Abstract
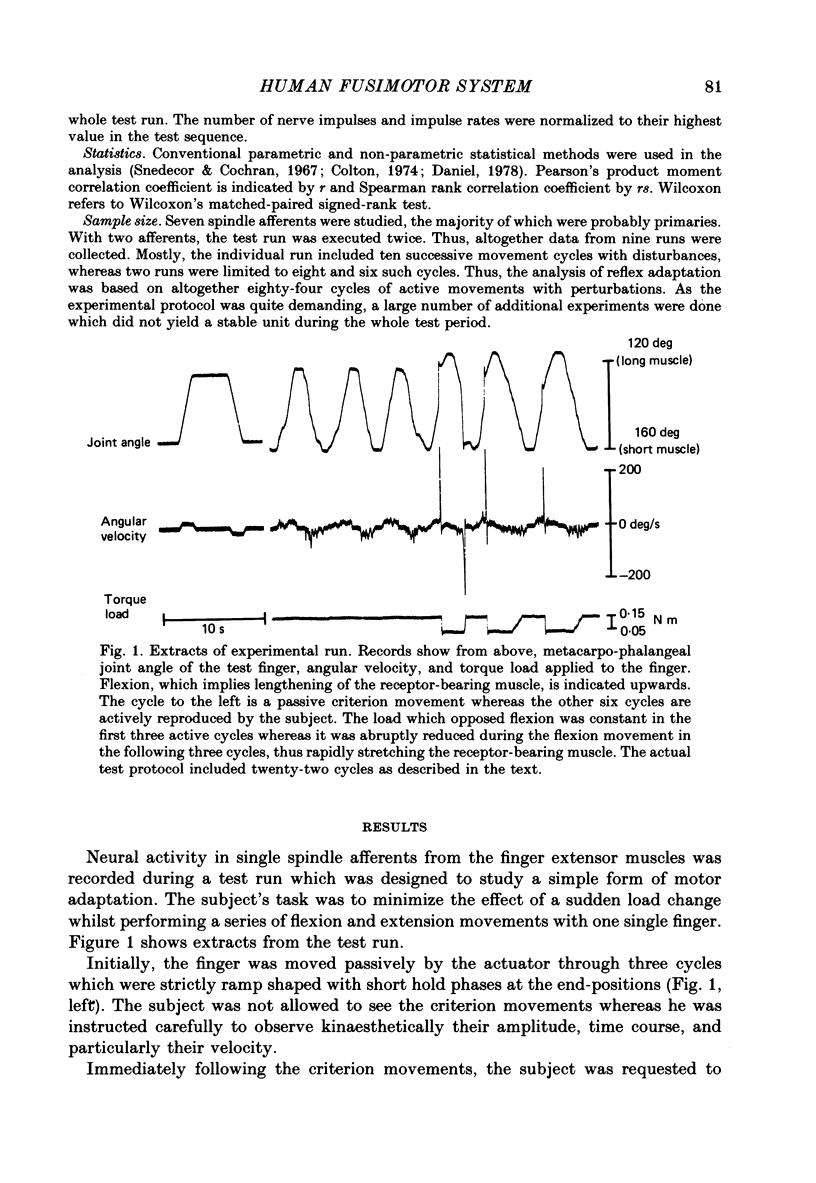
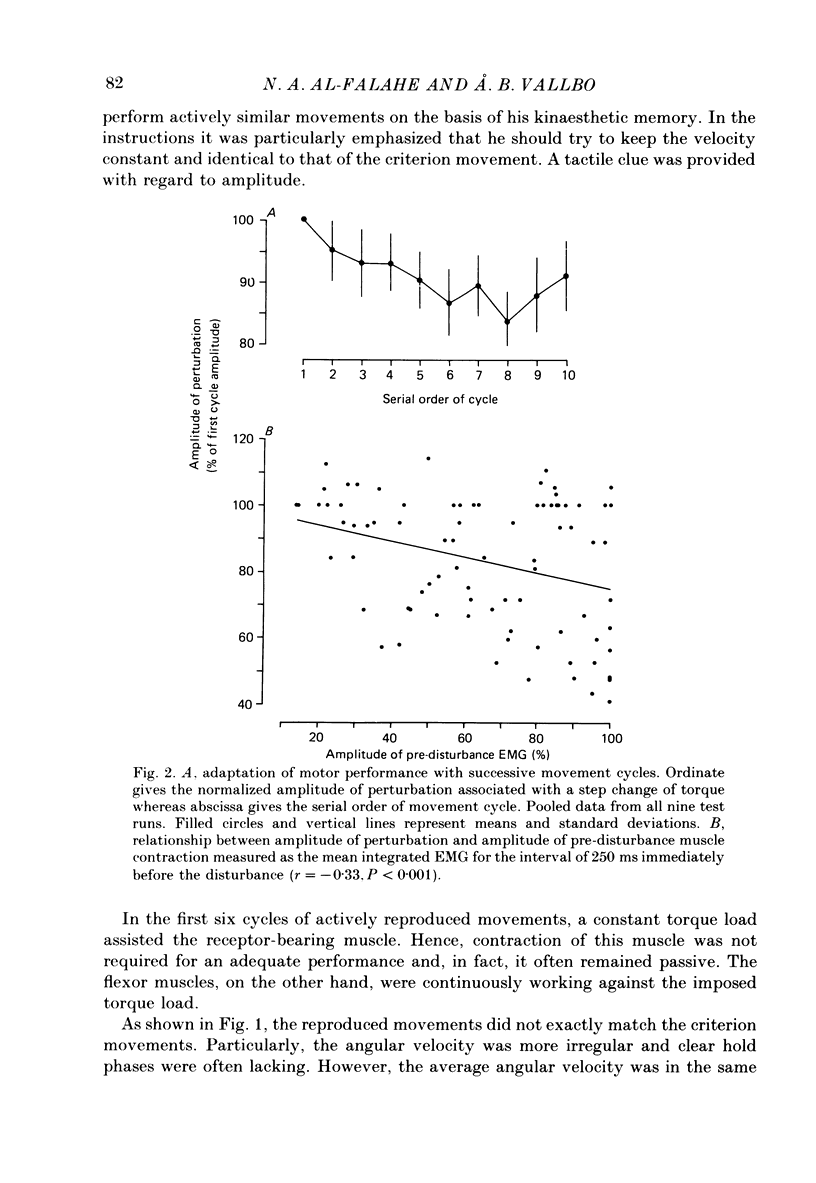
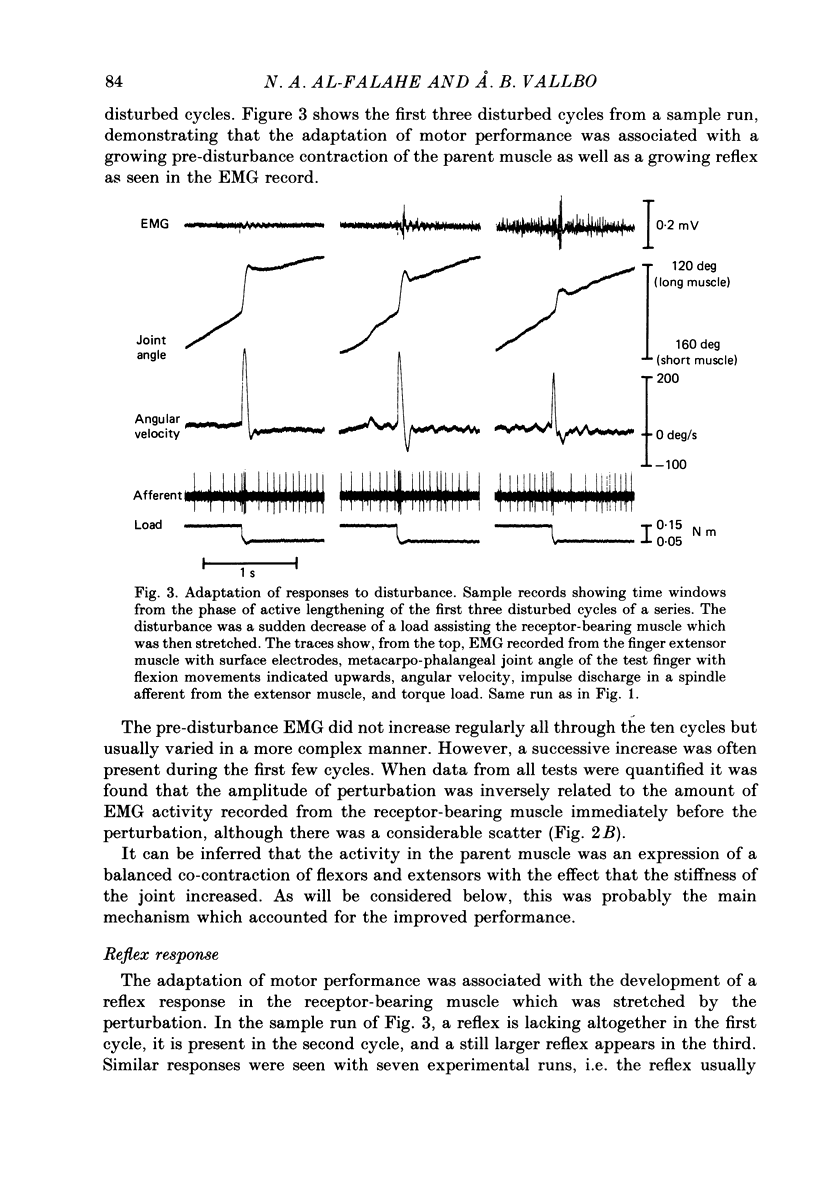
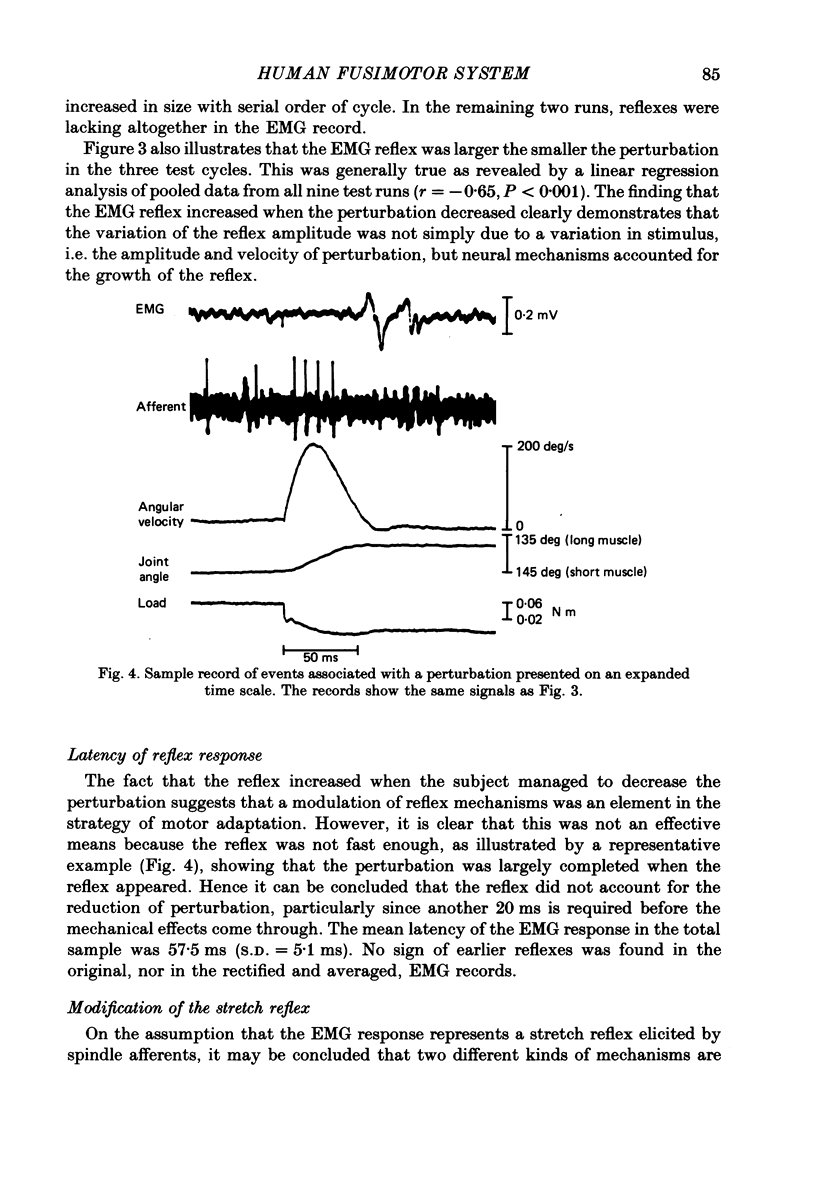
1. Single-unit activity was recorded with the microneurographic technique from the radial nerve of attending human subjects. During active finger movements, impulses in spindle afferents from the extensor digitorum muscle were analysed along with joint movements, size of imposed load and EMG activity of the receptor-bearing muscle. 2. In a simple motor adaptation task the subjects were requested to perform ramp-and-hold movements of prescribed amplitudes and velocities at a single metacarpo-phalangeal joint. A test run consisted of a series of movement cycles when the flexor muscle was continuously loaded with a constant torque, immediately followed by cycles when this load was abruptly decreased during the flexion movement, producing a fast stretch of the receptor-bearing muscle. The subjects' task was to strive for movements of constant velocity and particularly to minimize the effect of the disturbance. In order to allow prediction on the basis of immediately preceding cycles, the disturbance was always injected at the same angular position in a number of successive cycles. 3. Motor adaptation was manifested as a successive decrease of the perturbation amplitude, usually associated with the development of a continuous and growing EMG activity in the parent muscle and a growing reflex response of long latency (60 ms). Short-latency reflexes were not seen. 4. The main mechanism