Abstract
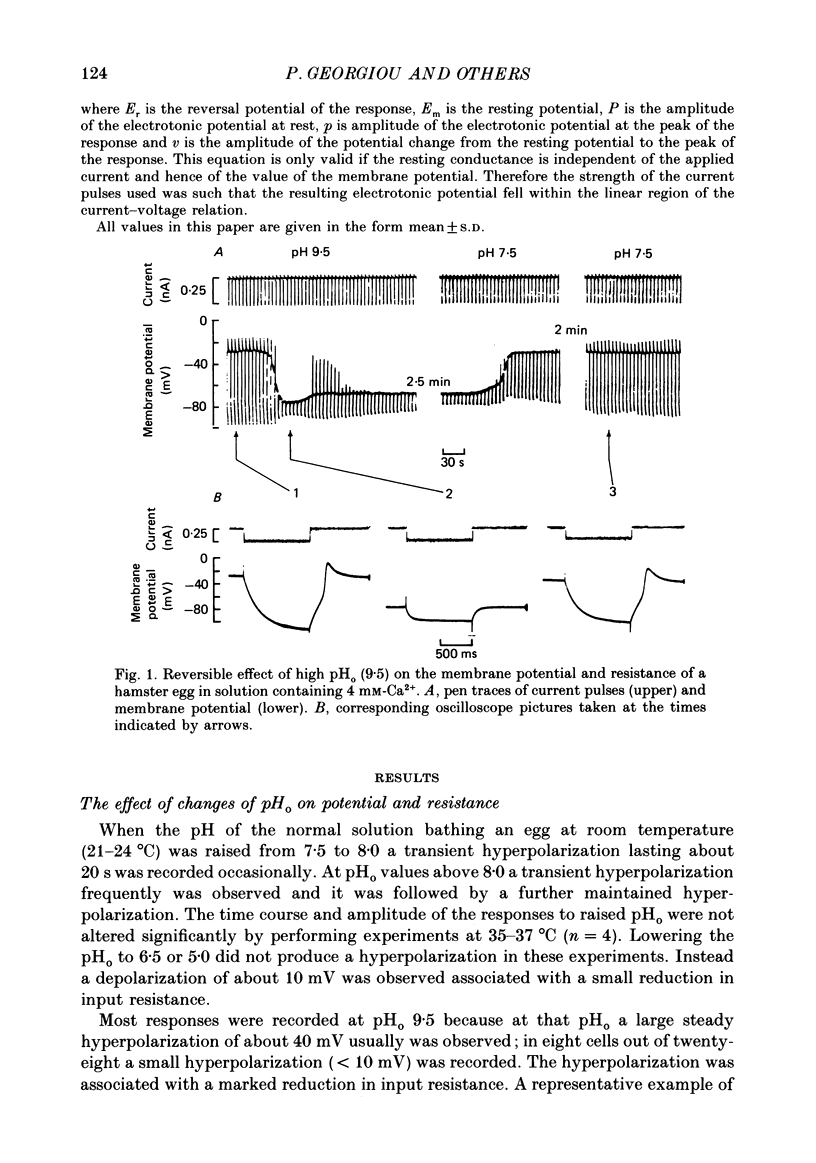
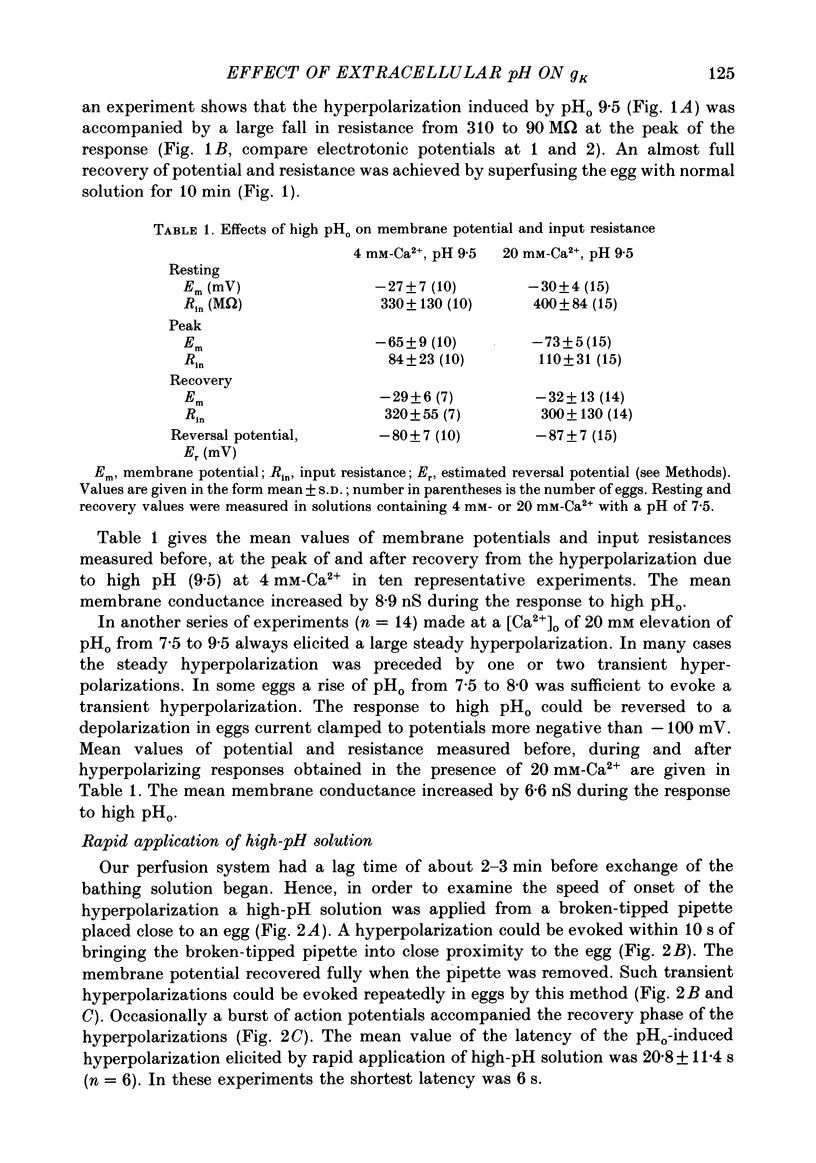
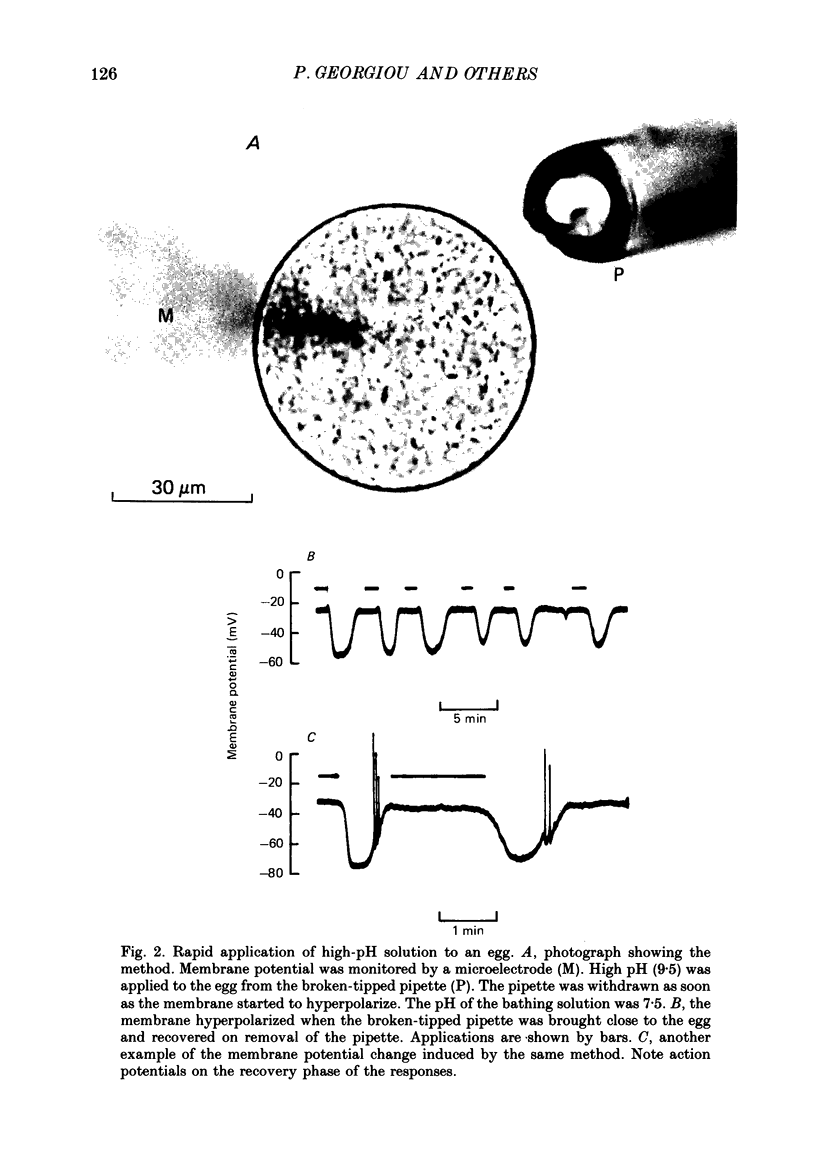
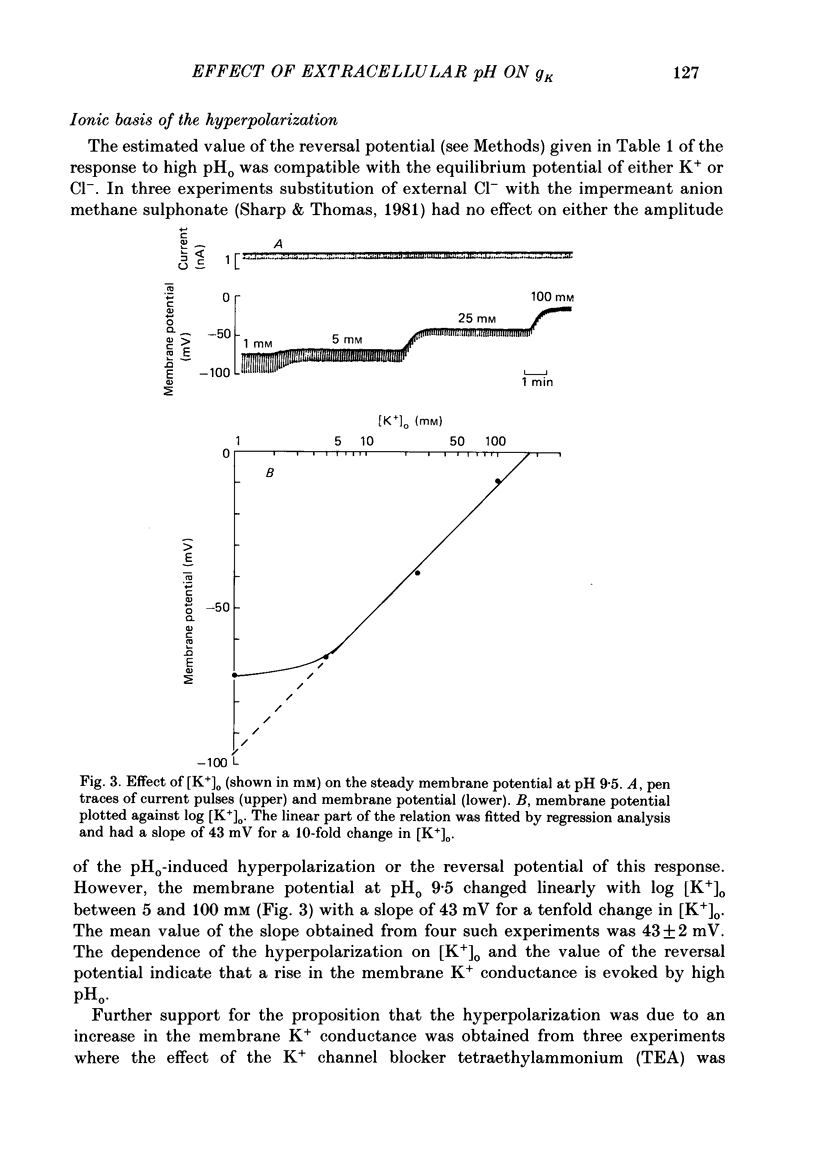
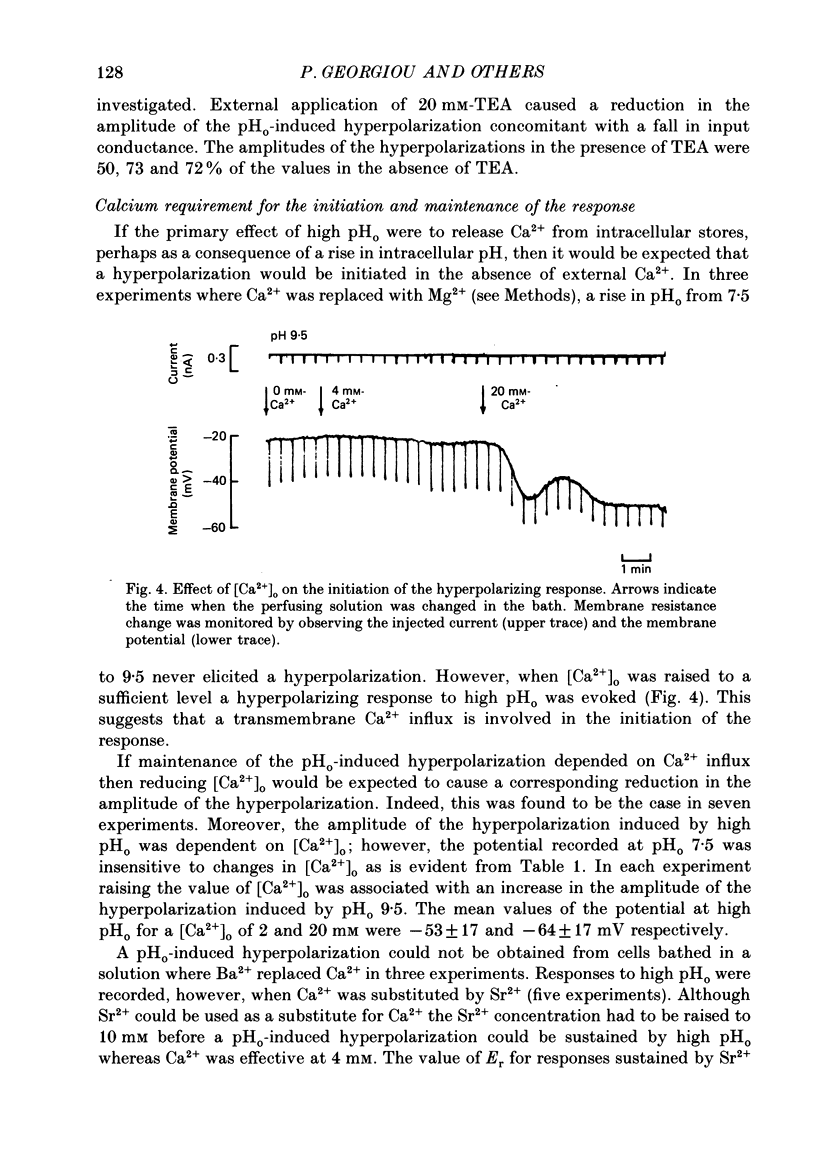
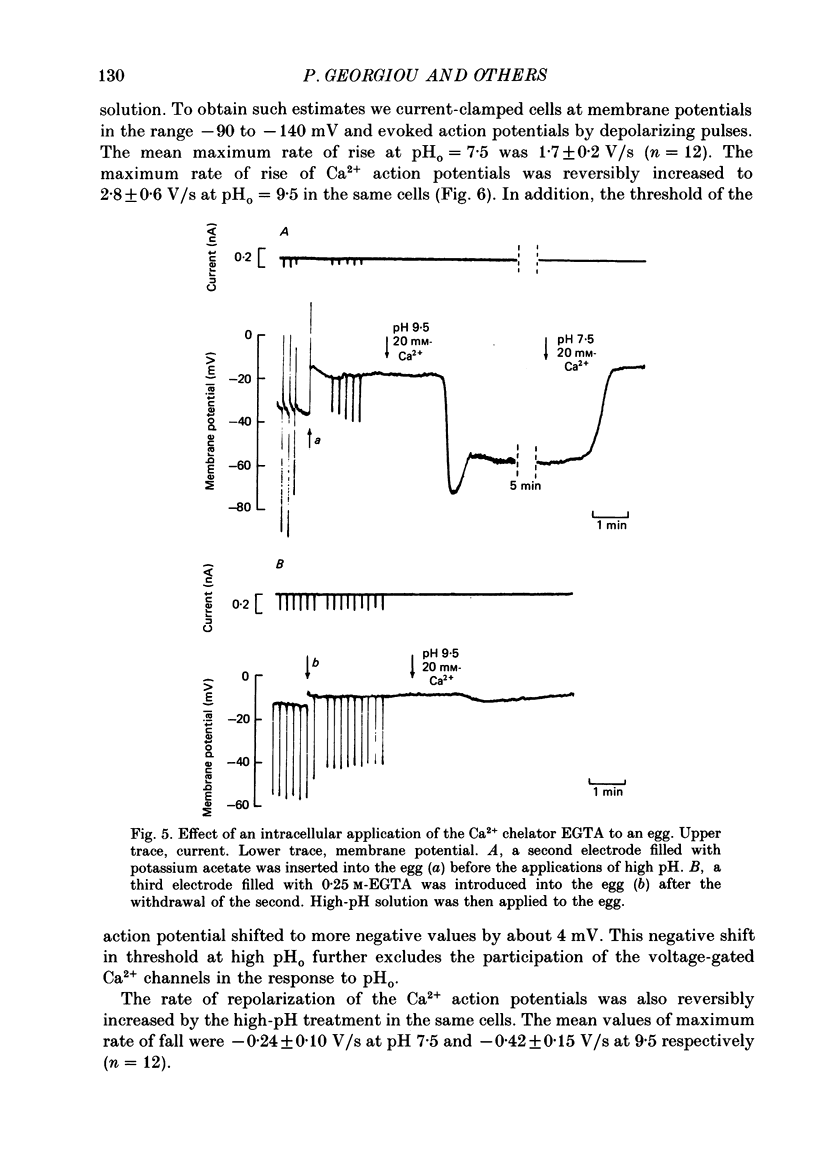
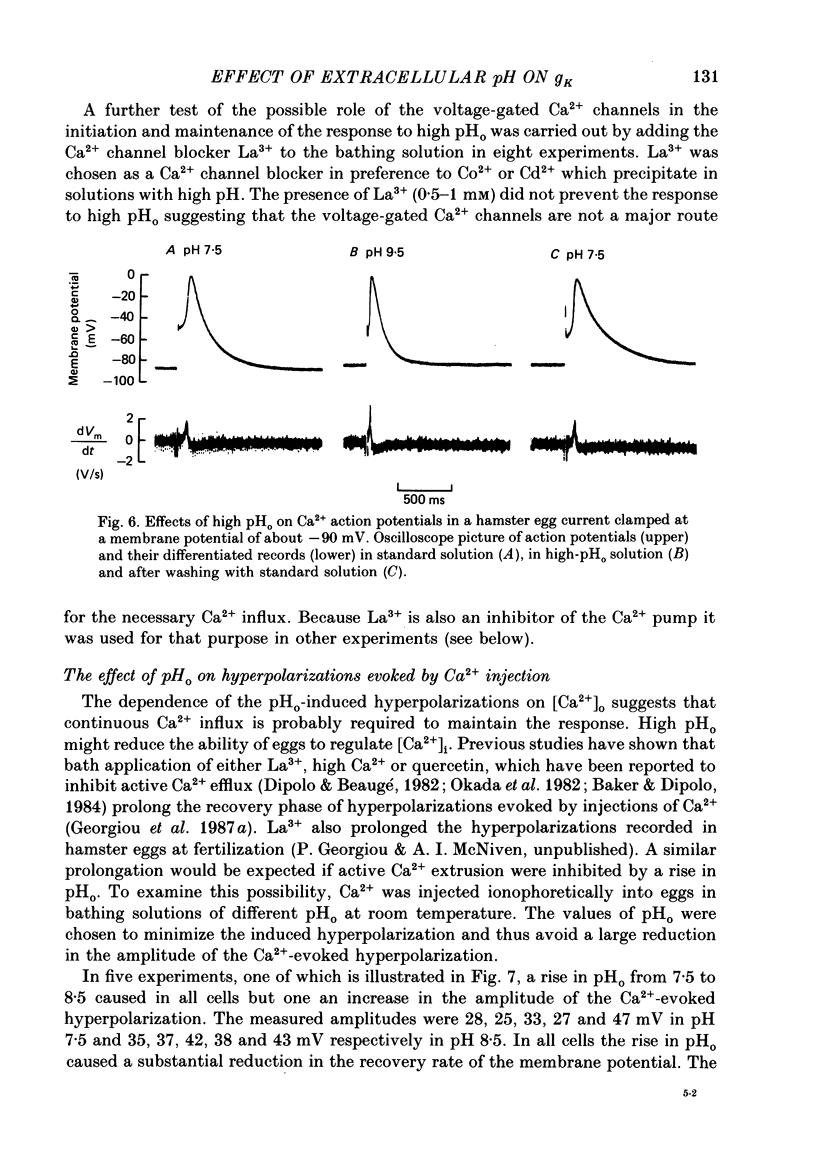
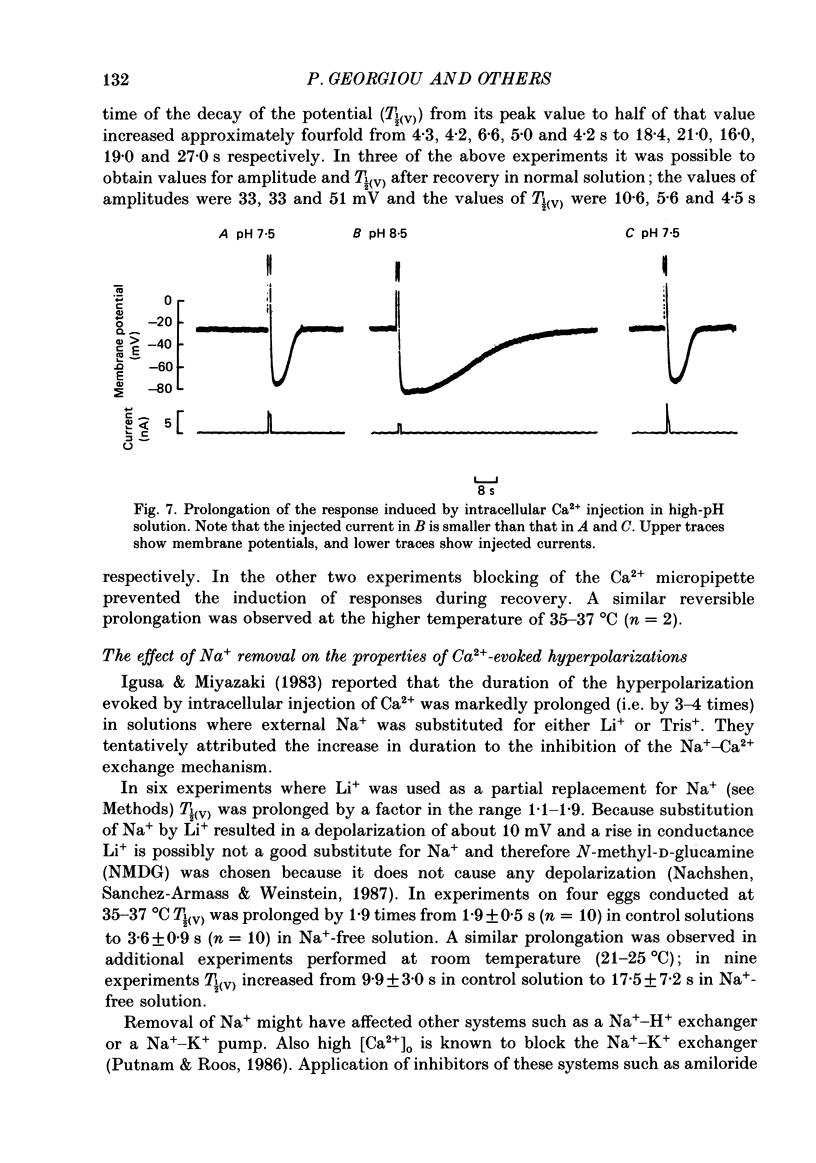
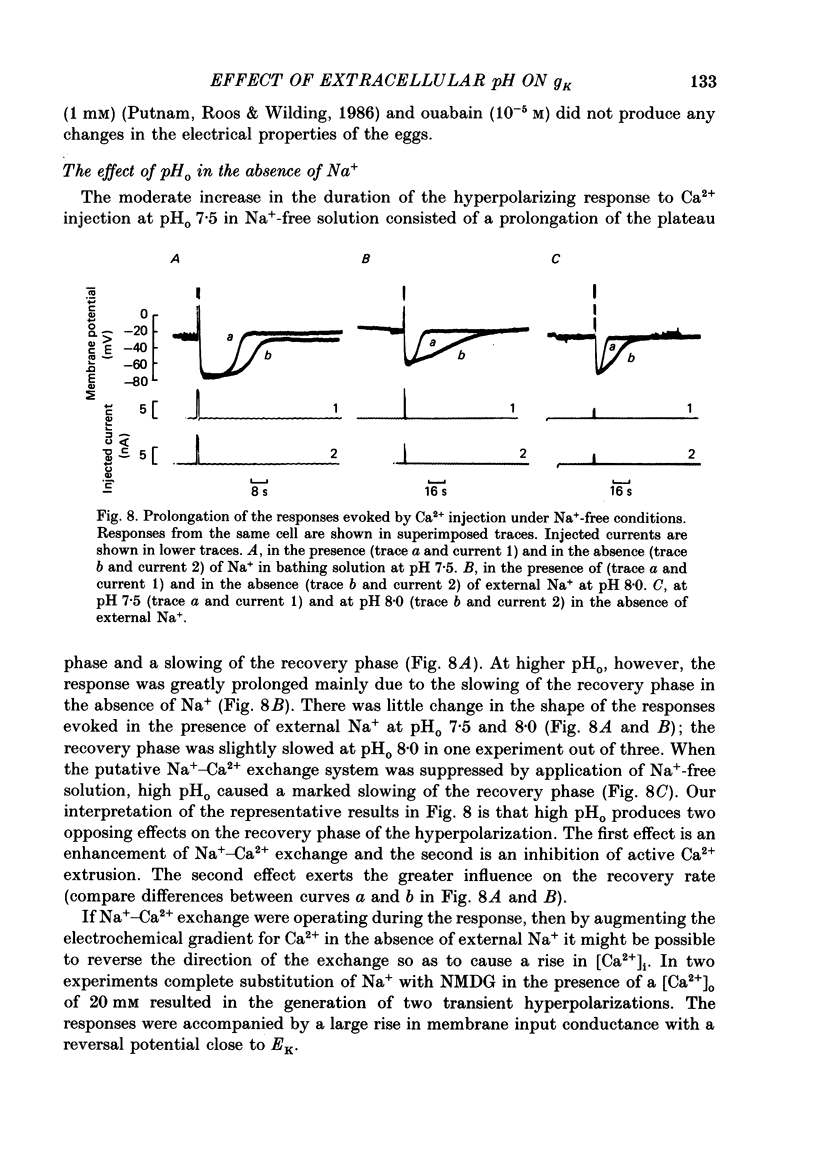
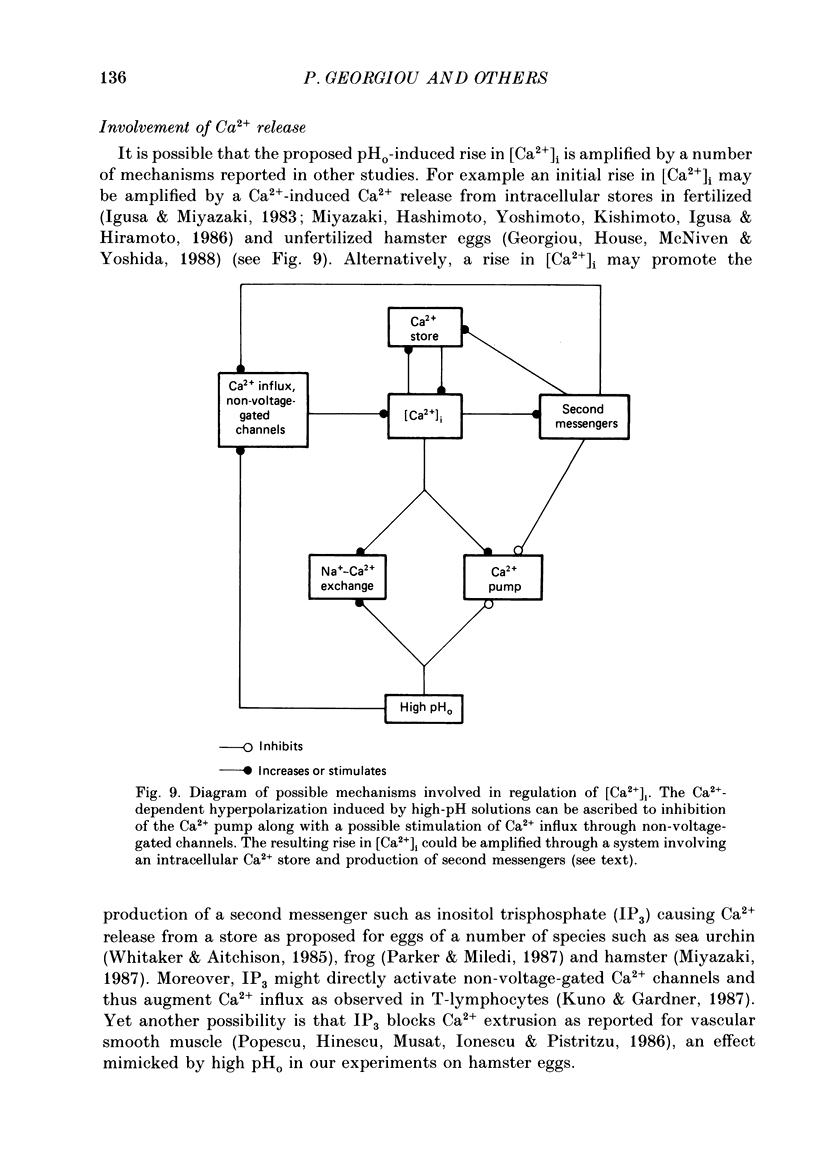
1. The effect of external pH (pHo) on the membrane potential and resistance of unfertilized zona-free hamster eggs was investigated by intracellular recording techniques. 2. A hyperpolarization of the hamster egg membrane was induced by raising the extracellular pH above 8.0. This hyperpolarization was accompanied by a rise in membrane conductance and was reversible by washing the egg. 3. The estimated value of the reversal potential of the hyperpolarizing response to a solution with pHo 9.5 was about -85 mV. The membrane potential changed linearly with log [K+]o with a slope of 43 +/- 2 mV (mean +/- S.D.; n = 4) for a 10-fold change in [K+]o, while it was unaltered by the removal of Cl- from the solution. 