Abstract
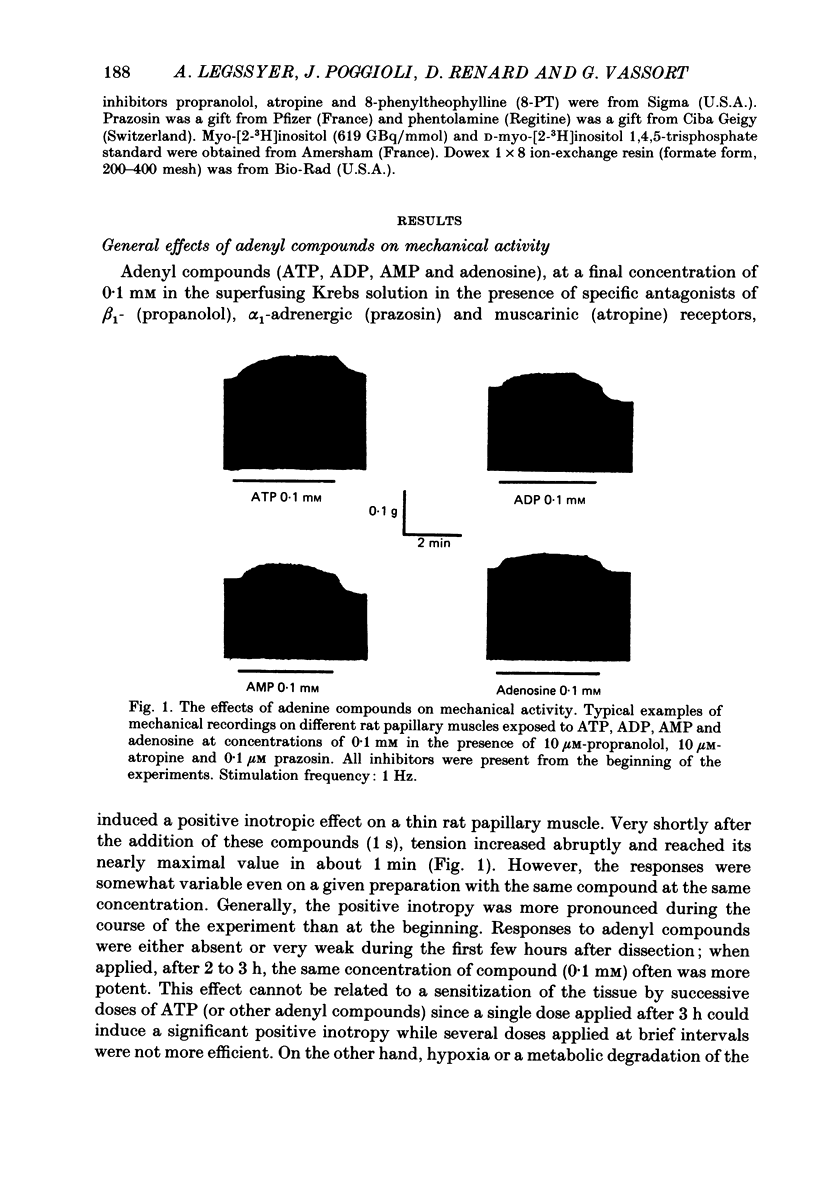
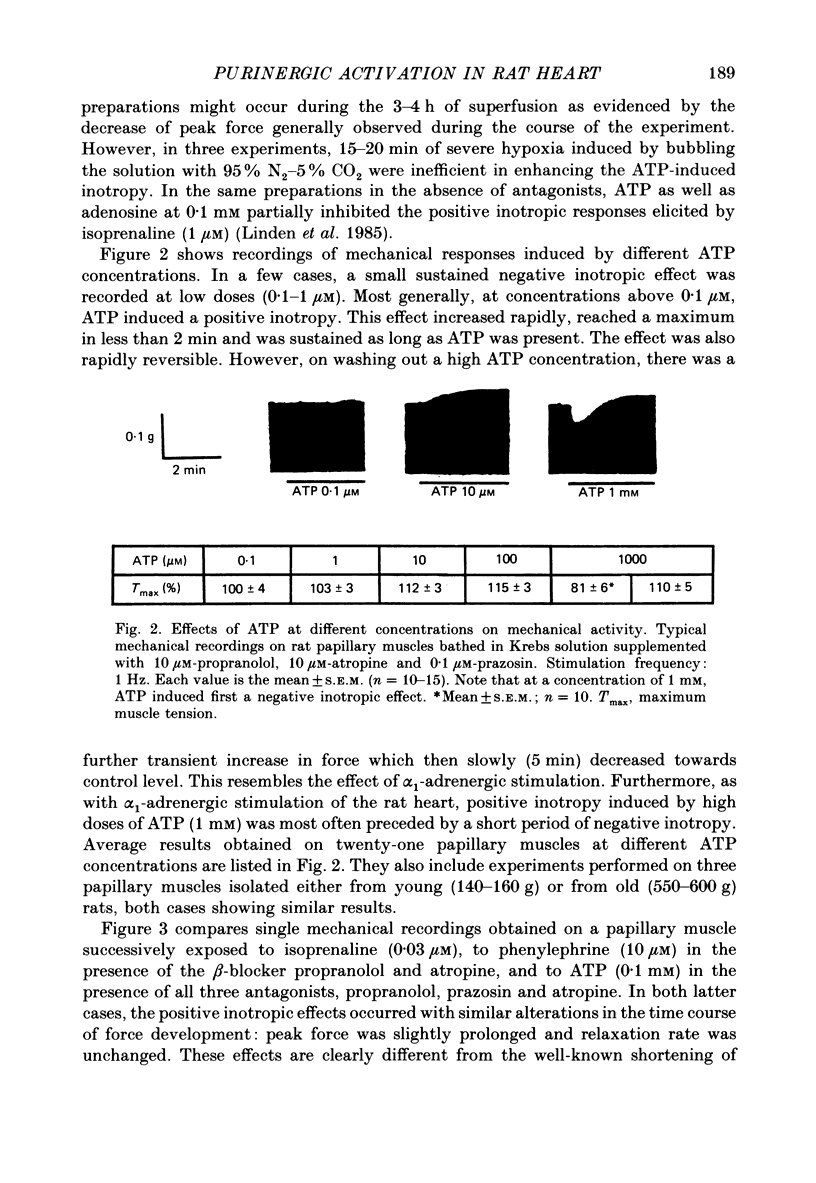
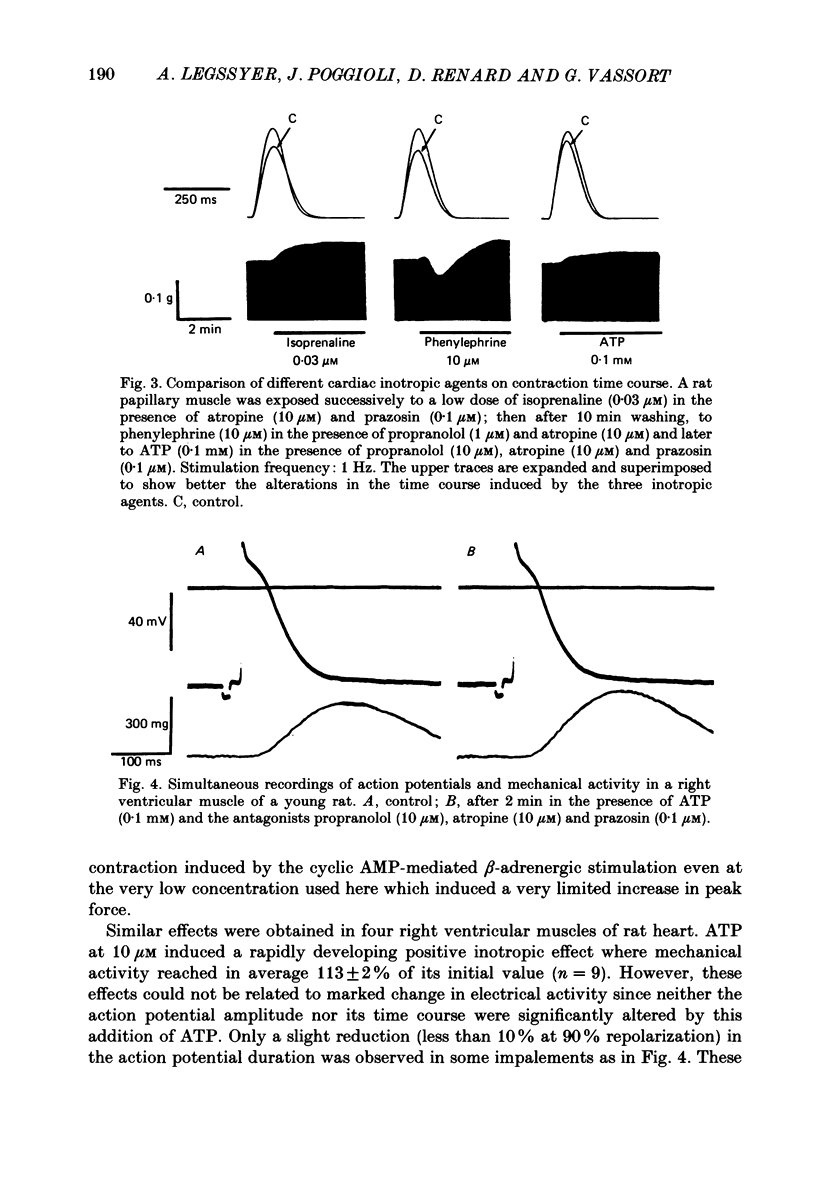
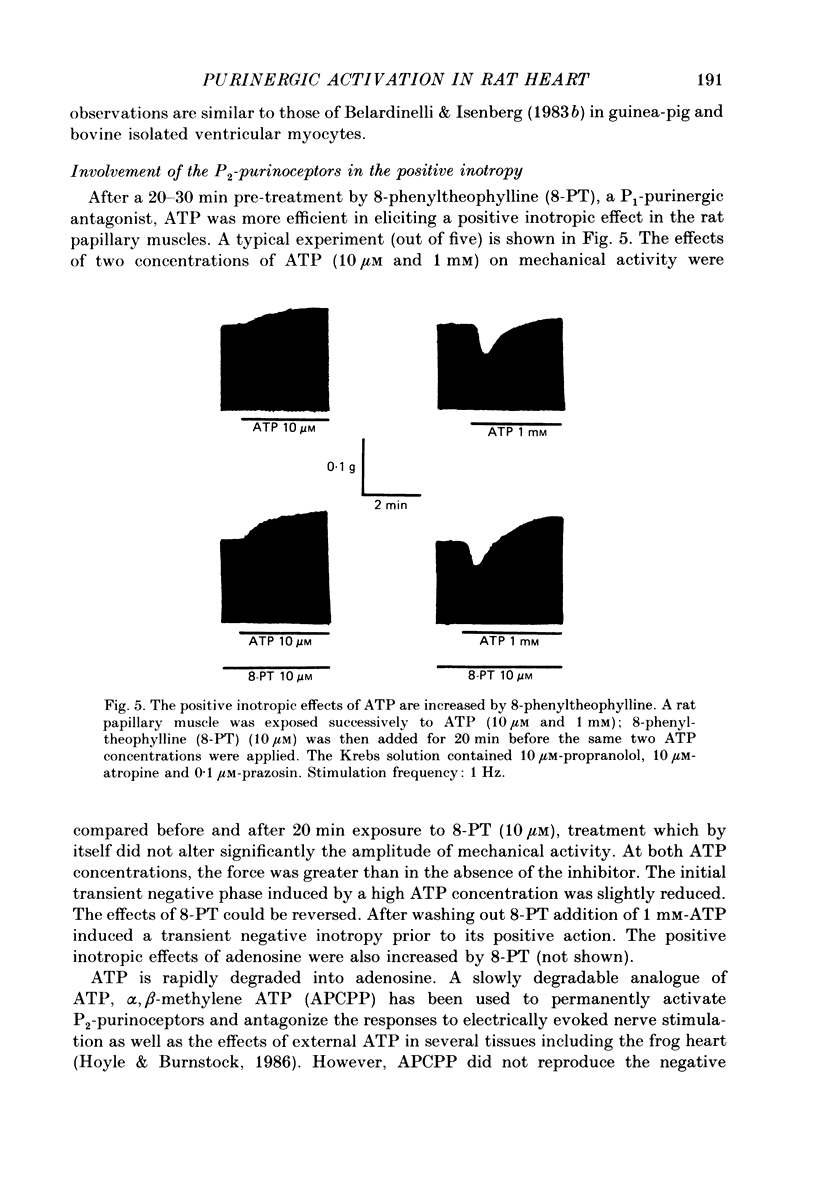
1. The effects of adenosine 5'-triphosphate (ATP) and other adenine compounds were examined on rat papillary and right ventricular muscles in the presence of 10 microM-propranolol, 10 microM-atropine and 0.1 microM-prazosin or 10 microM-phentolamine. 2. Adenosine, adenosine 5'-monophosphate (AMP), adenosine 5'-diphosphate (ADP), ATP and alpha,beta-methylene ATP (APCPP) produced small positive inotropic effects, sometimes preceded by transient negative effects. 3. 8-Phenyltheophylline (8-PT), a P1-purinoceptor antagonist antagonized the negative effects and increased the positive inotropy induced by ATP and adenosine. 4. In the presence of APCPP, a P2-purinergic agonist, ATP had only negative inotropic effects. 5. Adenosine and ATP increased inositol 1, 4, 5- and inositol 1, 3, 4-trisphosphate as well as inositol mono- and bisphosphate formation. Maximal effects were obtained at concentrations of 0.5 mM. 6. APCPP increased inositol phosphate formation while 8-PT did not prevent the