Abstract
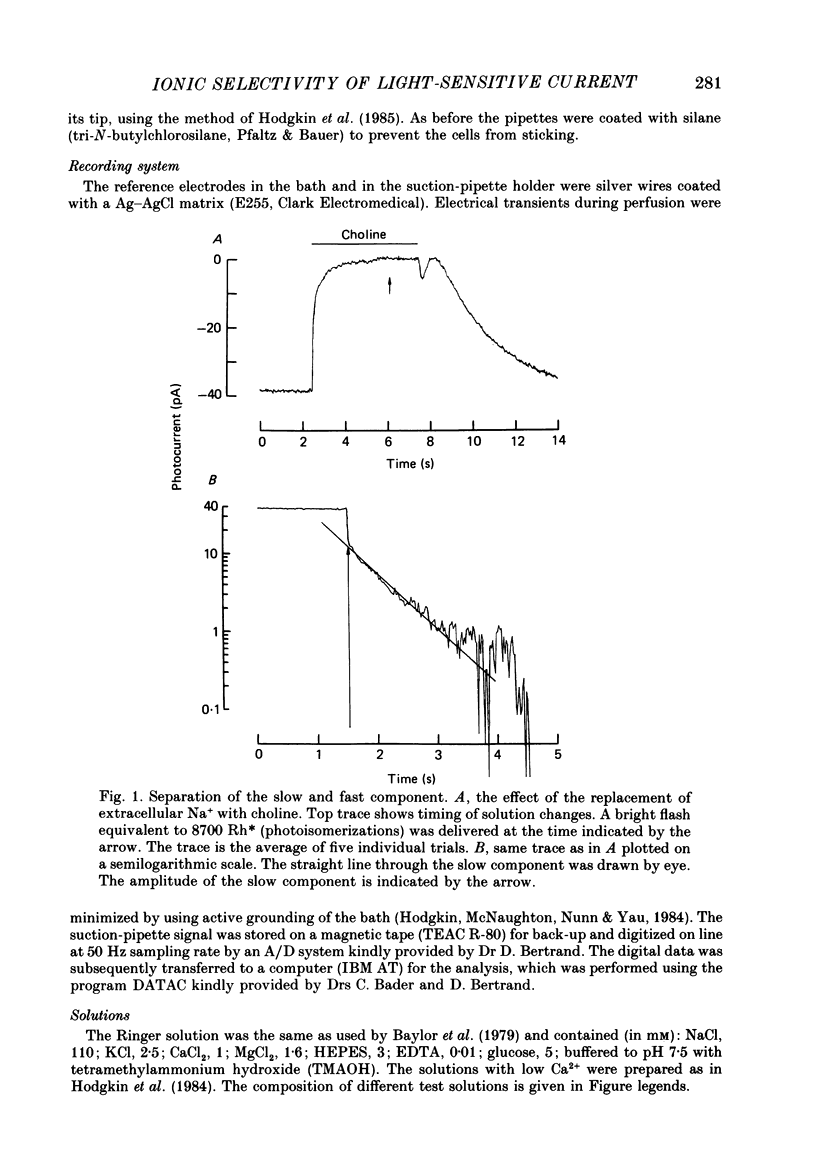
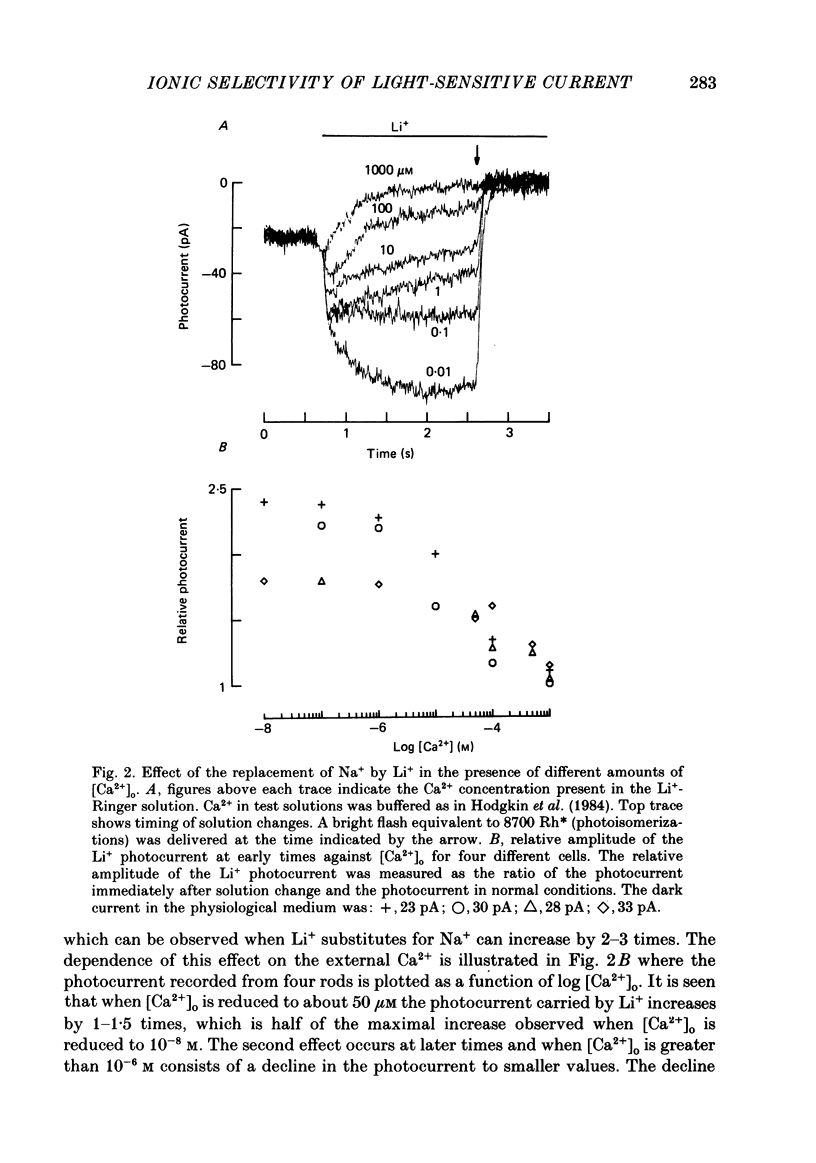
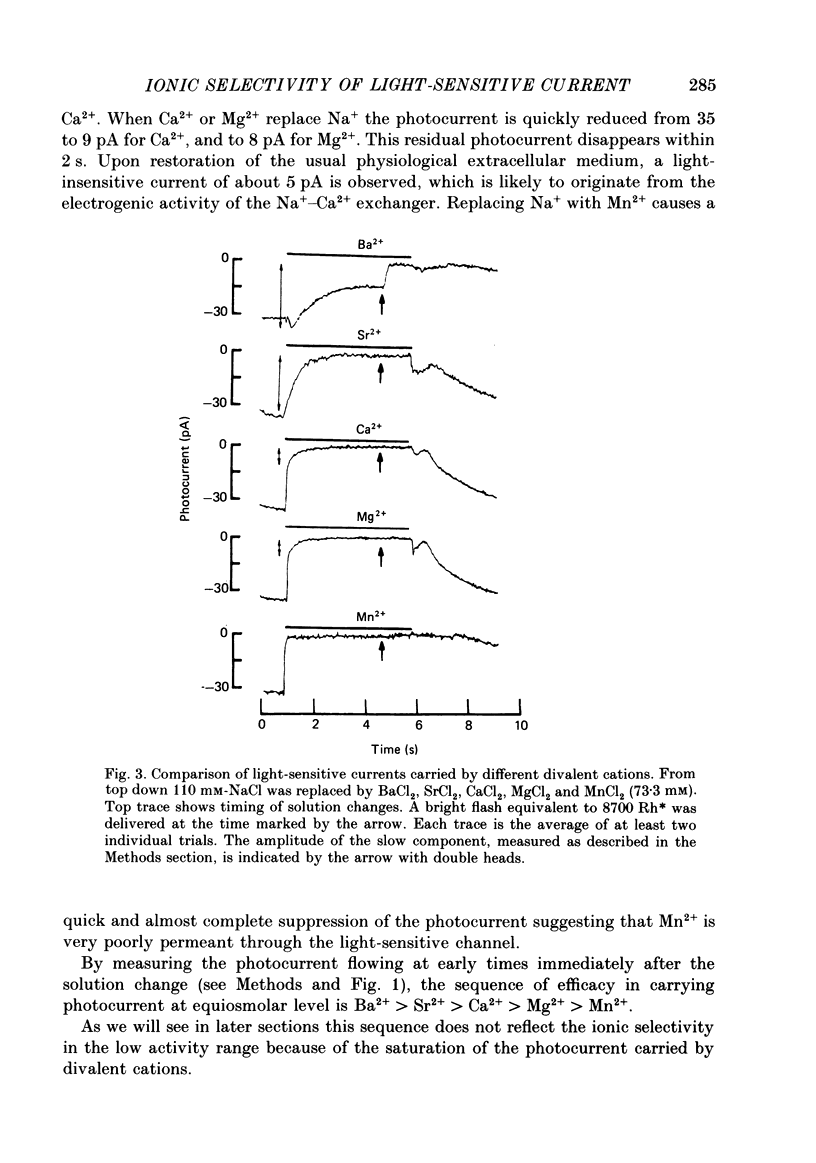
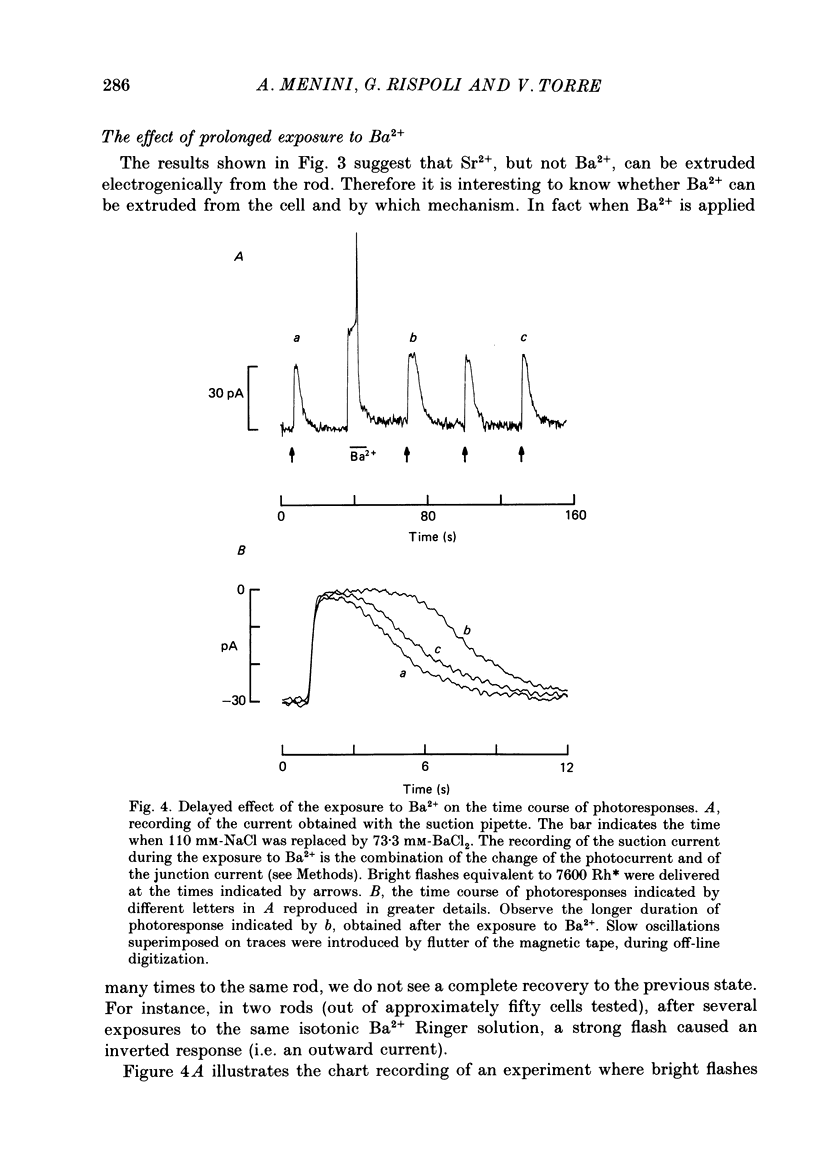
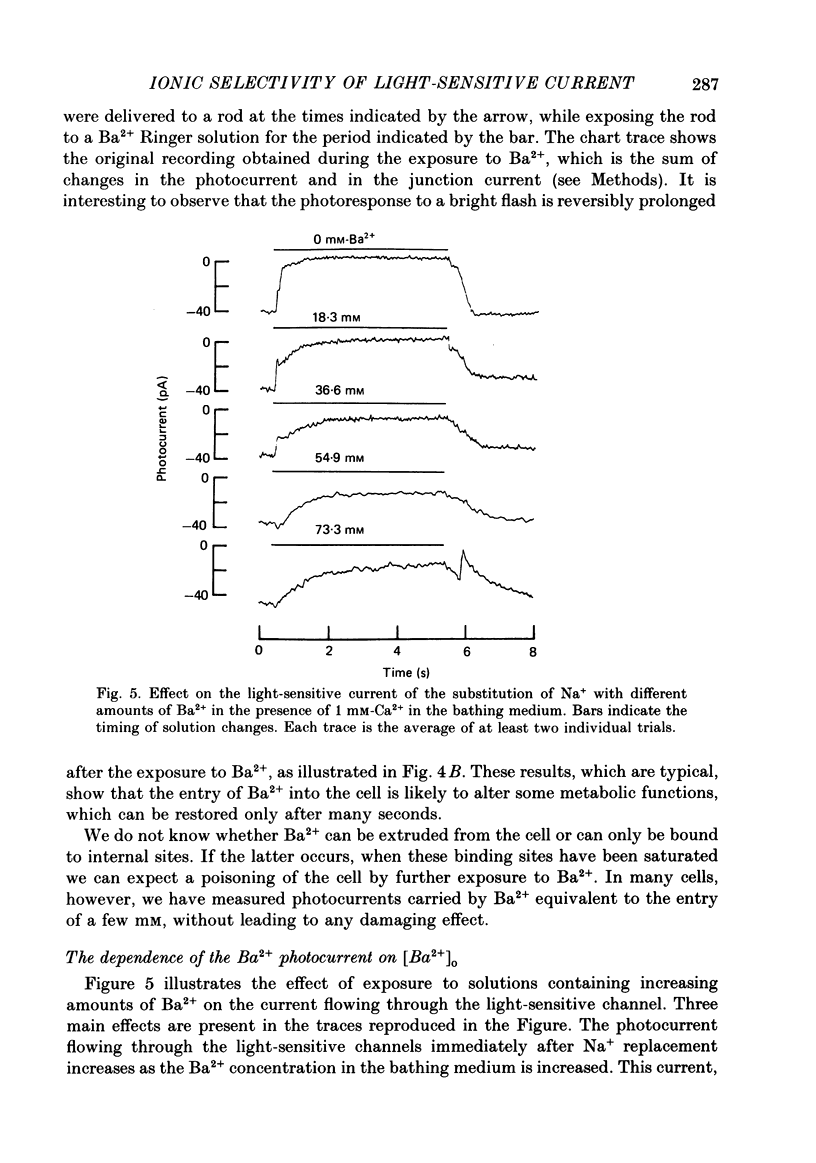
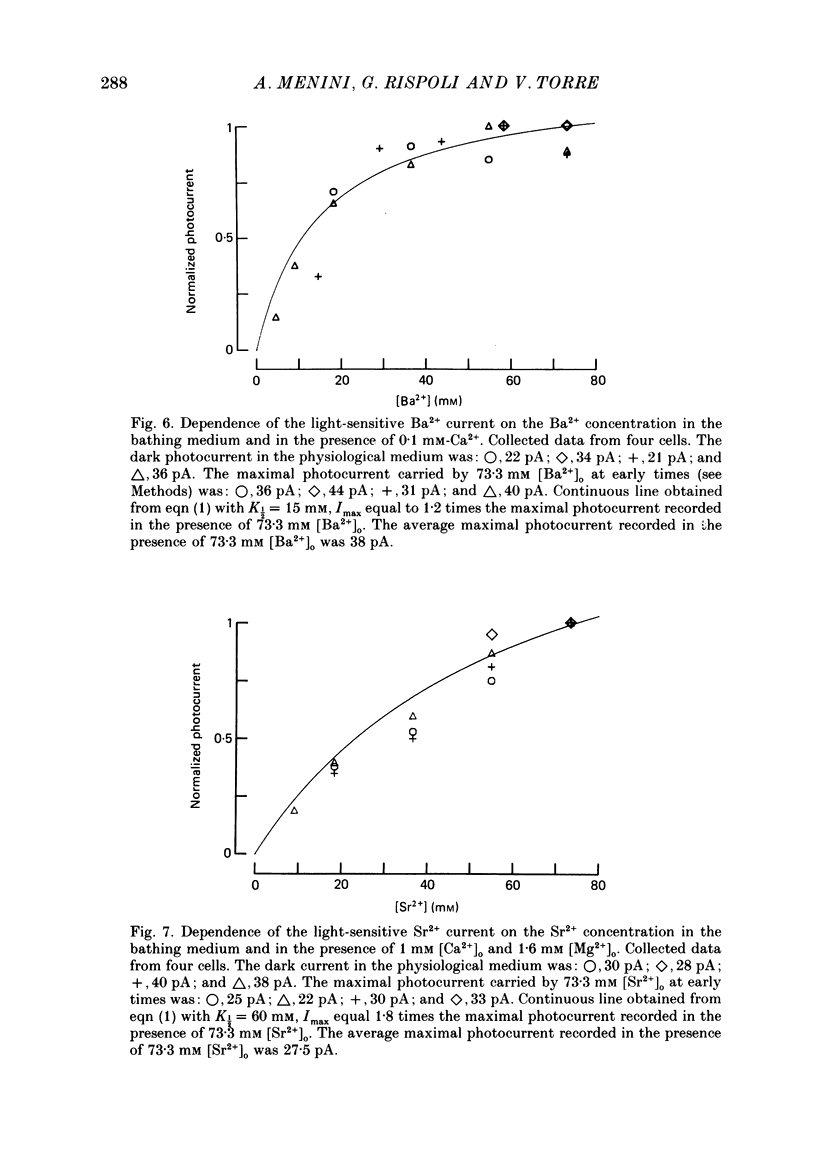
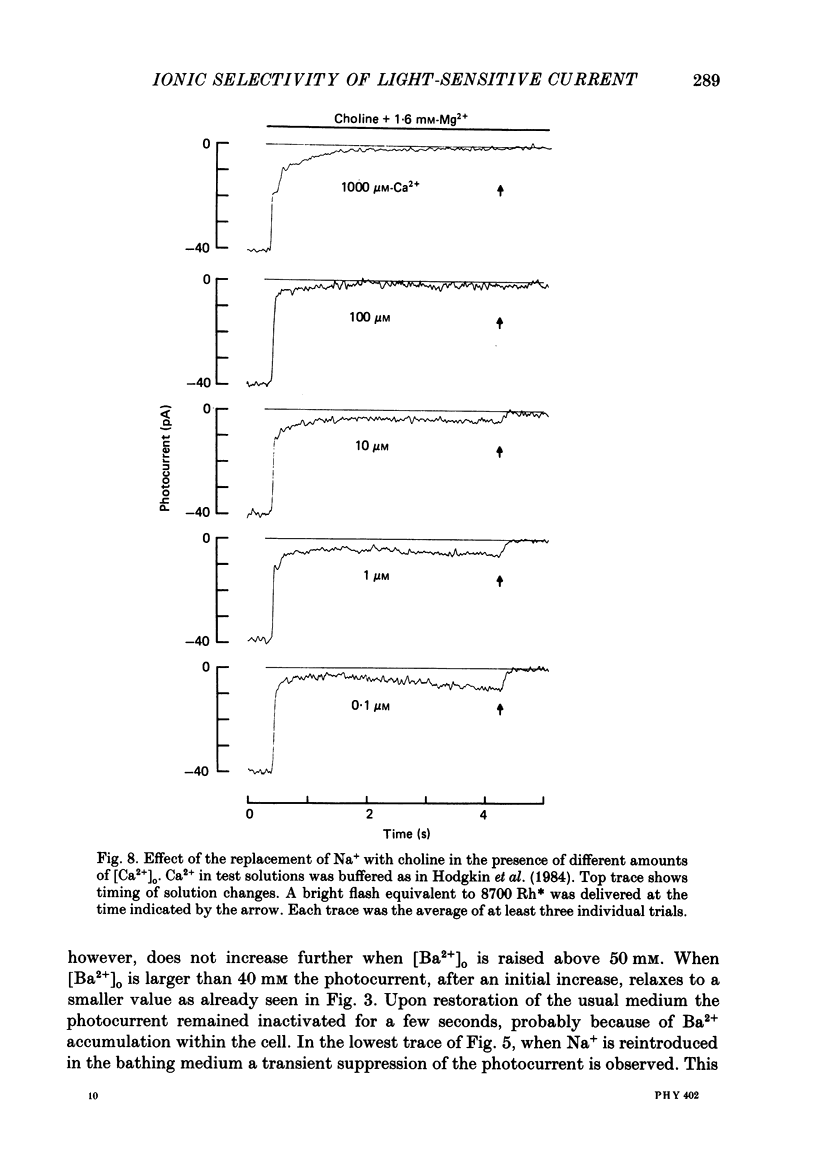
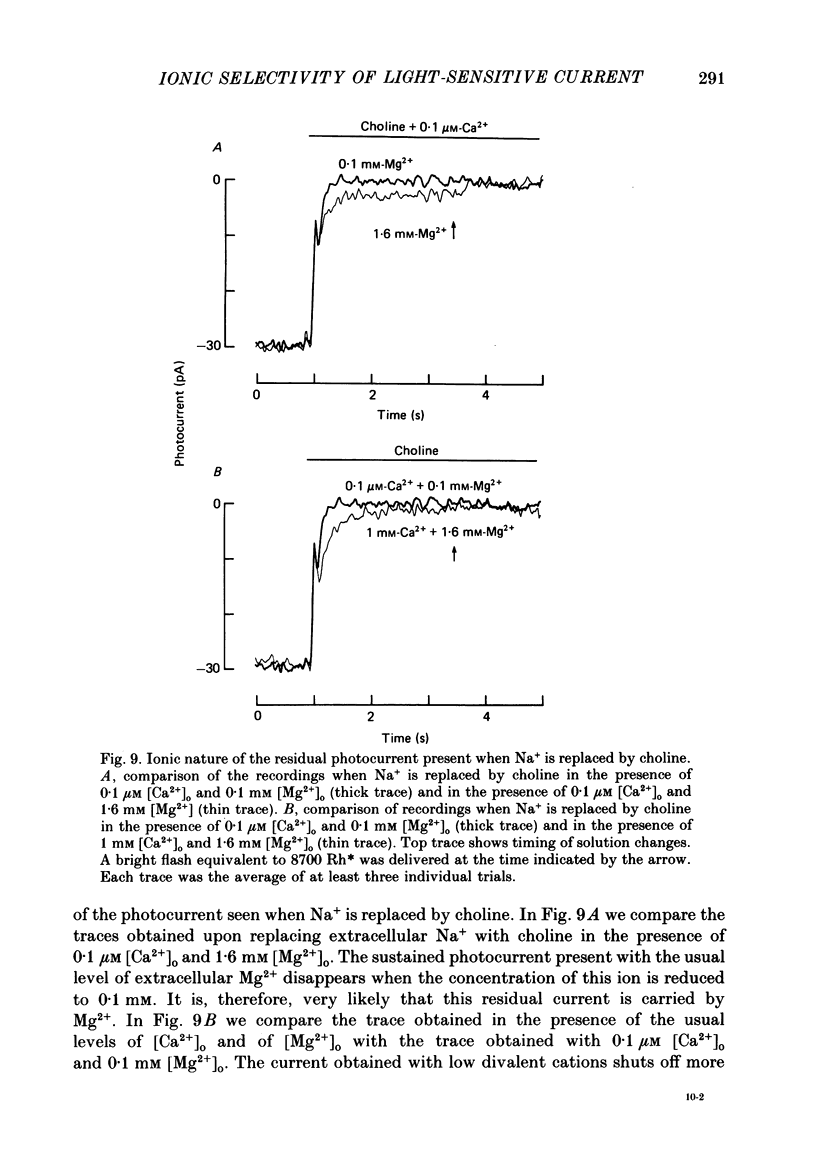
1. Using the method of Hodgkin, McNaughton & Nunn (1985) for rapidly changing the