4. The amplitude of the pHo-induced hyperpolarization decreased substantially as [Ca2+]o was lowered from 20 to 1 mM. Sr2+ could substitute for Ca2+ in sustaining the response to high pHo, whereas Ba2+ or Mg2+ could not. 5. Injection of the Ca2+ chelator EGTA into the egg prevented the pHo-induced hyperpolarization suggesting that a rise in [Ca2+]i is required. 6. The rate of rise of Ca2+ action potentials was reversibly enhanced by raising pHo. However, influx through the voltage-gated Ca2+ channels is not involved in initiation and maintenance of the pHo-induced response, as responses were not affected by the Ca2+ channel blocker La3+. 7. The duration of the hyperpolarization evoked by intracellular Ca2+ injection in eggs bathed in normal solution or Na+-free solution was greatly prolonged by raising pHo. 8. It is suggested that a rise in external pH produces an increase in [Ca2+]i, activating a Ca2+-mediated K+ conductance which hyperpolarizes the egg membrane. 9. It is concluded that both a Na+-Ca2+ exchange system and a Ca2+ pump are responsible for Ca2+ extrusion and that inhibition of the Ca2+ pump by high pHo is the chief mechanism underlying the pH-induced hyperpolarization in hamster eggs. Although the Na+-Ca2+ exchange system is facilitated at high pHo, the effect of this facilitation of efflux is outweighed by the inhibition of the Ca2+ pump.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLANDAU R., JENSEN L., RUMERY R. Determination of the pH values of the reproductive-tract fluids of the rat during heat. Fertil Steril. 1958 May-Jun;9(3):207–214. doi: 10.1016/s0015-0282(16)33061-8. [DOI] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Campbell D. T., Hahin R. Altered sodium and gating current kinetics in frog skeletal muscle caused by low external pH. J Gen Physiol. 1984 Nov;84(5):771–788. doi: 10.1085/jgp.84.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., McNaughton P. A. The effects of phosphodiesterase inhibitors and lanthanum ions on the light-sensitive current of toad retinal rods. J Physiol. 1986 Jan;370:91–109. doi: 10.1113/jphysiol.1986.sp015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. The calcium pump and sodium-calcium exchange in squid axons. Annu Rev Physiol. 1983;45:313–324. doi: 10.1146/annurev.ph.45.030183.001525. [DOI] [PubMed] [Google Scholar]