accounting for the improved performance was a co-contraction of the agonist-antagonist muscle pair during voluntary movements, producing an increased muscular stiffness. The reflex did not contribute to the motor adaptation because it was not fast enough to curtail the perturbation. 5. The development and the growth of the reflex were not due to a growing fusimotor drive during adaptation, because spindle discharge actually decreased when the reflex increased. The size of spindle response was related to the amplitude of perturbation rather than to the amplitude of the reflex. These findings suggest that reflex modifications were due to central excitability changes which paralleled the muscle contraction. 6. Spindle firing rate during active movements was generally higher in disturbed cycles compared to undisturbed cycles, indicating a higher fusimotor drive. Since muscle contraction was present mainly in the former, this finding may simply represent a case of fusimotor activation along with skeletomotor activation. No indication of an independence between the two was found. 7. The findings lend no support for the view that the size of the stretch reflex in a behavioural task is adjusted by selective changes of the fusimotor drive.
Full text
PDF












Selected References
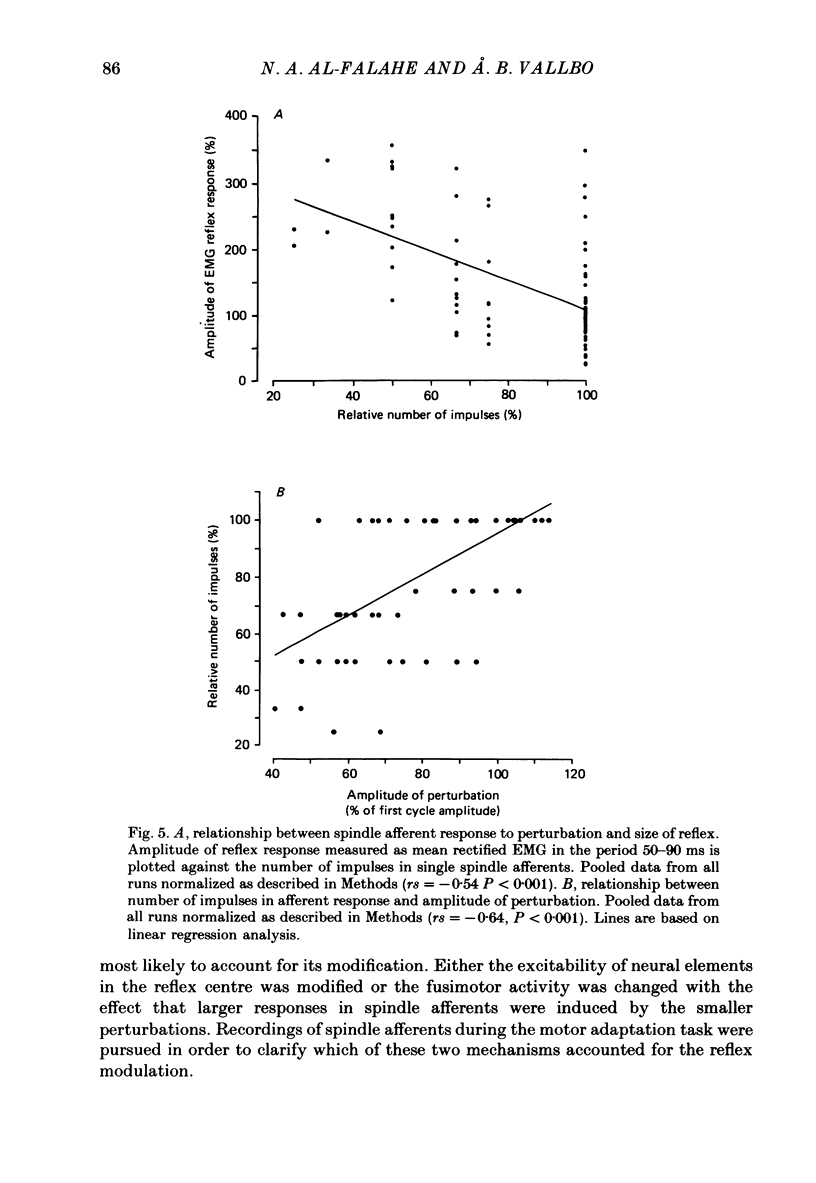
These references are in PubMed. This may not be the complete list of references from this article.
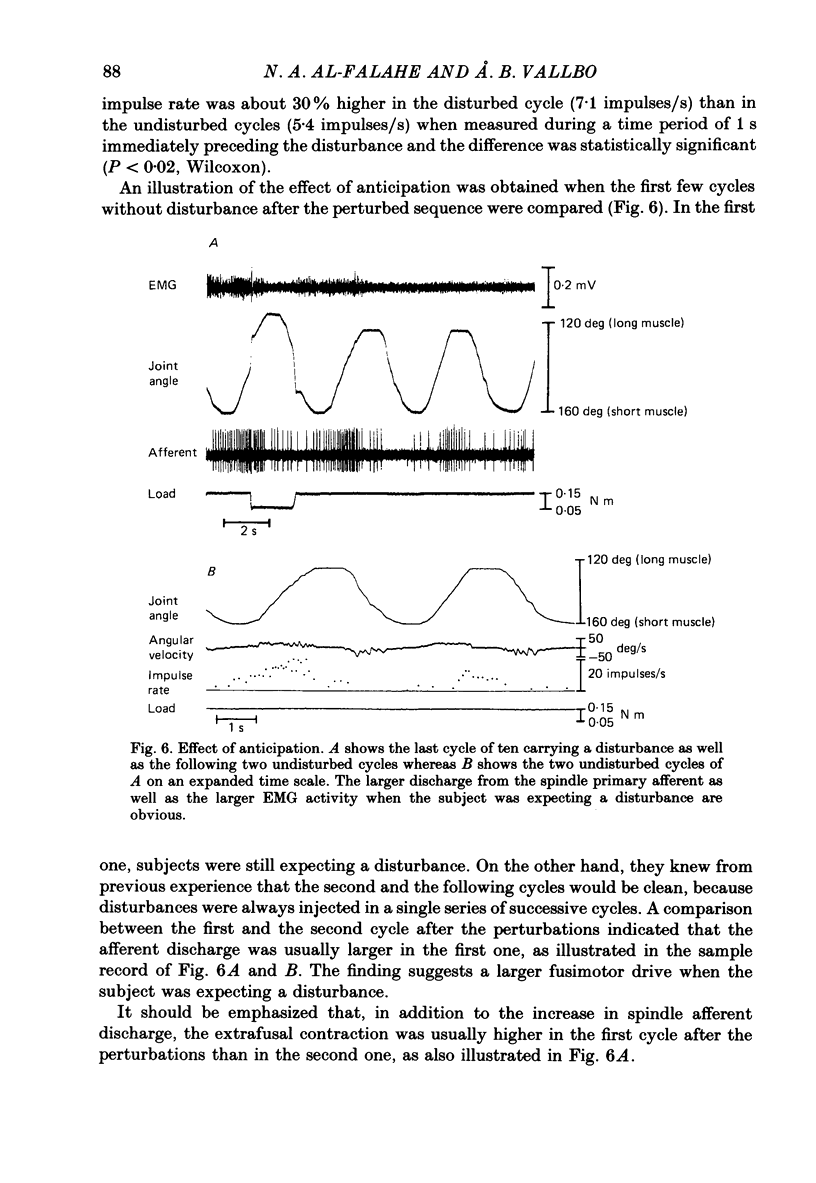