effects of adenosine and ATP. 7. It is suggested that P2-purinoceptor activation induces both a positive inotropy and an increase in inositol-lipid metabolism in rat ventricular muscles.
Full text
PDF


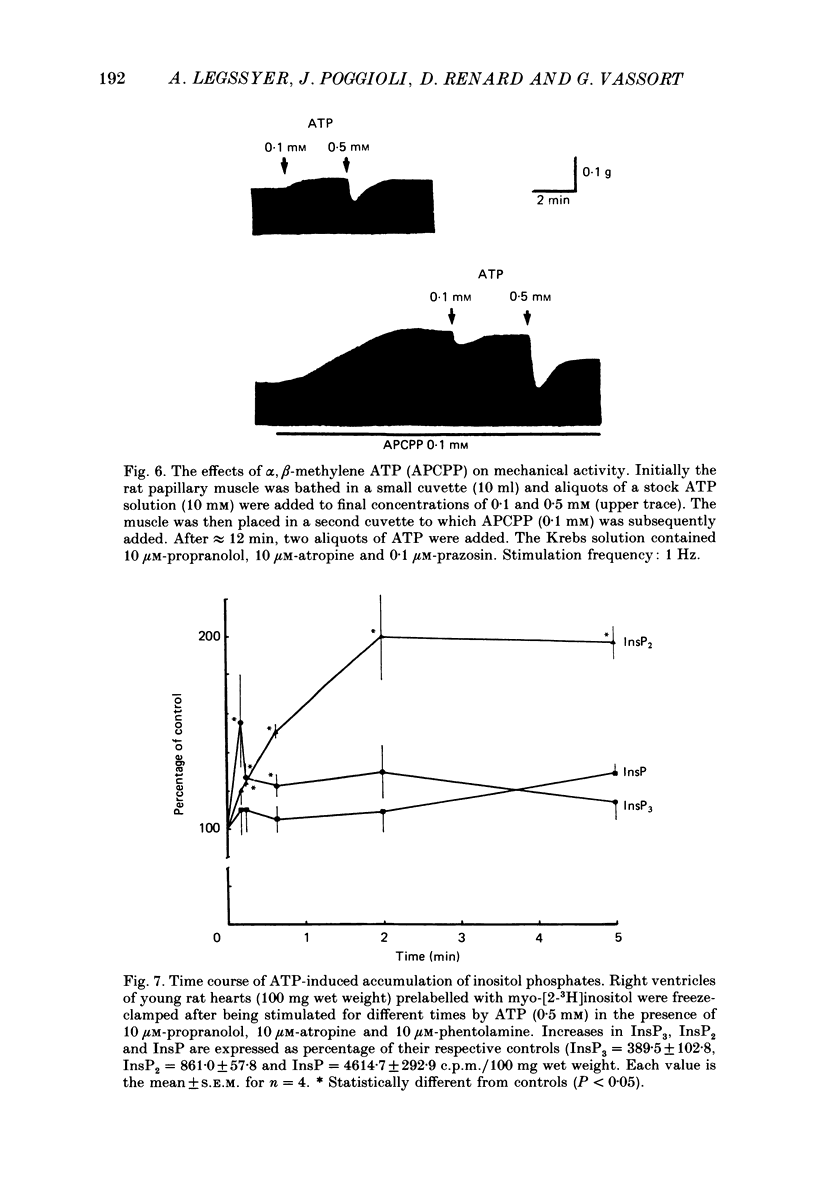
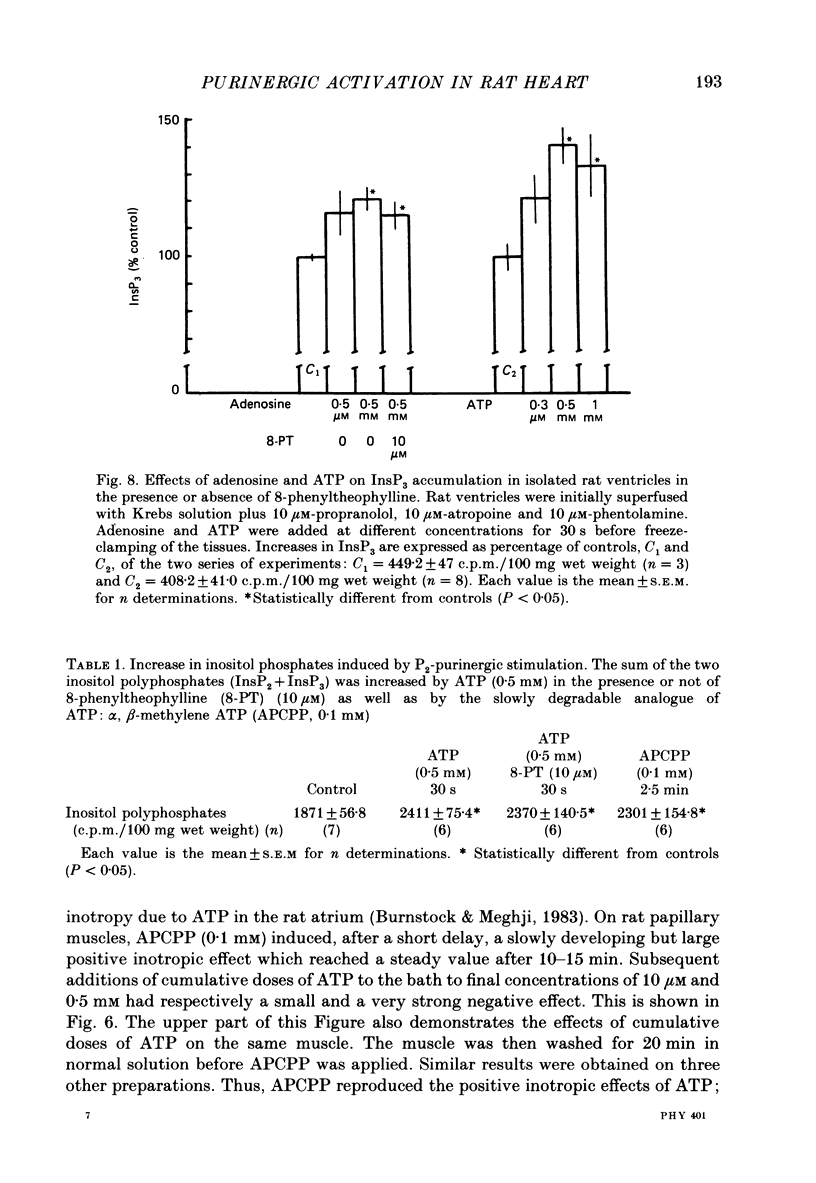
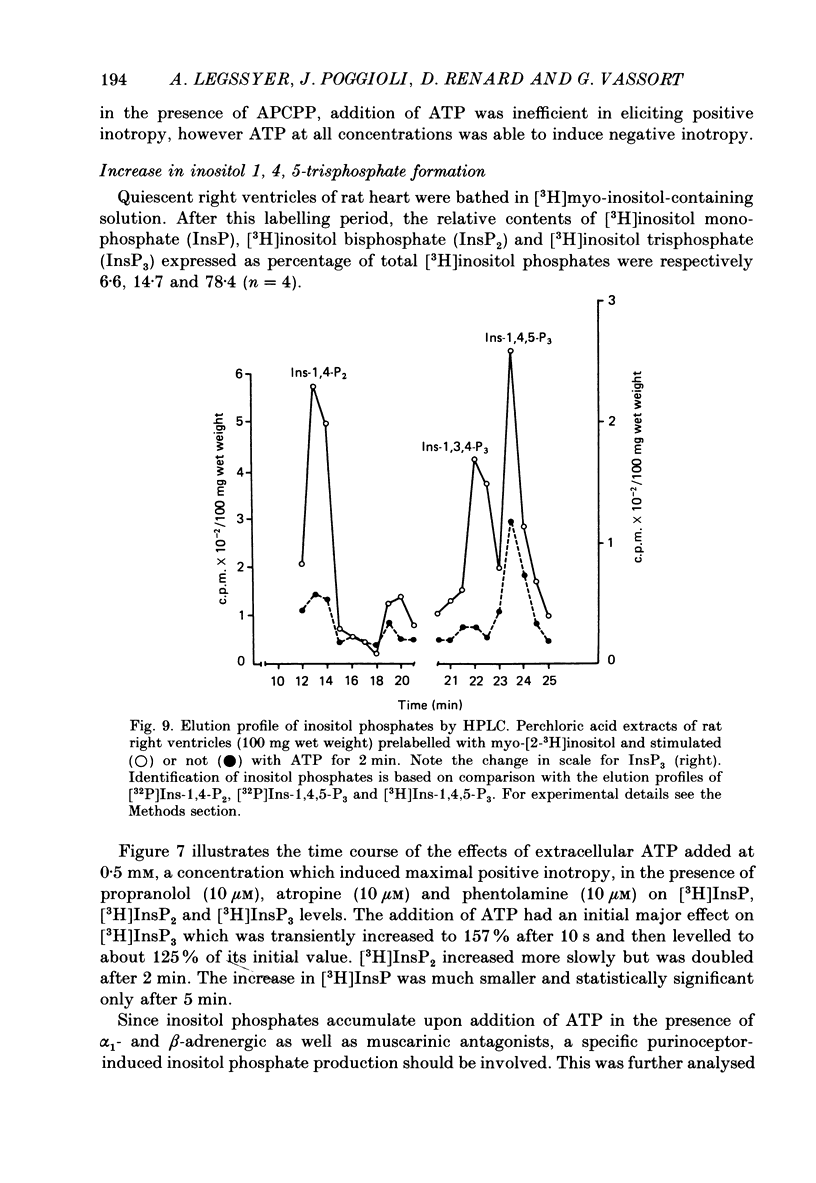





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L., Isenberg G. Actions of adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ Res. 1983 Sep;53(3):287–297. doi: 10.1161/01.res.53.3.287. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Isenberg G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol. 1983 May;244(5):H734–H737. doi: 10.1152/ajpheart.1983.244.5.H734. [DOI] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born G. V., Kratzer M. A. Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits and man. J Physiol. 1984 Sep;354:419–429. doi: 10.1113/jphysiol.1984.sp015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashear R. E., Mandelbaum I., Ross J. C. Selected cardiovascular effects of adenosine diphosphate. Am Heart J. 1970 Jul;80(1):63–69. doi: 10.1016/0002-8703(70)90038-4. [DOI] [PubMed] [Google Scholar]
