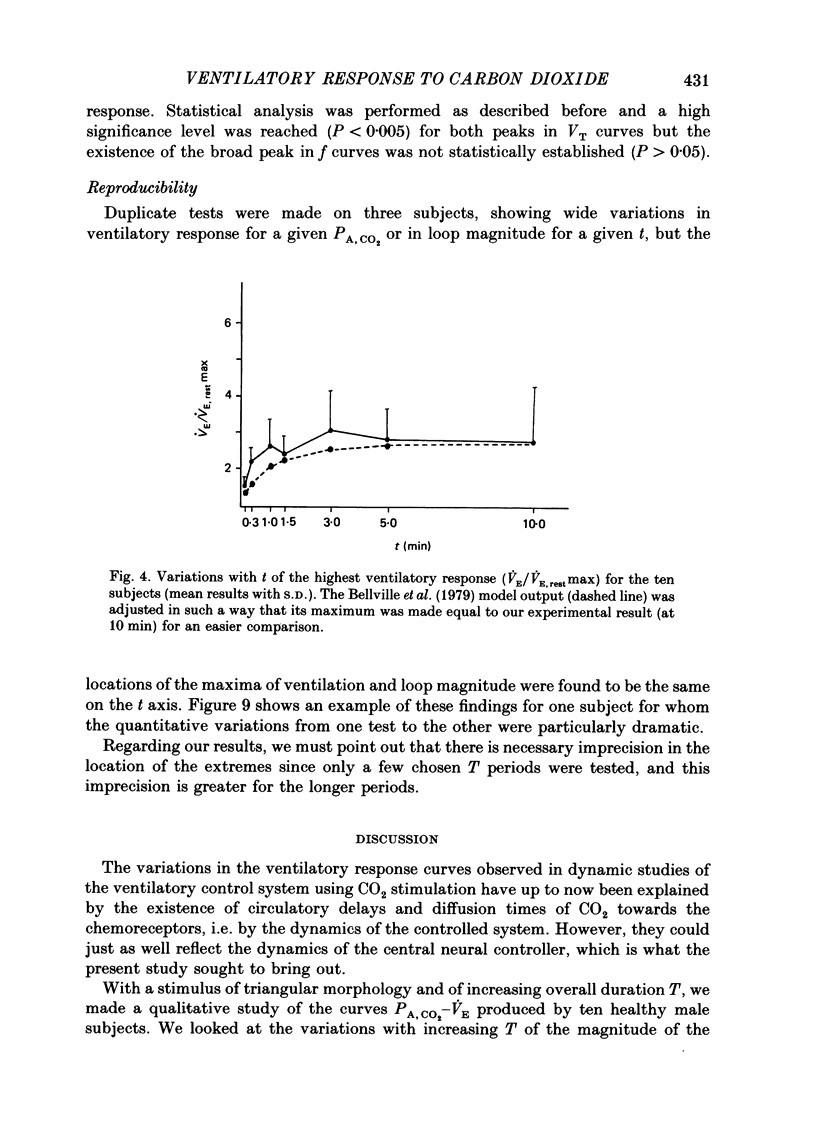
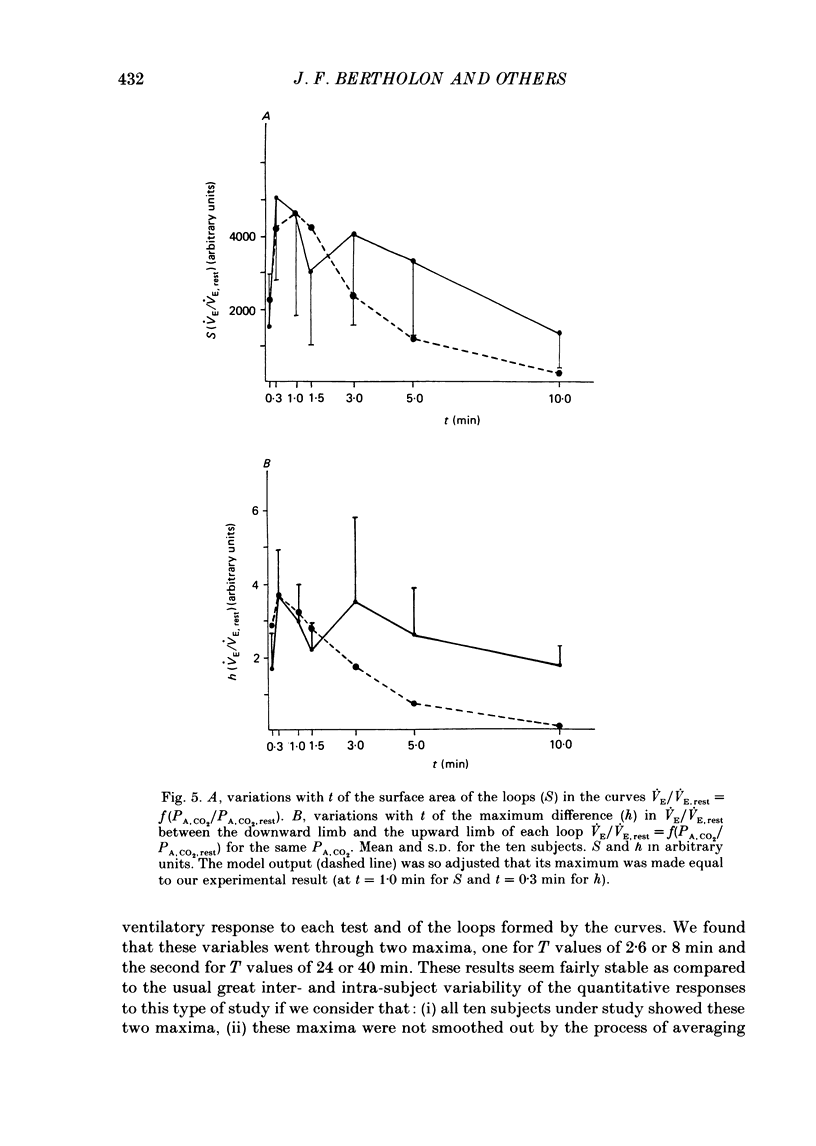
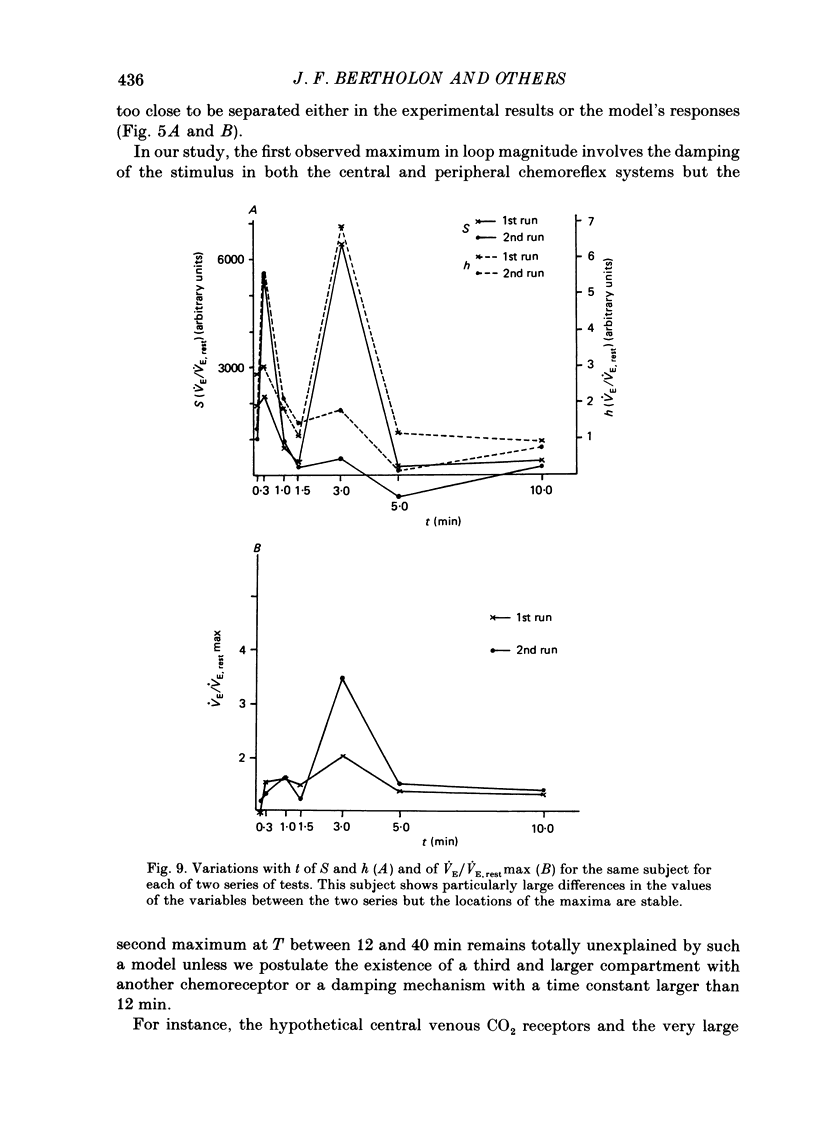
1. The dynamics of the ventilatory response to carbon dioxide inhalation were studied in ten healthy young men using four different inspired fractions of carbon dioxide (FI, CO2) in air (0.015, 0.030, 0.045 and 0.060) successively increasing and decreasing stepwise. 2. Seven such different progressions were performed for each subject and each of seven different durations of the steps (t) ranging between 0.1 (i.e. one ventilatory cycle) and 10 min ('steady-state' conditions). The overall duration of one test (T) was taken as the sum of the seven successive FI, CO2 steps (t) plus one step, t, of air breathing. Thus, the values of T ranged between 0.8 (i.e. eight ventilatory cycles) and 80 min. Three subjects were tested twice. 3. We measured, as a function of T, the magnitude of the loops formed by the curves PA, CO2-VE and the value of the highest ventilatory response (VE max) to each progression. For all ten subjects, both functions had two maxima, one for T values of 2.6 or 8.0 min and one for T values of 24 or 40 min, and one minimum at T equal to 12 min. 4. The same measurements were made on tidal volume-response curves (PA, CO2-VT) and ventilatory frequency-response curves (PA, CO2-f) and yielded the same results except for the ventilatory frequency-response curves, for which we only found a statistically insignificant single maximum for T values of 24 or 40 min. 5. The locations of the maxima in loop magnitude and VE max were similar in duplicate tests in three subjects, whereas the quantitative values of these variables showed wide differences. 6. We compared our results with what is expected from the current linear dynamic model of ventilatory control submitted to the same forcing function: the first maximum in the loop magnitude is predicted by the model, but the second is not. The model shows no peak in the evolution of VE max. 7. We conclude that controlled system dynamics, which are the only ones included in dynamic models of ventilatory control, cannot by themselves account for our observations, and that one should take into consideration the dynamics of the controlling neuronal network.