extracellular medium, we analysed the effect of divalent cations on the photocurrent of isolated retinal rods of the tiger salamander. 2. When the extracellular NaCl was replaced by equiosmolar amounts of BaCl2, SrCl2, CaCl2, MgCl2 and MnCl2 the efficacy in carrying the photocurrent at early times was Ba2+ greater than Sr2+ greater than Ca2+ greater than Mg2+ greater than Mn2+. At early times Ba2+ could carry a photocurrent similar to or larger than that carried by Na+. 3. The photocurrent carried by Ba2+ increased by about 50% when [Ca2+]o was reduced from 1 to 0.1 mM. In the presence of 0.1 mM-Ca2+ in the extracellular medium the photocurrent carried by Ba2+ saturated when [Ba2+]o was close to 50 mM and was half-activated at 15 mM [Ba2+]o. 4. The photocurrent which can be carried by Sr2+ is not larger than that carried by Ba2+ and does not saturate for [Sr2+]o up to 70 mM. 5. When extracellular Na+ is replaced by the impermeant organic ion choline it is possible to observe a transient photocurrent which is carried by Ca2+. This current has a maximal value of about 11 pA and has a half-activation constant of about 50 microM. 6. Movements of Mg2+ across the light-sensitive channel can be seen only when extracellular Ca2+ is reduced below 10 microM. Under these conditions the maximal photocurrent which can be carried by Mg2+ at early times is about 8 pA and has a half-activation of about 2 mM. Under normal conditions Mn2+ is hardly permeable through the light-sensitive channel. 7. It is concluded that the selectivity of the light-sensitive channel in the low ionic concentration range is Ca2+ greater than Sr2+ greater than Ba2+ greater than Mg2+ greater than Na+.