- Burke D., Hagbarth K. E., Löfstedt L. Muscle spindle responses in man to changes in load during accurate position maintenance. J Physiol. 1978 Mar;276:159–164. doi: 10.1113/jphysiol.1978.sp012225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976 Oct;261(3):695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., McKeon B., Skuse N. F., Westerman R. A. Anticipation and fusimotor activity in preparation for a voluntary contraction. J Physiol. 1980 Sep;306:337–348. doi: 10.1113/jphysiol.1980.sp013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney P. D., Preston J. B. Classification and response characteristics of muscle spindle afferents in the primate. J Neurophysiol. 1976 Jan;39(1):1–8. doi: 10.1152/jn.1976.39.1.1. [DOI] [PubMed] [Google Scholar]
- Cheney P. D., Preston J. B. Effects of fusimotor stimulation on dynamic and position sensitivities of spindle afferents in the primate. J Neurophysiol. 1976 Jan;39(1):20–30. doi: 10.1152/jn.1976.39.1.20. [DOI] [PubMed] [Google Scholar]
- Crago P. E., Houk J. C., Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol. 1976 Sep;39(5):925–935. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- Darton K., Lippold O. C., Shahani M., Shahani U. Long-latency spinal reflexes in humans. J Neurophysiol. 1985 Jun;53(6):1604–1618. doi: 10.1152/jn.1985.53.6.1604. [DOI] [PubMed] [Google Scholar]
- Davis J. N., Sears T. A. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol. 1970 Aug;209(3):711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin B. B., Vallbo A. B. Twitch contraction for identification of human muscle afferents. Acta Physiol Scand. 1987 Sep;131(1):129–138. doi: 10.1111/j.1748-1716.1987.tb08214.x. [DOI] [PubMed] [Google Scholar]