- Brückner R., Fenner A., Meyer W., Nobis T. M., Schmitz W., Scholz H. Cardiac effects of adenosine and adenosine analogs in guinea-pig atrial and ventricular preparations: evidence against a role of cyclic AMP and cyclic GMP. J Pharmacol Exp Ther. 1985 Sep;234(3):766–774. [PubMed] [Google Scholar]
- Burnstock G., Meghji P. The effect of adenyl compounds on the rat heart. Br J Pharmacol. 1983 May;79(1):211–218. doi: 10.1111/j.1476-5381.1983.tb10514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Chiba S., Himori N. Different inotropic responses to adenosine on the atrial and ventricular muscle of the dog heart. Jpn J Pharmacol. 1975 Aug;25(4):489–491. doi: 10.1254/jjp.25.489. [DOI] [PubMed] [Google Scholar]
- De Young M. B., Scarpa A. Extracellular ATP induces Ca2+ transients in cardiac myocytes which are potentiated by norepinephrine. FEBS Lett. 1987 Oct 19;223(1):53–58. doi: 10.1016/0014-5793(87)80508-2. [DOI] [PubMed] [Google Scholar]
- Dobson J. G., Jr Interaction between adenosine and inotropic interventions in guinea pig atria. Am J Physiol. 1983 Sep;245(3):H475–H480. doi: 10.1152/ajpheart.1983.245.3.H475. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R. Extracellular ATP activates polyphosphoinositide breakdown and Ca2+ mobilization in Ehrlich ascites tumor cells. Arch Biochem Biophys. 1986 Feb 15;245(1):84–95. doi: 10.1016/0003-9861(86)90192-x. [DOI] [PubMed] [Google Scholar]
- Dutta P., Mustafa S. J. Saturable binding of adenosine to the dog heart microsomal fraction: competitive inhibition by aminophylline. J Pharmacol Exp Ther. 1979 Dec;211(3):496–501. [PubMed] [Google Scholar]
- Fleetwood G., Gordon J. L. Purinoceptors in the rat heart. Br J Pharmacol. 1987 Jan;90(1):219–227. doi: 10.1111/j.1476-5381.1987.tb16843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney F. W., Singh J. Inotropic responses of the frog ventricle to adenosine triphosphate and related changes in endogenous cyclic nucleotides. J Physiol. 1980 Jul;304:21–42. doi: 10.1113/jphysiol.1980.sp013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. A., Rovetto M. J. Rat cardiac myocyte adenosine transport and metabolism. Am J Physiol. 1987 Jan;252(1 Pt 2):H54–H63. doi: 10.1152/ajpheart.1987.252.1.H54. [DOI] [PubMed] [Google Scholar]
