Abstract
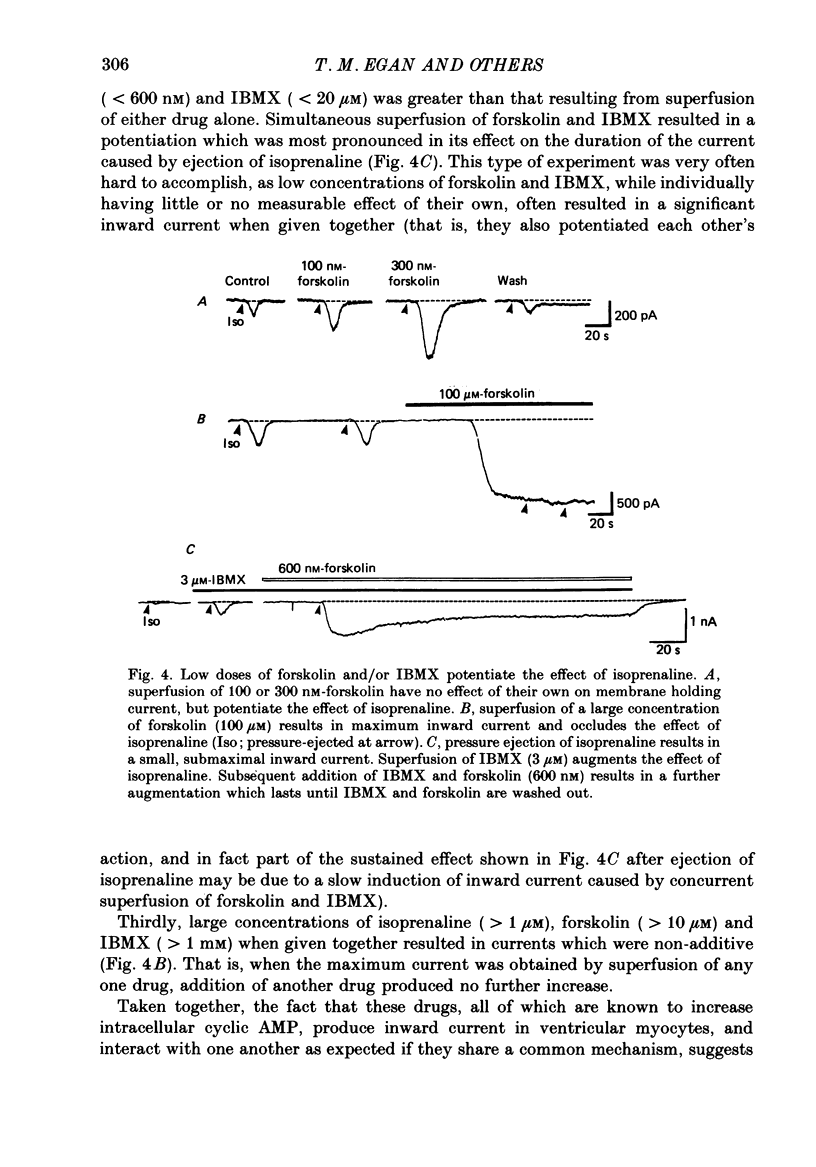
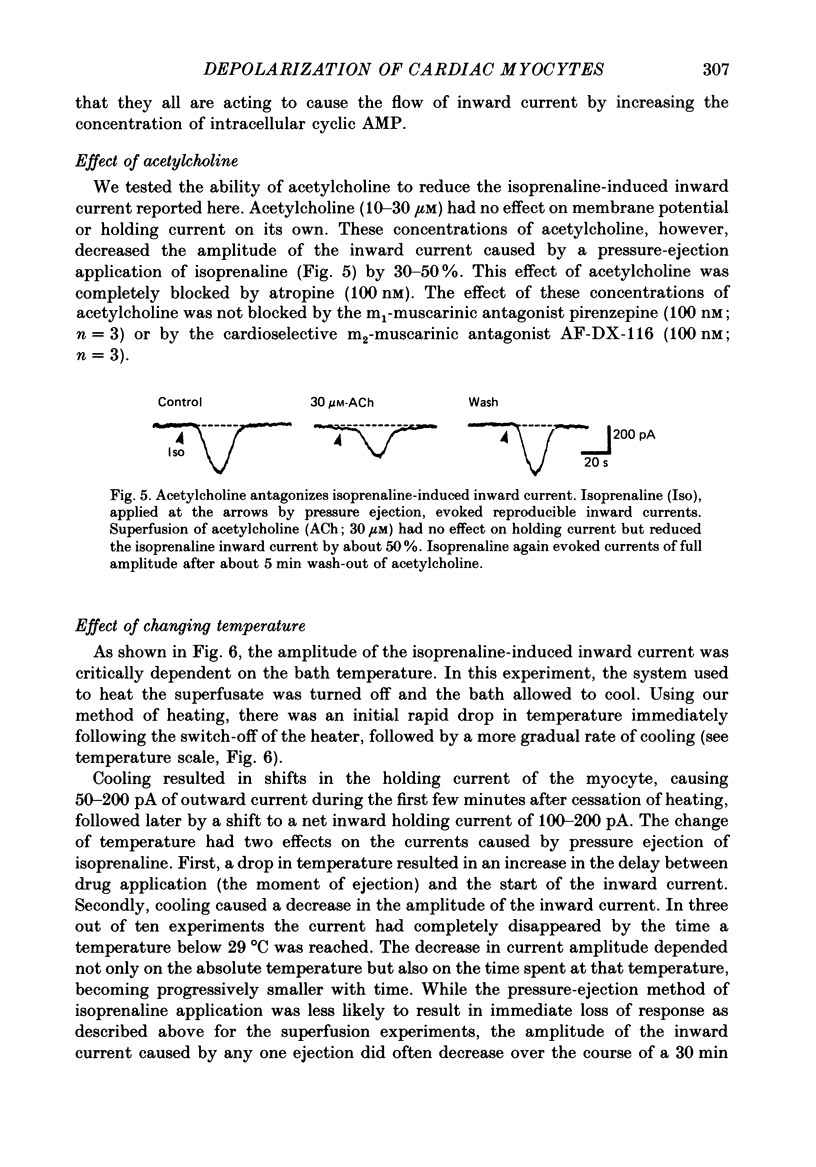
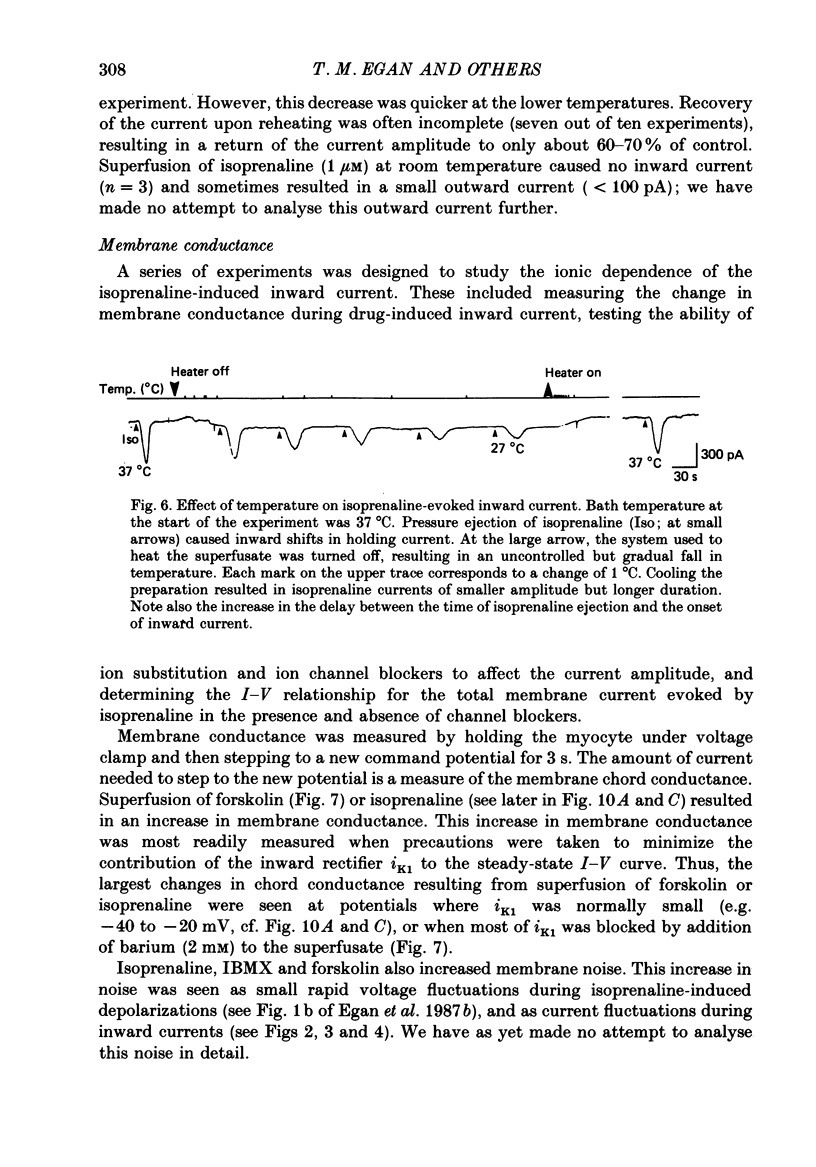
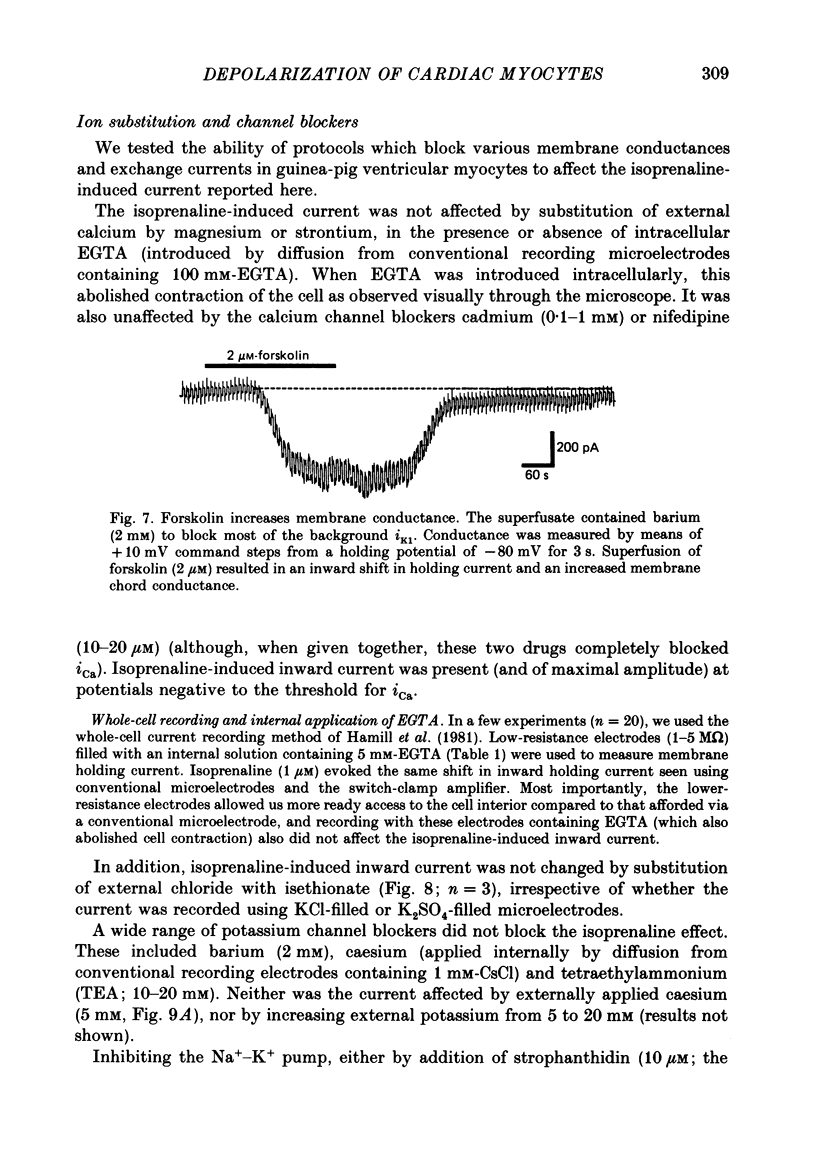
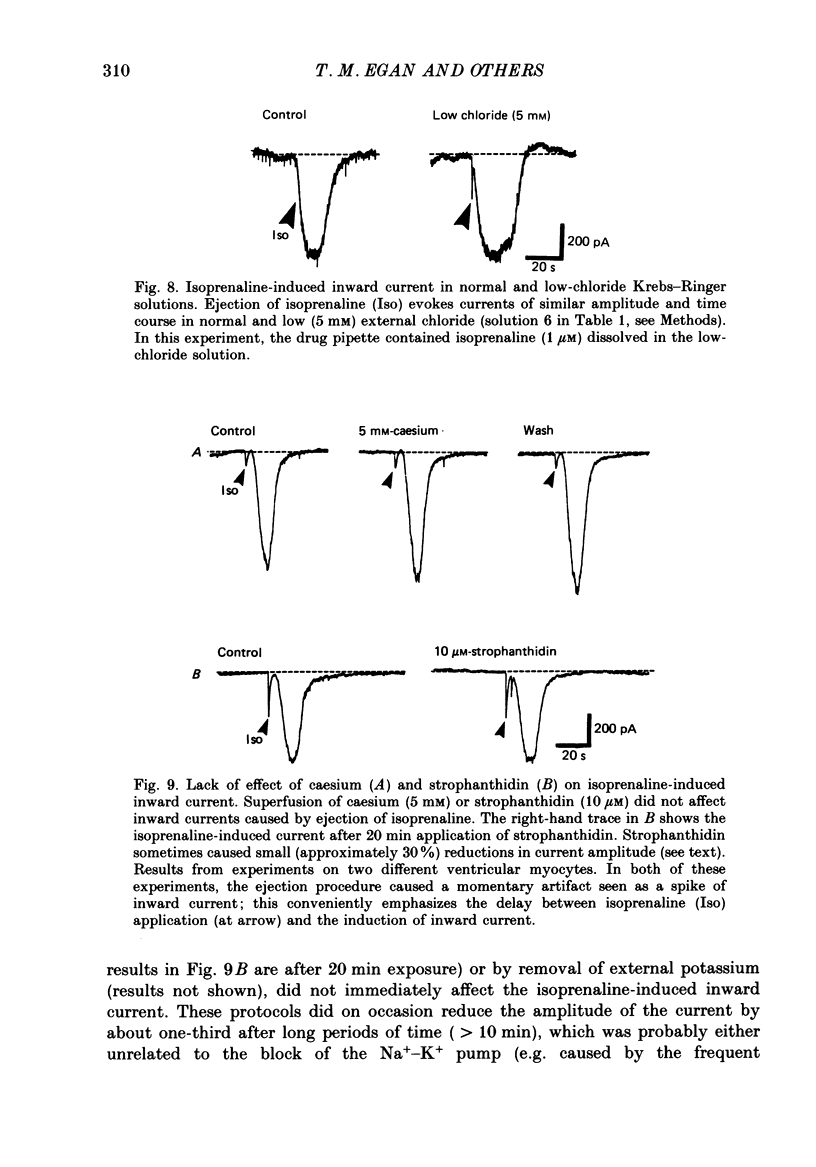
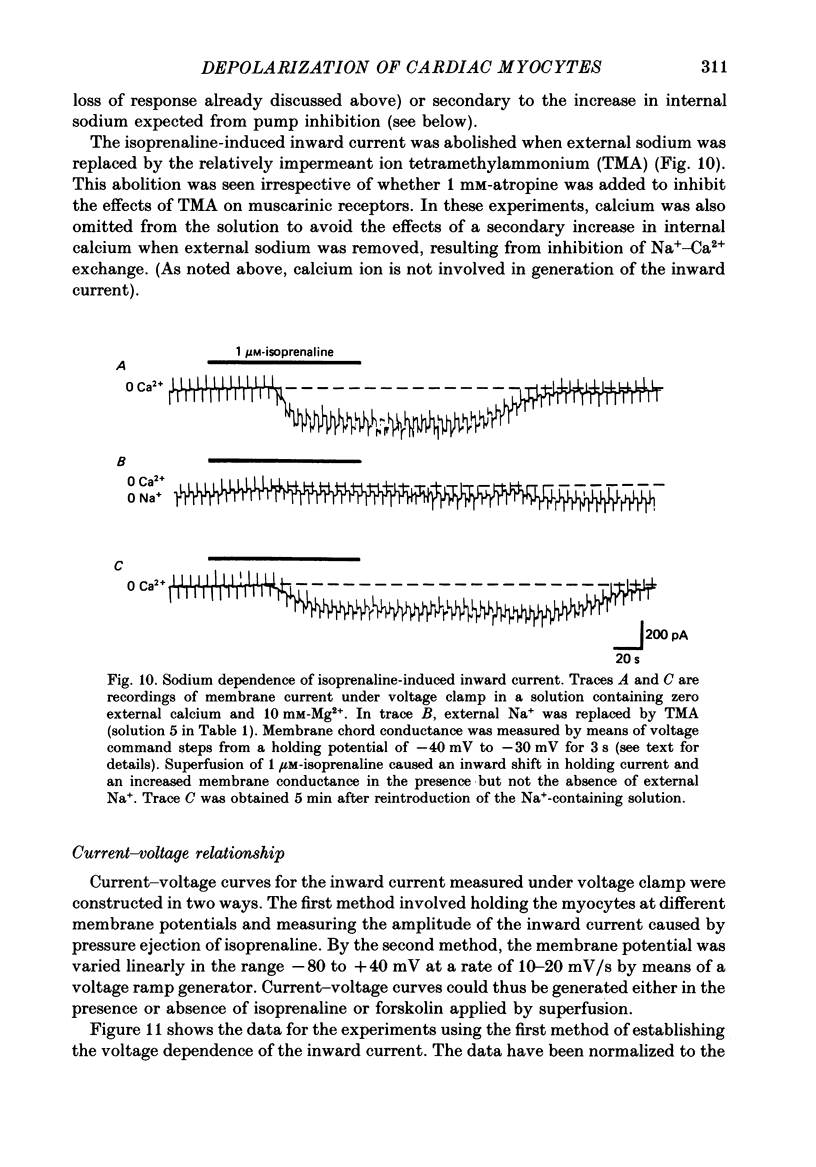
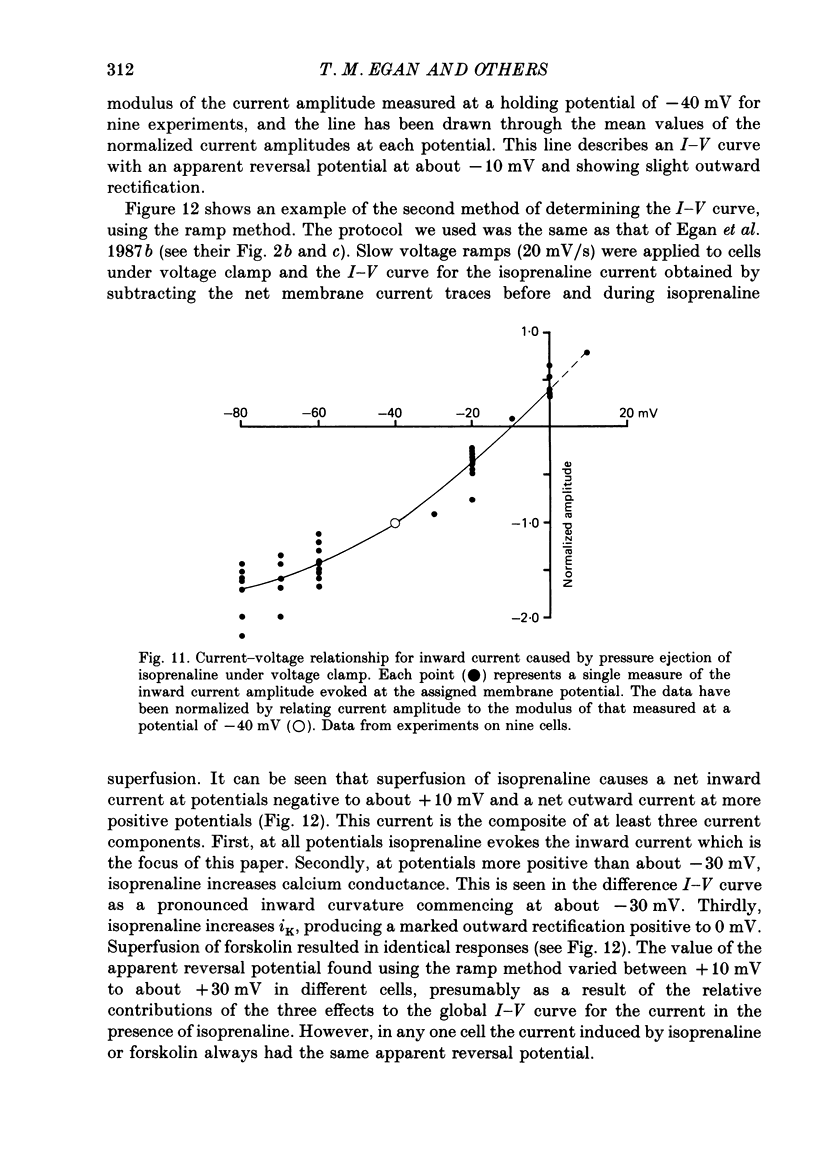
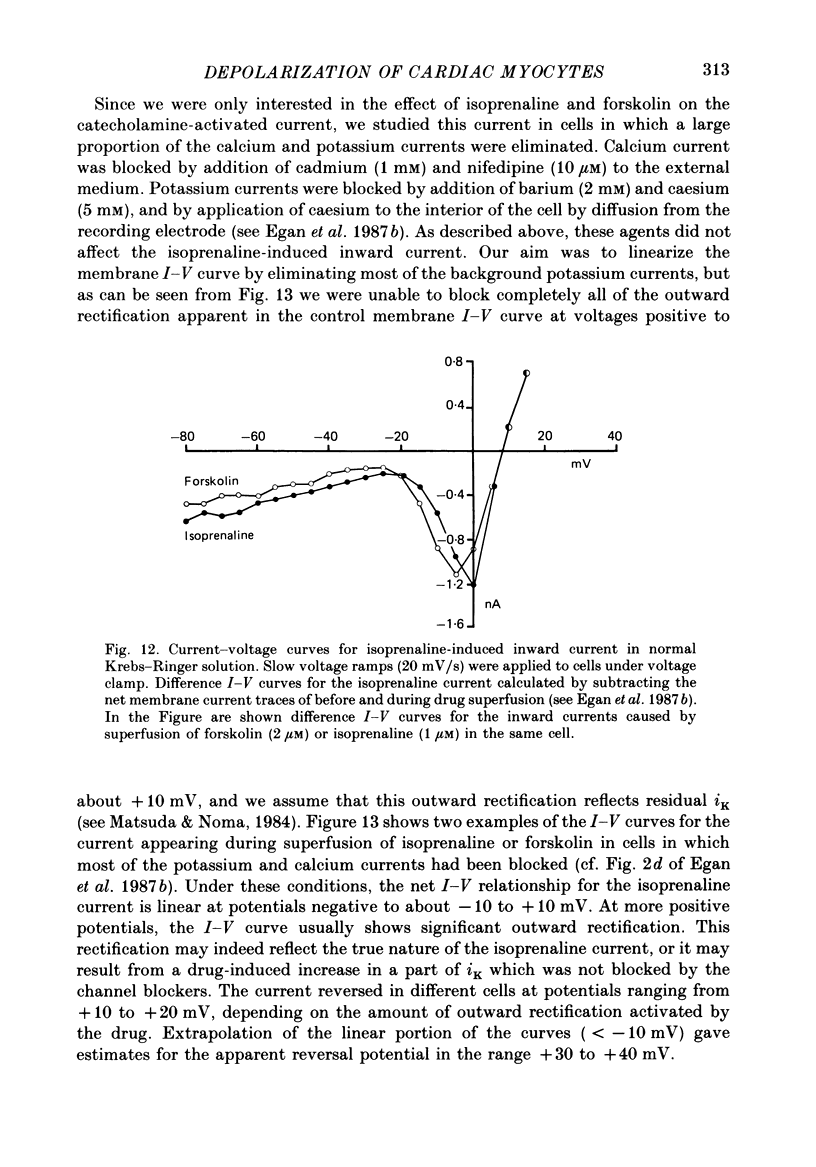
1. Isoprenaline (10 nM to 1 microM) and forskolin (0.6-100 microM) depolarized single guinea-pig myocytes studied in vitro. Under voltage clamp both agents caused an inward current to flow. 2. These effects were abolished by propranolol (100 nM) and the beta1-antagonist metoprolol (100-200 nM), but not by the beta2-agonist [corrected] salbutamol (1 microM). 3. The interaction of isoprenaline with forskolin, caffeine or isobutylmethylxanthine (IBMX) on current amplitude was as expected if all of these drugs were causing inward current by increasing intracellular levels of cyclic adenosine monophosphate (cyclic