Full text
PDF



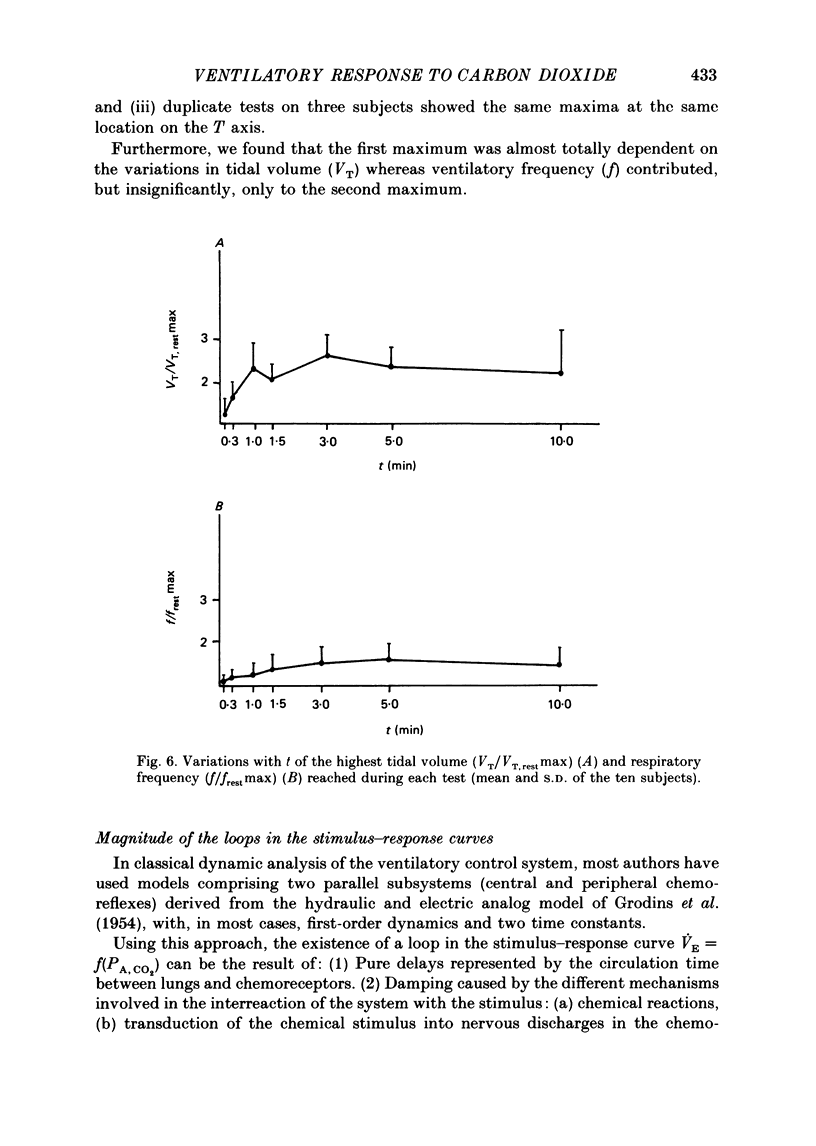
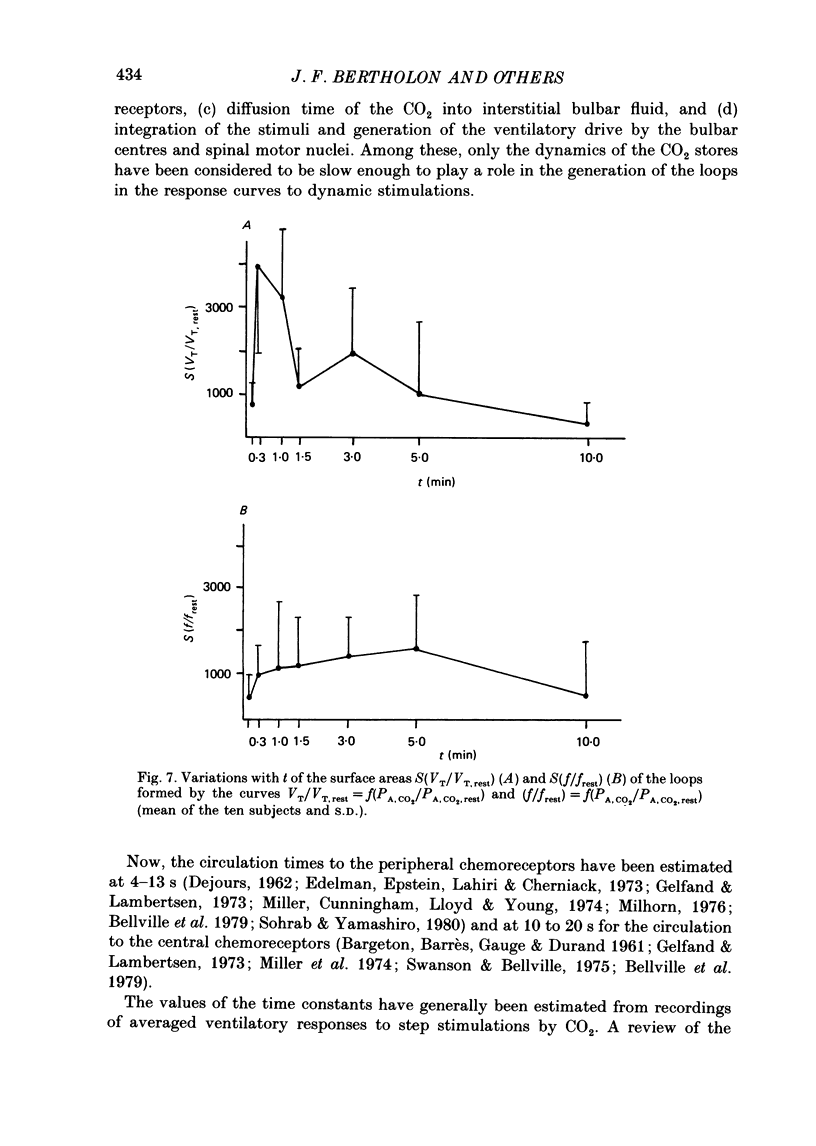
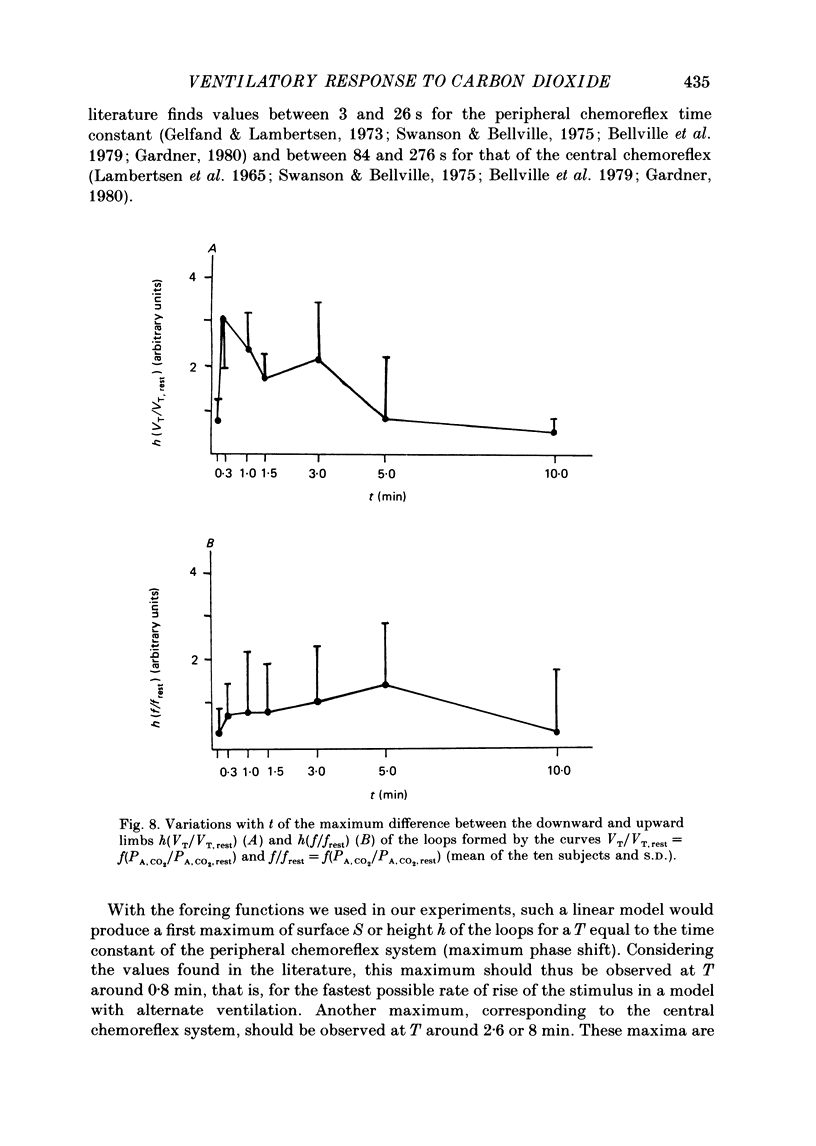




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARGETON D., BARRES G., GAUGE P., DURAND J. [Role of the pACO2 in the regulation of ventilation during rest studied under timed conditions]. J Physiol (Paris) 1961 Mar-Apr;53:261–262. [PubMed] [Google Scholar]
- Bellville J. W., Fleischli G., Defares J. G. A new method of studying regulation of respiration--the response to sinusoidally varying CO2 inhalation. Comput Biomed Res. 1969 Jun;2(4):329–349. doi: 10.1016/0010-4809(69)90019-6. [DOI] [PubMed] [Google Scholar]
- Bellville J. W., Whipp B. J., Kaufman R. D., Swanson G. D., Aqleh K. A., Wiberg D. M. Central and peripheral chemoreflex loop gain in normal and carotid body-resected subjects. J Appl Physiol Respir Environ Exerc Physiol. 1979 Apr;46(4):843–853. doi: 10.1152/jappl.1979.46.4.843. [DOI] [PubMed] [Google Scholar]
- Bernards J. A., Dejours P., Lacaisse A. Ventilatory effects in man of breathing successively CO-2-free, CO-2-enriched and CO-2-free gas mixtures with low, normal or high oxygen concentration. Respir Physiol. 1966;1(4):390–397. doi: 10.1016/0034-5687(66)90006-5. [DOI] [PubMed] [Google Scholar]
- CRAIG F. N. Pulmonary ventilation during exercise and inhalation of carbon dioxide. J Appl Physiol. 1955 Mar;7(5):467–471. doi: 10.1152/jappl.1955.7.5.467. [DOI] [PubMed] [Google Scholar]
- Cherniack N. S., Longobardo G. S. Oxygen and carbon dioxide gas stores of the body. Physiol Rev. 1970 Apr;50(2):196–243. doi: 10.1152/physrev.1970.50.2.196. [DOI] [PubMed] [Google Scholar]