- Eklund G., Hagbarth K. E., Hägglund J. V., Wallin E. U. The 'late' reflex responses to muscle stretch: the 'resonance hypothesis' versus the 'long-loop hypothesis'. J Physiol. 1982 May;326:79–90. doi: 10.1113/jphysiol.1982.sp014178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts E. V., Fromm C. Transcortical reflexes and servo control of movement. Can J Physiol Pharmacol. 1981 Jul;59(7):757–775. doi: 10.1139/y81-112. [DOI] [PubMed] [Google Scholar]
- Evarts E. V., Granit R. Relations of reflexes and intended movements. Prog Brain Res. 1976;44:1–14. doi: 10.1016/S0079-6123(08)60719-0. [DOI] [PubMed] [Google Scholar]
- Evarts E. V., Tanji J. Gating of motor cortex reflexes by prior instruction. Brain Res. 1974 May 17;71(2-3):479–494. doi: 10.1016/0006-8993(74)90992-5. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C. Response to sudden torques about ankle in man. II. Postmyotatic reactions. J Neurophysiol. 1980 Jan;43(1):86–101. doi: 10.1152/jn.1980.43.1.86. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C. Response to sudden torques about ankle in man: myotatic reflex. J Neurophysiol. 1979 Jan;42(1 Pt 1):91–106. doi: 10.1152/jn.1979.42.1.91. [DOI] [PubMed] [Google Scholar]
- HAMMOND P. H. Involuntary activity in biceps following the sudden application of velocity to the abducted forearm. J Physiol. 1955 Feb 28;127(2):23–5P. [PMC free article] [PubMed] [Google Scholar]
- HAMMOND P. H., MERTON P. A., SUTTON G. G. Nervous gradation of muscular contraction. Br Med Bull. 1956 Sep;12(3):214–218. doi: 10.1093/oxfordjournals.bmb.a069553. [DOI] [PubMed] [Google Scholar]
