Abstract
1. Afferent responses were recorded from filaments of the trigeminal nerve in each of two platypuses (Ornithorhynchus anatinus) anaesthetized with alpha-chloralose. All receptive fields were located along the lateral border of the upper bill. Discrete receptive fields could be identified as belonging to two distinct classes of sensory receptor. 2. The most prominent response was an irregular resting discharge which could be increased or decreased by weak electric pulses. These receptors were insensitive to moderately strong mechanical stimulation, and it was concluded that they were electroreceptors. 3. Each electroreceptor had a single spot of maximum sensitivity on the bill surface. When the stimulating electrode over this spot was the cathode it excited the receptor for the duration of the stimulating pulse, using stimulus strengths as low as 20 mV. When it was the anode, it inhibited the discharge. Cathodal excitation was followed by rebound inhibition and anodal inhibition by rebound excitation. 4. Receptors responded to cathodal steps with an initial high-frequency burst of impulses, followed by a lower maintained rate of discharge. Rapidly changing pulses were similarly effective in exciting receptors, adding support to the claim that platypuses are able to detect moving prey by the electrical activity associated with muscle contraction. 5. The centres of the receptive fields of two electroreceptors were marked by the insertion of fine entomological pins. Histological examination established the presence of a large mucus-secreting gland at the marked spot. The epidermal duct of the gland contained an elaborate myelinated innervation, with morphologically distinct axon terminals that we identify as the electroreceptors. 6. As well as electroreceptors, the skin of the bill contained three kinds of mechanoreceptors: slow-adapting receptors, rapidly adapting, vibration-sensitive receptors and receptors with an intermediate adaptation rate. The slowly adapting