AMP). Low concentrations of forskolin (less than 600 nM) or IBMX (less than 20 microM) potentiated the effect of isoprenaline, whereas isoprenaline caused no further inward current in cells in which high concentrations of forskolin (600 nM-100 microM) or IBMX (20 microM-1 mM) were already evoking maximum inward current. 4. Isoprenaline-induced inward current was reduced 30-50% by acetylcholine (10-30 microM). This action of acetylcholine was blocked by atropine (100 nM). 5. The effect of isoprenaline on holding current was critically dependent on temperature. The onset of the current was delayed and its amplitude reduced as the myocyte was cooled from 37 degrees C to ambient temperature (22-24 degrees C). 6. Isoprenaline-induced inward current was not affected by the potassium channel blockers barium (2 mM) or tetraethylammonium (TEA; 10-20 mM). The amplitude of the inward current did not vary as a function of [K+]o. 7. The inward current was not affected by the calcium channel blockers cadmium 1 mM, or nifedipine (10 microM), or when internal calcium was reduced by including EGTA in the recording electrode filling solution. 8. The amplitude of the current was also unaffected by caesium (5 mM), which blocks the hyperpolarization-activated, non-specific channel if, or by strophanthidin (10 microM) which blocks the Na+-K+ pump. It was unchanged by substitution of external chloride by isethionate. 9. The inward current was absent when external sodium was replaced by the impermeant ion tetramethylammonium (TMA). 10. Isoprenaline- and forskolin-induced inward currents were associated with an increase in both membrane chord conductance and noise. The increase in conductance was most readily measured at potentials where the inwardly rectifying potassium channel, iK1, was small, or when iK1 was blocked by the addition of barium (2 mM).