- Forrester T., Williams C. A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol. 1977 Jun;268(2):371–390. doi: 10.1113/jphysiol.1977.sp011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Yatani A., Tsuda Y. Effects of ATP on the membrane currents and tension components of bullfrog atrial muscle. Nihon Seirigaku Zasshi. 1976 Dec 1;38(12):503–506. [PubMed] [Google Scholar]
- Griffith S. G., Meghji P., Moody C. J., Burnstock G. 8-phenyltheophylline: a potent P1-purinoceptor antagonist. Eur J Pharmacol. 1981 Oct 15;75(1):61–64. doi: 10.1016/0014-2999(81)90346-0. [DOI] [PubMed] [Google Scholar]
- HOLLANDER P. B., WEBB J. L. Effects of adenine nucleotides on the contractility and membrane potentials of rat atrium. Circ Res. 1957 Jul;5(4):349–353. doi: 10.1161/01.res.5.4.349. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Adenosine receptors in frog sinus venosus: slow inhibitory potentials produced by adenine compounds and acetylcholine. J Physiol. 1979 Aug;293:23–49. doi: 10.1113/jphysiol.1979.sp012877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins S. V. The action of ATP in the guinea-pig heart. Biochem Pharmacol. 1973 Feb 1;22(3):335–339. doi: 10.1016/0006-2952(73)90414-0. [DOI] [PubMed] [Google Scholar]