Full text
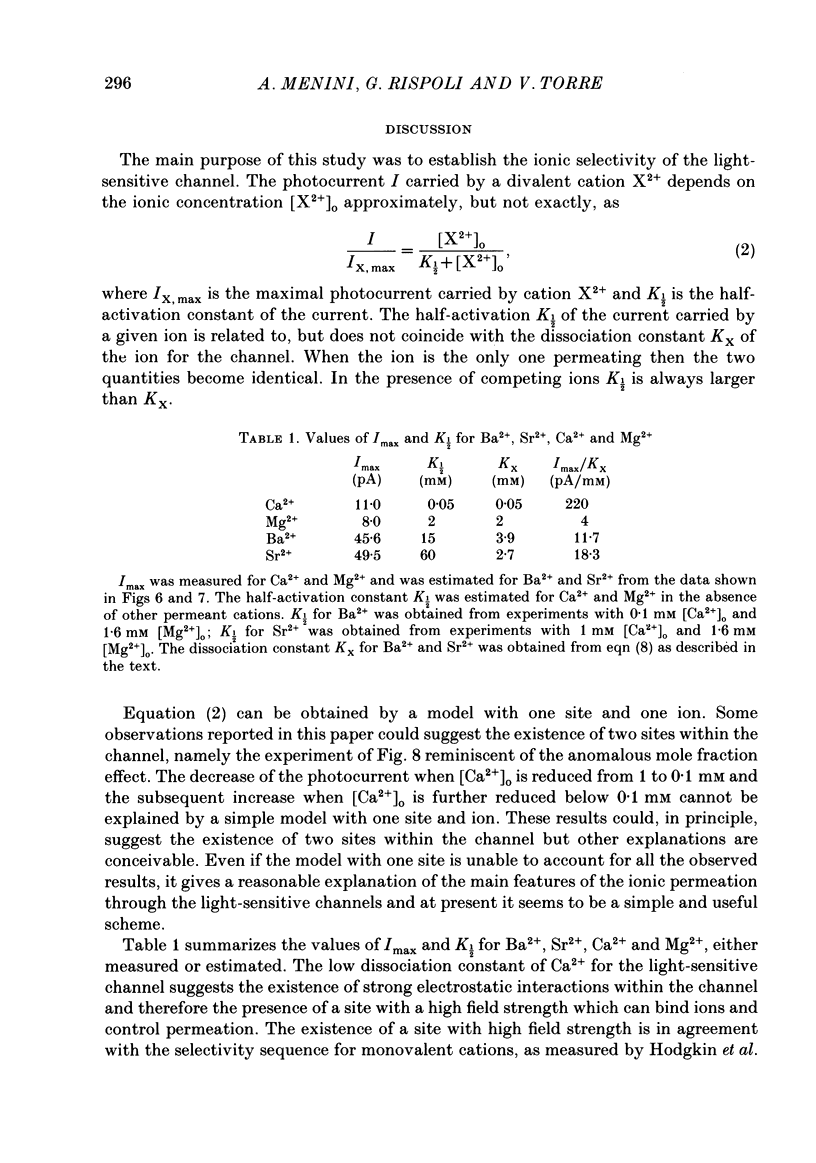
PDF









Selected References
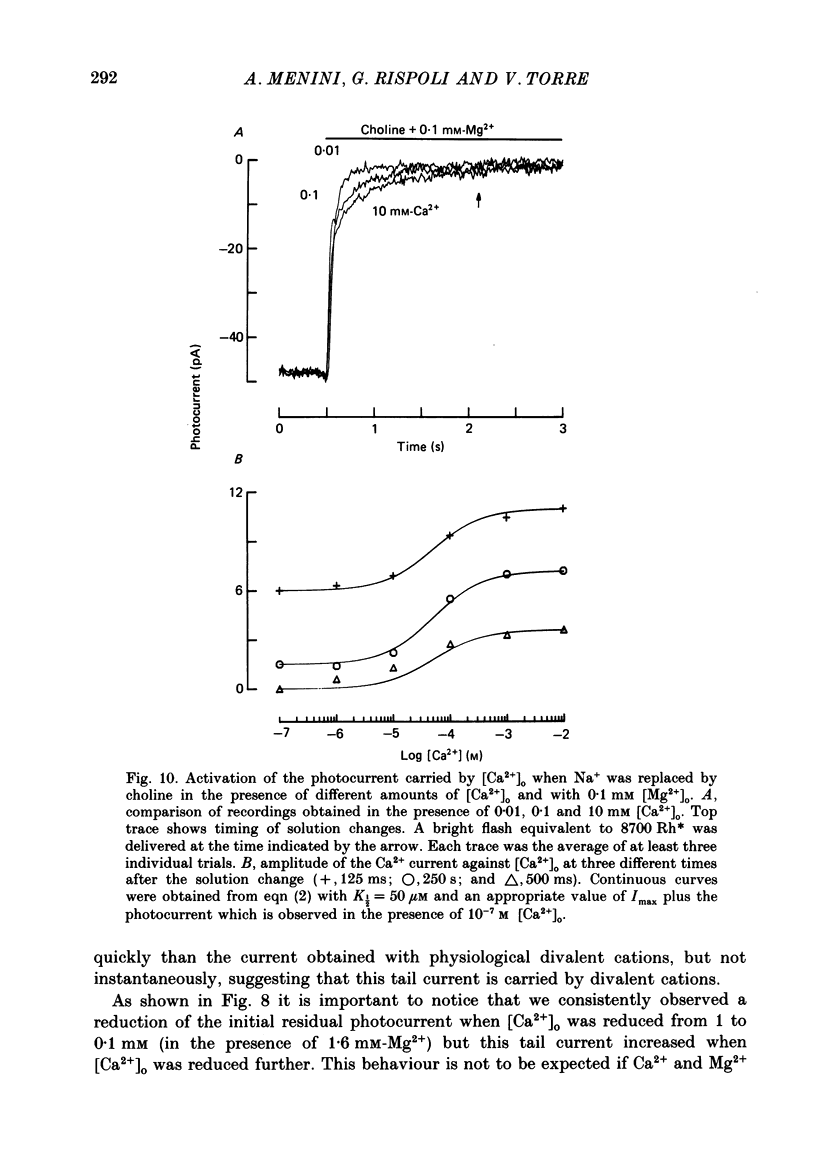
These references are in PubMed. This may not be the complete list of references from this article.
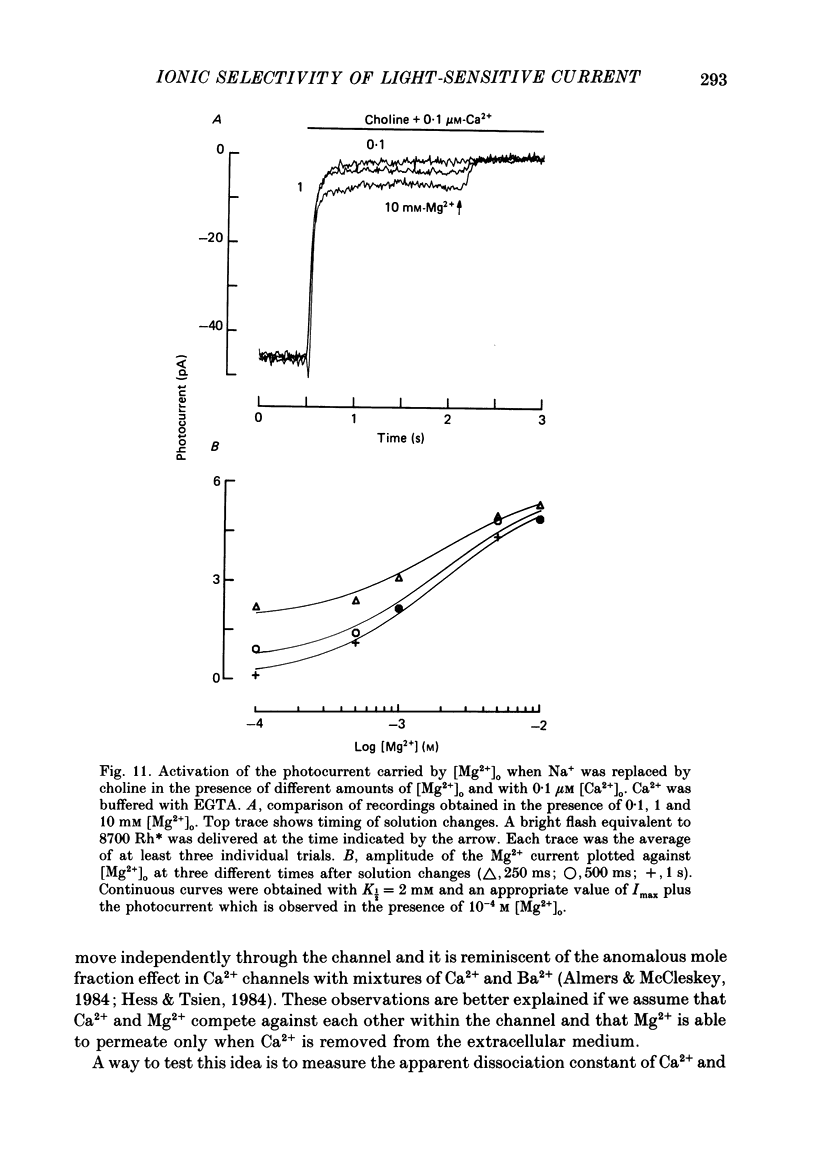
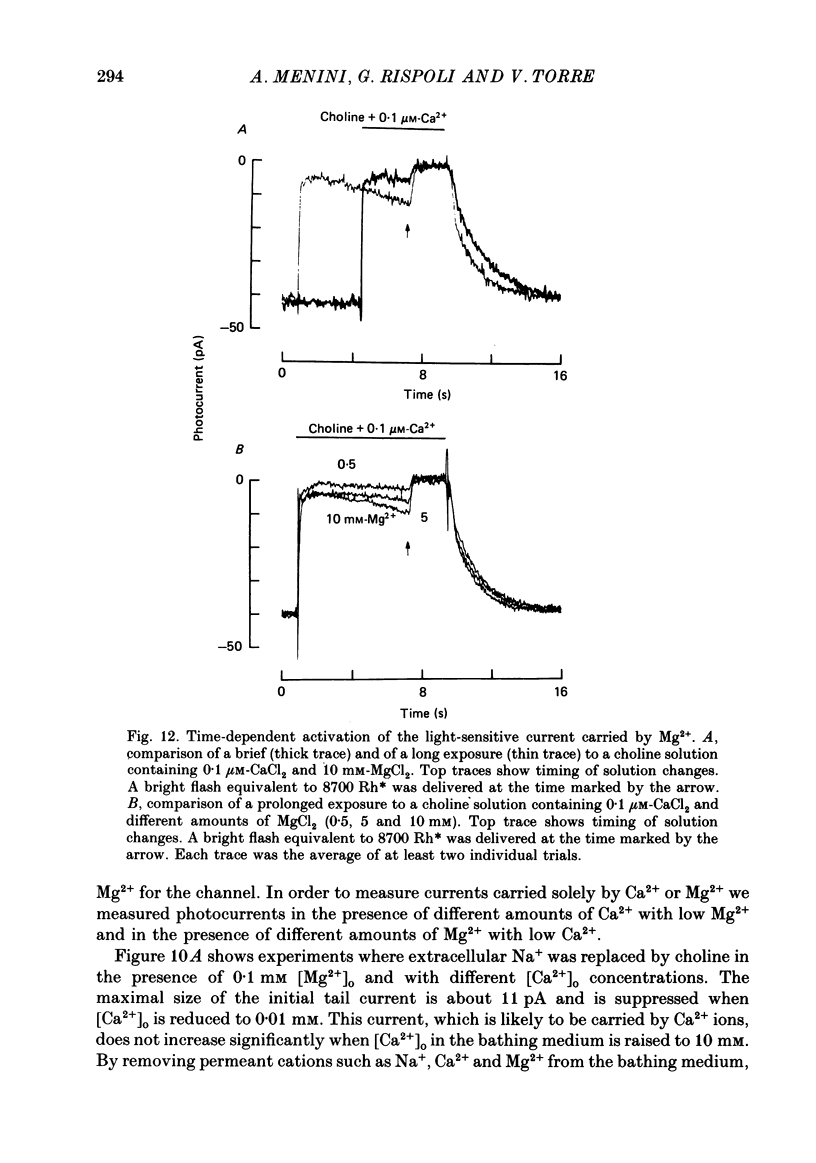
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Nunn B. J. Location and function of voltage-sensitive conductances in retinal rods of the salamander, Ambystoma tigrinum. J Physiol. 1984 Sep;354:203–223. doi: 10.1113/jphysiol.1984.sp015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Caretta A., Cervetto L., Torre V. Ionic movements through light-sensitive channels of toad rods. J Physiol. 