receptors were characterized by their low threshold to mechanical stimuli, irregular discharge and significant dynamic sensitivity. Vibration receptors showed maintained responses to sinusoidal vibration of the skin up to 600 Hz. 7. These experiments confirm an earlier report that the platypus bill is an electrodetector organ. The presence of electroreceptors of a unique structure and supplied by the trigeminal nerve indicates that electroreception has evolved independently in monotremes. This in turn emphasizes that monotremes are a highly evolved group which split off from the main mammalian stem a long time ago.
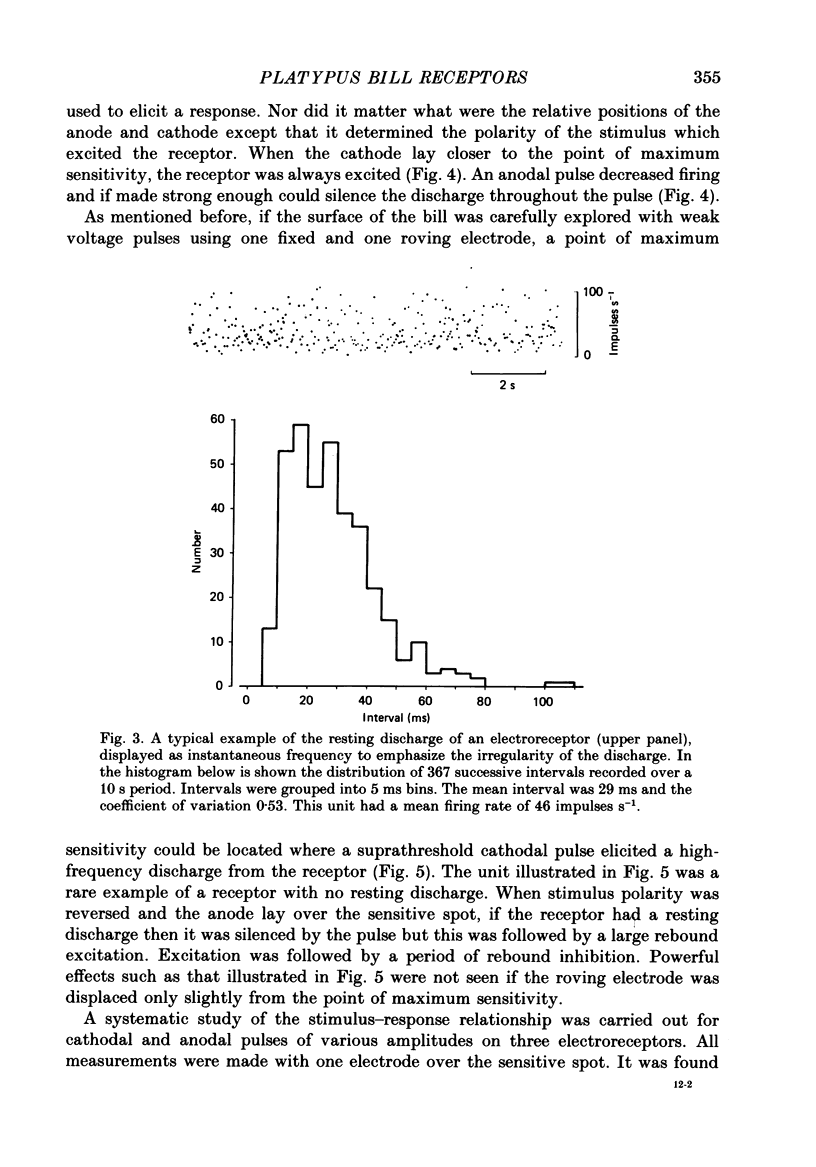
Full text
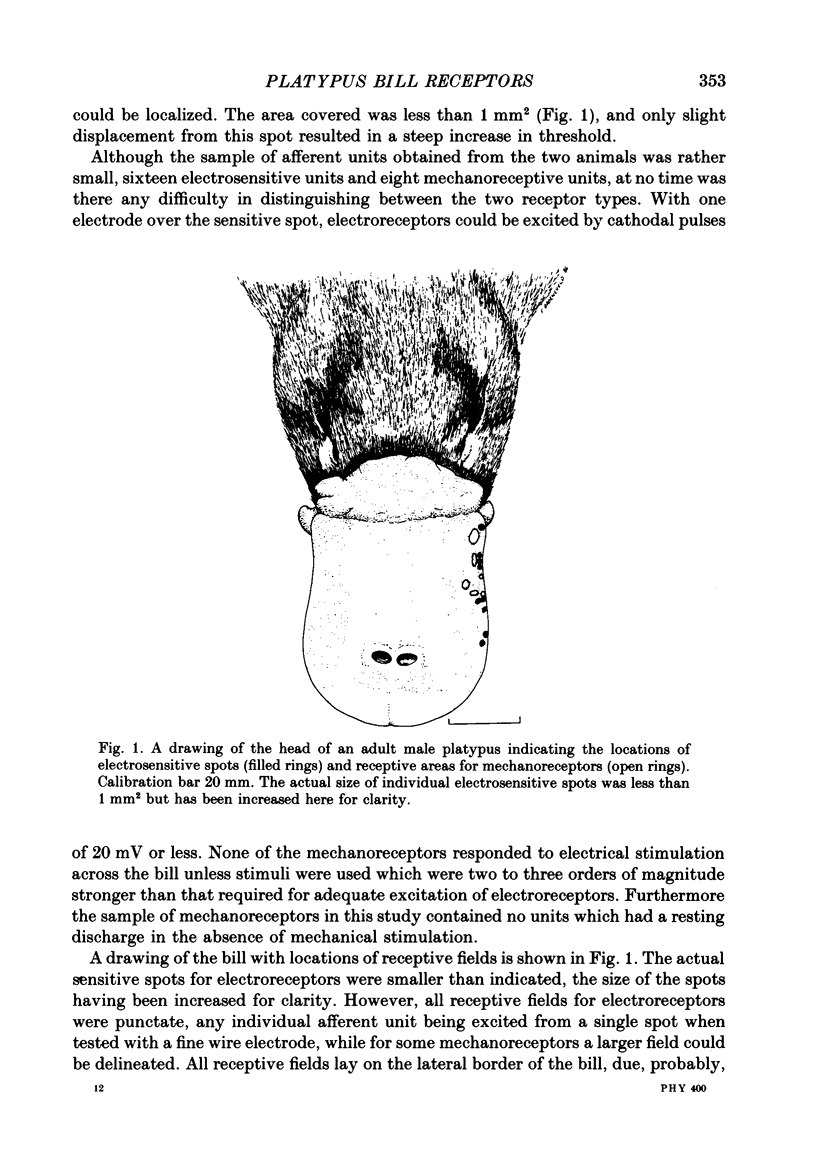
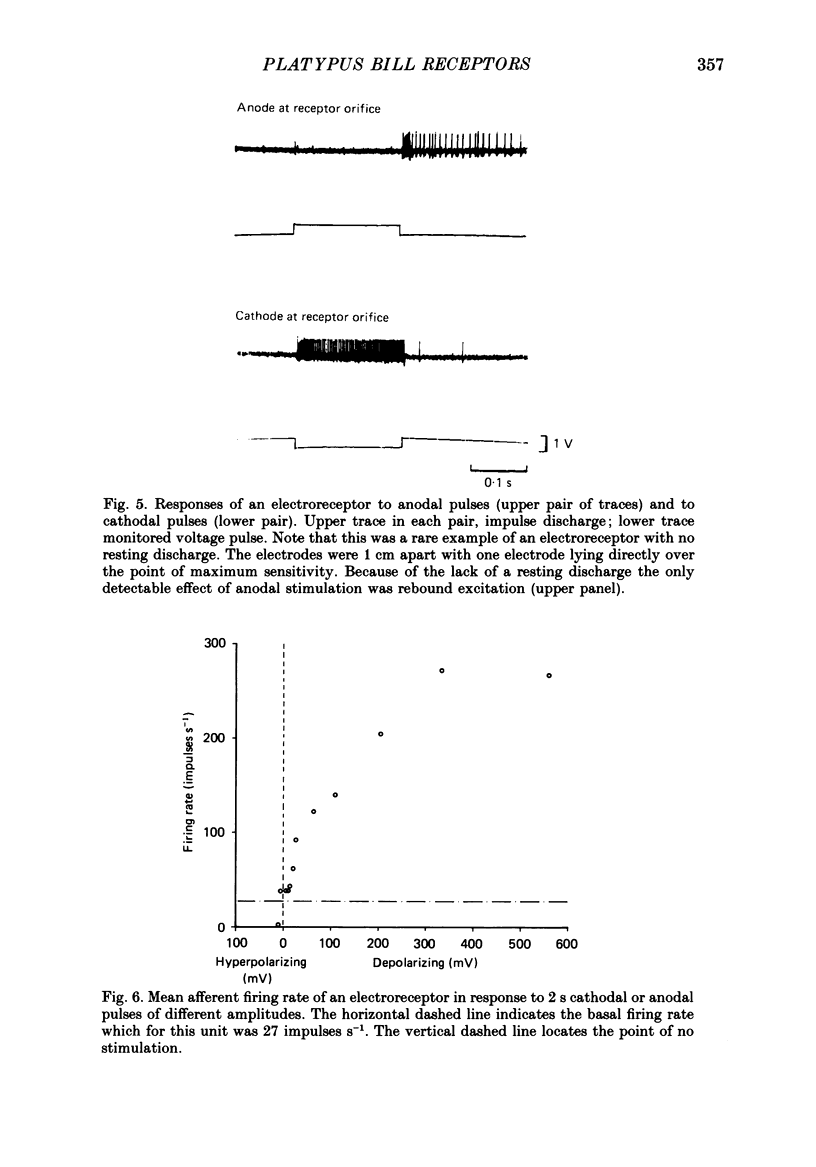
PDF













Images in this article
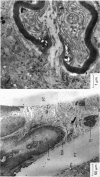
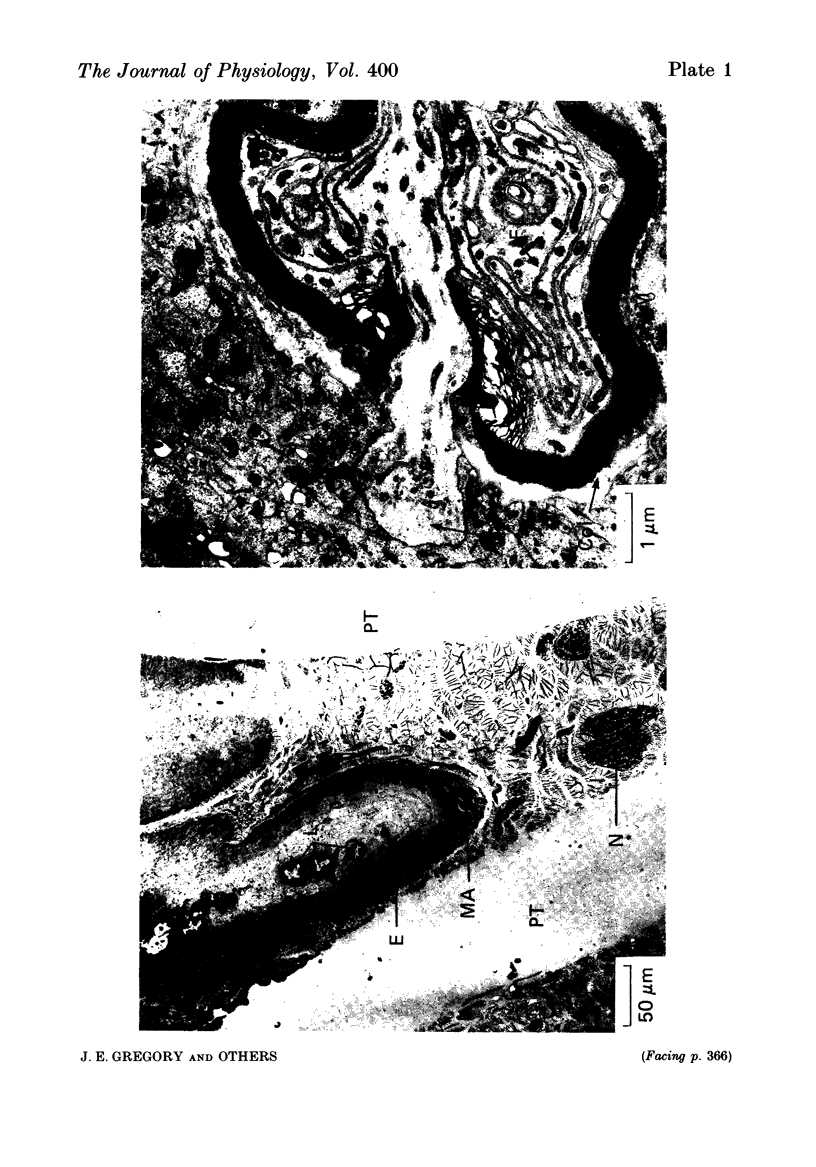
Selected References
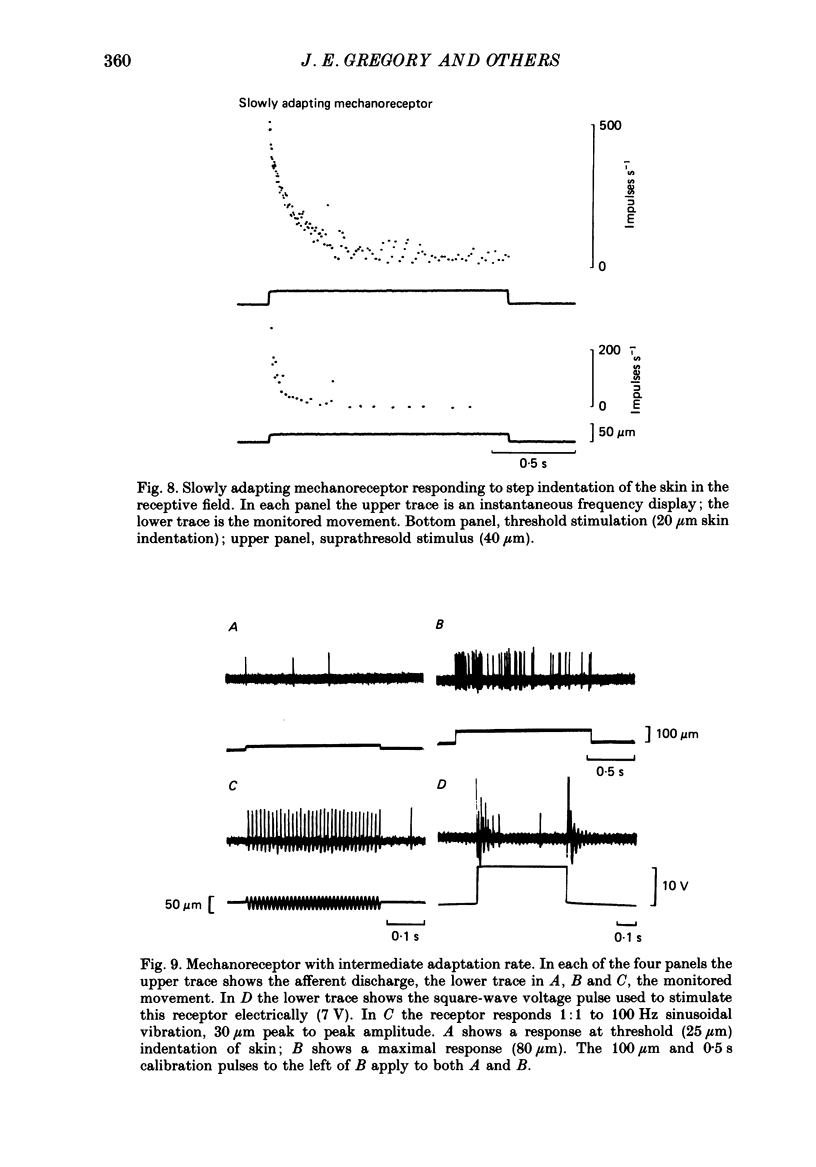