- Horstman D. A., Tennes K. A., Putney J. W., Jr ATP-induced calcium mobilization and inositol 1,4,5-triphosphate formation in H-35 hepatoma cells. FEBS Lett. 1986 Aug 18;204(2):189–192. doi: 10.1016/0014-5793(86)80809-2. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Evidence that ATP is a neurotransmitter in the frog heart. Eur J Pharmacol. 1986 May 27;124(3):285–289. doi: 10.1016/0014-2999(86)90229-3. [DOI] [PubMed] [Google Scholar]
- Ingerman C. M., Smith J. B., Silver M. J. Direct measurement of platelet secretion in whole blood. Thromb Res. 1979;16(3-4):335–344. doi: 10.1016/0049-3848(79)90081-1. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J., Hollen C. E., Patel A. The mechanism by which adenosine and cholinergic agents reduce contractility in rat myocardium. Correlation with cyclic adenosine monophosphate and receptor densities. Circ Res. 1985 May;56(5):728–735. doi: 10.1161/01.res.56.5.728. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Terenius L., Hökfelt T., Goldstein M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett. 1983 Dec 2;42(2):167–172. doi: 10.1016/0304-3940(83)90401-9. [DOI] [PubMed] [Google Scholar]
- Niedergerke R., Page S. Two physiological agents that appear to facilitate calcium discharge from the sarcoplasmic reticulum in frog heart cells: adrenalin an ATP. Proc R Soc Lond B Biol Sci. 1981 Nov 13;213(1192):325–344. doi: 10.1098/rspb.1981.0069. [DOI] [PubMed] [Google Scholar]
- Nosek T. M., Williams M. F., Zeigler S. T., Godt R. E. Inositol trisphosphate enhances calcium release in skinned cardiac and skeletal muscle. Am J Physiol. 1986 May;250(5 Pt 1):C807–C811. doi: 10.1152/ajpcell.1986.250.5.C807. [DOI] [PubMed] [Google Scholar]
- Phaneuf S., Berta P., Casanova J., Cavadore J. C. ATP stimulates inositol phosphates accumulation and calcium mobilization in a primary culture of rat aortic myocytes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):454–460. doi: 10.1016/0006-291x(87)91375-1. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Sulpice J. C., Vassort G. Inositol phosphate production following alpha 1-adrenergic, muscarinic or electrical stimulation in isolated rat heart. FEBS Lett. 1986 Oct 6;206(2):292–298. doi: 10.1016/0014-5793(86)80999-1. [DOI] [PubMed] [Google Scholar]