- Cleave J. P., Levine M. R., Fleming P. J., Long A. M. Hopf bifurcations and the stability of the respiratory control system. J Theor Biol. 1986 Apr 7;119(3):299–318. doi: 10.1016/s0022-5193(86)80143-6. [DOI] [PubMed] [Google Scholar]
- DEJOURS P. Chemoreflexes in breathing. Physiol Rev. 1962 Jul;42:335–358. doi: 10.1152/physrev.1962.42.3.335. [DOI] [PubMed] [Google Scholar]
- Dejours P., Puccinelli R., Armand J., Dicharry M. Concept and measurement of ventilatory sensitivity to carbon dioxide. J Appl Physiol. 1965 Sep;20(5):890–897. doi: 10.1152/jappl.1965.20.5.890. [DOI] [PubMed] [Google Scholar]
- Dutton R. E., Hodson W. A., Davies D. G., Fenner A. Effect of the rate of rise of carotid body PCO2 on the time course of ventilation. Respir Physiol. 1967 Dec;3(3):367–379. doi: 10.1016/0034-5687(67)90065-5. [DOI] [PubMed] [Google Scholar]
- Edelman N. H., Epstein P. E., Lahiri S., Cherniack N. S. Ventilatory responses to transient hypoxia and hypercapnia in man. Respir Physiol. 1973 Apr;17(3):302–314. doi: 10.1016/0034-5687(73)90005-4. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. Central neural stimulation of respiration in unanesthetized decerebrate cats. J Appl Physiol. 1976 Jan;40(1):23–28. doi: 10.1152/jappl.1976.40.1.23. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P., Millhorn D. E. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981 Feb;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. L., Cowan J. D. Large-scale activity in neural nets II: A model for the brainstem respiratory oscillator. Biol Cybern. 1975;17(1):39–51. doi: 10.1007/BF00326708. [DOI] [PubMed] [Google Scholar]
- Folgering H. T., Bernards J. A., Biesta J. H., Smolders F. Mathematical analysis of the response of lung ventilation to CO2 in normoxia and hyperoxia. Pflugers Arch. 1974 Mar 25;347(4):347–350. [PubMed] [Google Scholar]