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. V., Clusin W. T. Transduction at electroreceptors: origins of sensitivity. Soc Gen Physiol Ser. 1979;33:91–116. [PubMed] [Google Scholar]
- Bohringer R. C., Rowe M. J. The organization of the sensory and motor areas of cerebral cortex in the platypus (Ornithorhynchus anatinus). J Comp Neurol. 1977 Jul 1;174(1):1–14. doi: 10.1002/cne.901740102. [DOI] [PubMed] [Google Scholar]
- Bullock T. H. Electroreception. Annu Rev Neurosci. 1982;5:121–170. doi: 10.1146/annurev.ne.05.030182.001005. [DOI] [PubMed] [Google Scholar]
- Chambers M. R., Andres K. H., von Duering M., Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- Dorward P. K., McIntyre A. K. Responses of vibration-sensitive receptors in the interosseous region of the duck's hind limb. J Physiol. 1971 Dec;219(1):77–87. doi: 10.1113/jphysiol.1971.sp009650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J. E., Iggo A., McIntyre A. K., Proske U. Electroreceptors in the platypus. 1987 Mar 26-Apr 1Nature. 326(6111):386–387. doi: 10.1038/326386a0. [DOI] [PubMed] [Google Scholar]
- Gregory J. E., McIntyre A. K., Proske U. Vibration-evoked responses from lamellated corpuscles in the legs of kangaroos. Exp Brain Res. 1986;62(3):648–653. doi: 10.1007/BF00236045. [DOI] [PubMed] [Google Scholar]
- HUNT C. C. On the nature of vibration receptors in the hind limb of the cat. J Physiol. 1961 Jan;155:175–186. doi: 10.1113/jphysiol.1961.sp006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., ISHIKO N. Sodium chloride sensitivity and electrochemical effects in a Lorenzinian ampulla. Nature. 1962 Apr 21;194:292–294. doi: 10.1038/194292b0. [DOI] [PubMed] [Google Scholar]
- Proske U. Vibration-sensitive mechanoreceptors in snake skin. Exp Neurol. 1969 Feb;23(2):187–194. doi: 10.1016/0014-4886(69)90055-7. [DOI] [PubMed] [Google Scholar]
- Scheich H., Langner G., Tidemann C., Coles R. B., Guppy A. Electroreception and electrolocation in platypus. 1986 Jan 30-Feb 5Nature. 319(6052):401–402. doi: 10.1038/319401a0. [DOI] [PubMed] [Google Scholar]