- Renard D., Poggioli J. Does the inositol tris/tetrakisphosphate pathway exist in rat heart? FEBS Lett. 1987 Jun 8;217(1):117–123. doi: 10.1016/0014-5793(87)81254-1. [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Sperelakis N. Slow Ca2+ and Na+ responses induced by isoproterenol and methylxanthines in isolated perfused guinea pig hearts exposed to elevated K+. J Mol Cell Cardiol. 1975 Apr;7(4):249–273. doi: 10.1016/0022-2828(75)90084-x. [DOI] [PubMed] [Google Scholar]
- Schrader J., Nees S., Gerlach E. Evidence for a cell surface adenosine receptor on coronary myocytes and atrial muscle cells. Studies with an adenosine derivative of high molecular weight. Pflugers Arch. 1977 Jul 19;369(3):251–257. doi: 10.1007/BF00582192. [DOI] [PubMed] [Google Scholar]
- Shah A., Kechejian S. J., Kavaler F., Fisher V. J. Effects of adenine nucleotides on contractility of normal and postischemic myocardium. Am Heart J. 1974 Jun;87(6):740–749. doi: 10.1016/0002-8703(74)90420-7. [DOI] [PubMed] [Google Scholar]
- Watt A. H. Hypertrophic cardiomyopathy: a disease of impaired adenosine-mediated autoregulation of the heart. Lancet. 1984 Jun 9;1(8389):1271–1273. doi: 10.1016/s0140-6736(84)92449-8. [DOI] [PubMed] [Google Scholar]