- Forster H. V., Klein J. P., Hamilton L. H., Kampine J. P. Regulation of PaCO2 and ventilation in humans inspiring low levels of CO2. J Appl Physiol Respir Environ Exerc Physiol. 1982 Feb;52(2):287–294. doi: 10.1152/jappl.1982.52.2.287. [DOI] [PubMed] [Google Scholar]
- GRODINS F. S., GRAY J. S., SCHROEDER K. R., NORINS A. L., JONES R. W. Respiratory responses to CO2 inhalation; a theoretical study of a nonlinear biological regulator. J Appl Physiol. 1954 Nov;7(3):283–308. doi: 10.1152/jappl.1954.7.3.283. [DOI] [PubMed] [Google Scholar]
- Gardner W. N. The pattern of breathing following step changes of alveolar partial pressures of carbon dioxide and oxygen in man. J Physiol. 1980 Mar;300:55–73. doi: 10.1113/jphysiol.1980.sp013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand R., Lambertsen C. J. Dynamic respiratory response to abrupt change of inspired CO2 at normal and high PO2. J Appl Physiol. 1973 Dec;35(6):903–913. doi: 10.1152/jappl.1973.35.6.903. [DOI] [PubMed] [Google Scholar]
- Gray J. S. The Multiple Factor Theory of the Control of Respiratory Ventilation. Science. 1946 Jun 28;103(2687):739–744. doi: 10.1126/science.103.2687.739. [DOI] [PubMed] [Google Scholar]
- KELLOGG R. H., PACE N., ARCHIBALD E. R., VAUGHAN B. E. Respiratory response to inspired CO2 during acclimatization to an altitude of 12, 470 feet. J Appl Physiol. 1957 Jul;11(1):65–71. doi: 10.1152/jappl.1957.11.1.65. [DOI] [PubMed] [Google Scholar]
- LANDMESSER C. M., COBB S., PECK A. S., CONVERSE J. G. Respiratory responses to carbon dioxide transients in normal volunteers. Anesthesiology. 1957 Nov-Dec;18(6):807–830. doi: 10.1097/00000542-195711000-00001. [DOI] [PubMed] [Google Scholar]