- HAMMOND P. H. The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response. J Physiol. 1956 Apr 27;132(1):17–8P. [PubMed] [Google Scholar]
- Hagbarth K. E. EMG studies of stretch reflexes in man. Electroencephalogr Clin Neurophysiol. 1967;(Suppl):74–79. [PubMed] [Google Scholar]
- Hagbarth K. E., Hägglund J. V., Wallin E. U., Young R. R. Grouped spindle and electromyographic responses to abrupt wrist extension movements in man. J Physiol. 1981 Mar;312:81–96. doi: 10.1113/jphysiol.1981.sp013617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Wallin G., Burke D., Löfstedt L. Effects of the Jendrassik manoeuvre on muscle spindle activity in man. J Neurol Neurosurg Psychiatry. 1975 Dec;38(12):1143–1153. doi: 10.1136/jnnp.38.12.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. Convergence on interneurones in the reciprocal Ia inhibitory pathway to motoneurones. Acta Physiol Scand Suppl. 1972;375:1–42. doi: 10.1111/j.1748-1716.1972.tb05298.x. [DOI] [PubMed] [Google Scholar]
- Johansson R. S., Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56(3):550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Johansson R. S., Westling G. Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp Brain Res. 1987;66(1):141–154. doi: 10.1007/BF00236210. [DOI] [PubMed] [Google Scholar]