- Dipolo R., Beaugé L. The effect of pH on Ca2+ extrusion mechanisms in dialyzed squid axons. Biochim Biophys Acta. 1982 May 21;688(1):237–245. doi: 10.1016/0005-2736(82)90599-5. [DOI] [PubMed] [Google Scholar]
- Georgiou P., Bountra C., Bland K. P., House C. R. Calcium-evoked opening of potassium channels in hamster eggs. Q J Exp Physiol. 1983 Oct;68(4):687–700. doi: 10.1113/expphysiol.1983.sp002758. [DOI] [PubMed] [Google Scholar]
- Georgiou P., Bountra C., McNiven A., House C. R. The effect of lanthanum, quercetin and dinitrophenol on calcium-evoked electrical responses in hamster eggs. Q J Exp Physiol. 1987 Apr;72(2):227–241. doi: 10.1113/expphysiol.1987.sp003066. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., House C. R., Silinsky E. M. Conductance changes associated with the secretory potential in the cockroach salivary gland. J Physiol. 1974 Feb;236(3):723–731. doi: 10.1113/jphysiol.1974.sp010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. The effect of ions on sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:371–398. doi: 10.1113/jphysiol.1987.sp016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y., Miyazaki S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol. 1983 Jul;340:611–632. doi: 10.1113/jphysiol.1983.sp014783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y., Miyazaki S. Periodic increase of cytoplasmic free calcium in fertilized hamster eggs measured with calcium-sensitive electrodes. J Physiol. 1986 Aug;377:193–205. doi: 10.1113/jphysiol.1986.sp016181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H., Toyoda Y., Chang M. C. Effect of hydrogen-ion concentration on in vitro fertilization of mouse, golden hamster, and rat eggs. Biol Reprod. 1974 May;10(4):487–493. doi: 10.1095/biolreprod10.4.487. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Hashimoto N., Yoshimoto Y., Kishimoto T., Igusa Y., Hiramoto Y. Temporal and spatial dynamics of the periodic increase in intracellular free calcium at fertilization of golden hamster eggs. Dev Biol. 1986 Nov;118(1):259–267. doi: 10.1016/0012-1606(86)90093-x. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Igusa Y. Ca-mediated activation of a K current at fertilization of golden hamster eggs. Proc Natl Acad Sci U S A. 1982 Feb;79(3):931–935. doi: 10.1073/pnas.79.