- LOESCHCHKE H. H., GERTZ K. H. Einfluss des O2-Druckes in der Einatmungsluft auf die Atemtätigkeit des Menschen, geprüft unter Konstanthaltung des alveolaren CO2-Druckes. Pflugers Arch. 1958;267(5):460–477. doi: 10.1007/BF00361733. [DOI] [PubMed] [Google Scholar]
- LOESCHCKE H. H., KATSAROS B., ALBERS C., MICHEL C. C. UBER DEN ZEITLICHEN VERLAUF VON ATEMZUGVOLUMEN, ATEM-PERIODENDAUER, ATEMINUTENVOLUMEN UND ENDEXSPIRATORISCHEM CO2-DRUCK BEI EINATRMUNG VON GASGEMISCHEN MIT ERHOETEM CO2-DRUCK. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;277:671–683. [PubMed] [Google Scholar]
- Lefrançois R., Gautier H., Pasquis P., Cevaer A. M., Hellot M. F., Leroy J. Chemoreflex ventilatory response to CO 2 in man at low and high altitudes. Respir Physiol. 1972 Apr;14(3):296–306. doi: 10.1016/0034-5687(72)90036-9. [DOI] [PubMed] [Google Scholar]
- Milhorn H. T., Jr, Reynolds W. J. 'Exponential peeling' of ventilatory transients following inhalation of 5, 6 and 7% CO2. Respir Physiol. 1976 Oct;28(1):75–87. doi: 10.1016/0034-5687(76)90086-4. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Cunningham D. J., Lloyd B. B., Young J. M. The transient respiratory effects in man of sudden changes in alveolar CO2 in hypoxia and in high oxygen. Respir Physiol. 1974 Feb;20(1):17–31. doi: 10.1016/0034-5687(74)90015-2. [DOI] [PubMed] [Google Scholar]
- Read D. J. A clinical method for assessing the ventilatory response to carbon dioxide. Australas Ann Med. 1967 Feb;16(1):20–32. doi: 10.1111/imj.1967.16.1.20. [DOI] [PubMed] [Google Scholar]
- Robbins P. A., Swanson G. D., Howson M. G. A prediction-correction scheme for forcing alveolar gases along certain time courses. J Appl Physiol Respir Environ Exerc Physiol. 1982 May;52(5):1353–1357. doi: 10.1152/jappl.1982.52.5.1353. [DOI] [PubMed] [Google Scholar]