- Lee R. G., Tatton W. G. Motor responses to sudden limb displacements in primates with specific CNS lesions and in human patients with motor system disorders. Can J Neurol Sci. 1975 Aug;2(3):285–293. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- Loo C. K., McCloskey D. I. Effects of prior instruction and anaesthesia on long-latency responses to stretch in the long flexor of the human thumb. J Physiol. 1985 Aug;365:285–296. doi: 10.1113/jphysiol.1985.sp015772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B. MUSCLE SPINDLES AND THEIR MOTOR CONTROL. Physiol Rev. 1964 Apr;44:219–288. doi: 10.1152/physrev.1964.44.2.219. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Latency measurements compatible with a cortical pathway for the stretch reflex in man. J Physiol. 1973 Apr;230(1):58P–59P. [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in human voluntary movement. Nature. 1972 Jul 21;238(5360):140–143. doi: 10.1038/238140a0. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in the human thumb. J Physiol. 1976 May;257(1):1–44. doi: 10.1113/jphysiol.1976.sp011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. The sensory mechanism of servo action in human muscle. J Physiol. 1977 Feb;265(2):521–535. doi: 10.1113/jphysiol.1977.sp011728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol. 1984 Mar;348:383–415. doi: 10.1113/jphysiol.1984.sp015116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The contrasting stretch reflex responses of the long and short flexor muscles of the human thumb. J Physiol. 1984 Mar;348:545–558. doi: 10.1113/jphysiol.1984.sp015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner L. M. Adapting reflexes controlling the human posture. Exp Brain Res. 1976 Aug 27;26(1):59–72. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- Phillips C. G. The Ferrier lecture, 1968. Motor apparatus of the baboon's hand. Proc R Soc Lond B Biol Sci. 1969 May 20;173(1031):141–174. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Wand P. Tendon organ discharge during voluntary movements in cats. J Physiol. 1980 Jun;303:385–390. doi: 10.1113/jphysiol.1980.sp013293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell J. C., Day B. L., Berardelli A., Marsden C. D. Habituation and conditioning of the human long latency stretch reflex. Exp Brain Res. 1986;63(1):197–204. doi: 10.1007/BF00235664. [DOI] [PubMed] [Google Scholar]
- Salmoni A. W., Schmidt R. A., Walter C. B. Knowledge of results and motor learning: a review and critical reappraisal. Psychol Bull. 1984 May;95(3):355–386. [PubMed] [Google Scholar]
- Tatton W. G., Forner S. D., Gerstein G. L., Chambers W. W., Liu C. N. The effect of postcentral cortical lesions on motor responses to sudden upper limb displacements in monkeys. Brain Res. 1975 Oct 10;96(1):108–113. doi: 10.1016/0006-8993(75)90580-6. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol. 1968 Jul;21(3):270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E., Torebjörk H. E., Wallin B. G. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979 Oct;59(4):919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M., Miles T. S. Ascending pathway of low-threshold muscle afferents to the cerebral cortex and its possible role in motor control. Physiol Rev. 1982 Oct;62(4 Pt 1):1234–1270. doi: 10.1152/physrev.1982.62.4.1234. [DOI] [PubMed] [Google Scholar]