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Igusa Y. Fertilization potential in golden hamster eggs consists of recurring hyperpolarizations. Nature. 1981 Apr 23;290(5808):702–704. doi: 10.1038/290702a0. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Sanchez-Armass S., Weinstein A. M. The regulation of cytosolic calcium in rat brain synaptosomes by sodium-dependent calcium efflux. J Physiol. 1986 Dec;381:17–28. doi: 10.1113/jphysiol.1986.sp016309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Tsuchiya W., Yada T. Calcium channel and calcium pump involved in oscillatory hyperpolarizing responses of L-strain mouse fibroblasts. J Physiol. 1982 Jun;327:449–461. doi: 10.1113/jphysiol.1982.sp014242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yamashita N. Ionic currents through the membrane of the mammalian oocyte and their comparison with those in the tunicate and sea urchin. J Physiol. 1977 May;267(2):465–495. doi: 10.1113/jphysiol.1977.sp011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Miledi R. Injection of inositol 1,3,4,5-tetrakisphosphate into Xenopus oocytes generates a chloride current dependent upon intracellular calcium. Proc R Soc Lond B Biol Sci. 1987 Oct 22;232(1266):59–70. doi: 10.1098/rspb.1987.0061. [DOI] [PubMed] [Google Scholar]
- Popescu L. M., Hinescu M. E., Musat S., Ionescu M., Pistritzu F. Inositol trisphosphate and the contraction of vascular smooth muscle cells. Eur J Pharmacol. 1986 Apr 9;123(1):167–169. doi: 10.1016/0014-2999(86)90701-6. [DOI] [PubMed] [Google Scholar]
- Putnam R. W., Roos A. Effect of calcium and other divalent cations on intracellular pH regulation of frog skeletal muscle. J Physiol. 1986 Dec;381:221–239. doi: 10.1113/jphysiol.1986.sp016324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam R. W., Roos A., Wilding T. J. Properties of the intracellular pH-regulating systems of frog skeletal muscle. J Physiol. 1986 Dec;381:205–219. doi: 10.1113/jphysiol.1986.sp016323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Szász I., Gerlóczy A., Gárdos G. Transport parameters and stoichiometry of active calcium ion extrusion in intact human red cells. Biochim Biophys Acta. 1977 Jan 4;464(1):93–107. doi: 10.1016/0005-2736(77)90373-x. [DOI] [PubMed] [Google Scholar]
- Sharp A. P., Thomas R. C. The effects of chloride substitution on intracellular pH in crab muscle. J Physiol. 1981 Mar;312:71–80. doi: 10.1113/jphysiol.1981.sp013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. S., Steinhardt R. A. Intracellular pH and the sodium requirement at fertilisation. Nature. 1979 Nov 1;282(5734):87–89. doi: 10.1038/282087a0. [DOI] [PubMed] [Google Scholar]
- VISHWAKARMA P. The pH and bicarbonate-ion content of the oviduct and uterine fluids. Fertil Steril. 1962 Sep-Oct;13:481–485. doi: 10.1016/s0015-0282(16)34633-7. [DOI] [PubMed] [Google Scholar]
- Webb D. J., Nuccitelli R. Direct measurement of intracellular pH changes in Xenopus eggs at fertilization and cleavage. J Cell Biol. 1981 Nov;91(2 Pt 1):562–567. doi: 10.1083/jcb.91.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M., Aitchison M. Calcium-dependent polyphosphoinositide hydrolysis is associated with exocytosis in vitro. FEBS Lett. 1985 Mar 11;182(1):119–124. doi: 10.1016/0014-5793(85)81167-4. [DOI] [PubMed] [Google Scholar]