- Robbins P. A. The ventilatory response of the human respiratory system to sine waves of alveolar carbon dioxide and hypoxia. J Physiol. 1984 May;350:461–474. doi: 10.1113/jphysiol.1984.sp015212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEFER K. E. Respiratory pattern and respiratory response to CO2. J Appl Physiol. 1958 Jul;13(1):1–14. doi: 10.1152/jappl.1958.13.1.1. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Jameson L. C., Mitchell G. S., Musch T. I., Dempsey J. A. Central-peripheral chemoreceptor interaction in awake cerebrospinal fluid-perfused goats. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jun;56(6):1541–1549. doi: 10.1152/jappl.1984.56.6.1541. [DOI] [PubMed] [Google Scholar]
- Sohrab S., Yamashiro S. M. Pseudorandom testing of ventilatory response to inspired carbon dioxide in man. J Appl Physiol Respir Environ Exerc Physiol. 1980 Dec;49(6):1000–1009. doi: 10.1152/jappl.1980.49.6.1000. [DOI] [PubMed] [Google Scholar]
- Stoll P. J. Respiratory system analysis based on sinusoidal variations of CO2 in inspired air. J Appl Physiol. 1969 Sep;27(3):389–399. doi: 10.1152/jappl.1969.27.3.389. [DOI] [PubMed] [Google Scholar]
- Swanson G. D., Bellville J. W. Step changes in end-tidal CO2: methods and implications. J Appl Physiol. 1975 Sep;39(3):377–385. doi: 10.1152/jappl.1975.39.3.377. [DOI] [PubMed] [Google Scholar]
- Swanson G. D., Carpenter T. M., Jr, Snider D. E., Bellville J. W. An on-line hybrid computing system for dynamic respiratory response studies. Comput Biomed Res. 1971 Apr;4(1):205–215. doi: 10.1016/0010-4809(71)90055-3. [DOI] [PubMed] [Google Scholar]
- Wilson H. R., Cowan J. D. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J. 1972 Jan;12(1):1–24. doi: 10.1016/S0006-3495(72)86068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