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatt M. R., Slayman C. L. KCl leakage from microelectrodes and its impact on the membrane parameters of a nonexcitable cell. J Membr Biol. 1983;72(3):223–234. doi: 10.1007/BF01870589. [DOI] [PubMed] [Google Scholar]
- Brown H. F., Noble S. J. Proceedings: Effects of adrenaline on membrane currents underlying pacemaker activity in frog atrial muscle. J Physiol. 1974 Apr;238(1):51P–53P. [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Changes by acetylcholine of membrane currents in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:201–217. doi: 10.1113/jphysiol.1986.sp015969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Characterization of the acetylcholine-induced potassium current in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:219–237. doi: 10.1113/jphysiol.1986.sp015970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Coombs J., Thompson S. Forskolin's effect on transient K current in nudibranch neurons is not reproduced by cAMP. J Neurosci. 1987 Feb;7(2):443–452. doi: 10.1523/JNEUROSCI.07-02-00443.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Désilets M., Baumgarten C. M. Isoproterenol directly stimulates the Na+-K+ pump in isolated cardiac myocytes. Am J Physiol. 1986 Jul;251(1 Pt 2):H218–H225. doi: 10.1152/ajpheart.1986.251.1.H218. [DOI] [PubMed] [Google Scholar]
- Désilets M., Baumgarten C. M. K+, Na+, and Cl- activities in ventricular myocytes isolated from rabbit heart. Am J Physiol. 1986 Aug;251(2 Pt 1):C197–C208. doi: 10.1152/ajpcell.1986.251.2.C197. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W. An isoprenaline activated sodium-dependent inward current in ventricular myocytes. Nature. 1987 Aug 13;328(6131):634–637. doi: 10.1038/328634a0. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble S. J. Acetylcholine and the mammalian 'slow inward' current: a computer investigation. Proc R Soc Lond B Biol Sci. 1987 Apr 22;230(1260):315–337. doi: 10.1098/rspb.1987.0022. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Rasch R. An effect of noradrenaline on resting potential and Na activity in sheep cardiac Purkinje fibres. Pflugers Arch. 1986 Feb;406(2):144–150. doi: 10.1007/BF00586675. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hauswirth O., Noble D., Tsien R. W. Adrenaline: mechanism of action on the pacemaker potential in cardiac Purkinje fibers. Science. 1968 Nov 22;162(3856):916–917. doi: 10.1126/science.162.3856.916. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Jewitt D. E., Reid D., Thomas M., Mercer C. J., Valori C., Shillingford J. P. Free noradrenaline and adrenaline excretion in relation to the development of cardiac arrhythmias and heart-failure in patients with acute myocardial infarction. Lancet. 1969 Mar 29;1(7596):635–641. doi: 10.1016/s0140-6736(69)92009-1. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Wiegers S. E. The ionic basis of concentration-related effects of noradrenaline on the action potential of calf cardiac purkinje fibres. J Physiol. 1982 Jan;322:541–558. doi: 10.1113/jphysiol.1982.sp014054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Electrical activity and contraction in cells isolated from rat and guinea-pig ventricular muscle: a comparative study. J Physiol. 1987 Oct;391:527–544. doi: 10.1113/jphysiol.1987.sp016754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Influence of a change in stimulation rate on action potentials, currents and contractions in rat ventricular cells. J Physiol. 1985 Jul;364:113–130. doi: 10.1113/jphysiol.1985.sp015734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. The effects of ryanodine, EGTA and low-sodium on action potentials in rat and guinea-pig ventricular myocytes: evidence for two inward currents during the plateau. Br J Pharmacol. 1984 Mar;81(3):543–550. doi: 10.1111/j.1476-5381.1984.tb10107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Stiles G. L., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptors: biochemical mechanisms of physiological regulation. Physiol Rev. 1984 Apr;64(2):661–743. doi: 10.1152/physrev.1984.64.2.661. [DOI] [PubMed] [Google Scholar]
- Terris S., Wasserstrom J. A., Fozzard H. A. Depolarizing effects of catecholamines in quiescent sheep cardiac Purkinje fibers. Am J Physiol. 1986 Nov;251(5 Pt 2):H1056–H1061. doi: 10.1152/ajpheart.1986.251.5.H1056. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Cavalié A. Cardiac calcium channels and their control by neurotransmitters and drugs. J Am Coll Cardiol. 1985 Dec;6(6):1409–1416. doi: 10.1016/s0735-1097(85)80233-3. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Videbaek J., Christensen N. J., Sterndorff B. Serial determination of plasma catecholamines in myocardial infarction. Circulation. 1972 Nov;46(5):846–855. doi: 10.1161/01.cir.46.5.846. [DOI] [PubMed] [Google Scholar]