1983 Oct;343:295–310. doi: 10.1113/jphysiol.1983.sp014893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Pasino E., Torre V. The sodium current underlying the responses of toad rods to light. J Physiol. 1981 Aug;317:223–242. doi: 10.1113/jphysiol.1981.sp013822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A., Cavaggioni A. Fast ionic flux activated by cyclic GMP in the membrane of cattle rod outer segments. Eur J Biochem. 1983 Apr 15;132(1):1–8. doi: 10.1111/j.1432-1033.1983.tb07317.x. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. Measurement of sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. The effect of ions on sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:371–398. doi: 10.1113/jphysiol.1987.sp016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R., Torre V. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J Physiol. 1986 Mar;372:315–349. doi: 10.1113/jphysiol.1986.sp016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolley R. N., Racz E. Calcium modulation of cyclic GMP synthesis in rat visual cells. Vision Res. 1982;22(12):1481–1486. doi: 10.1016/0042-6989(82)90213-9. [DOI] [PubMed] [Google Scholar]
- Matthews H. R., Torre V., Lamb T. D. Effects on the photoresponse of calcium buffers and cyclic GMP incorporated into the cytoplasm of retinal rods. Nature. 1985 Feb 14;313(6003):582–585. doi: 10.1038/313582a0. [DOI] [PubMed] [Google Scholar]
- Owen W. G. Ionic conductances in rod photoreceptors. Annu Rev Physiol. 1987;49:743–764. doi: 10.1146/annurev.ph.49.030187.003523. [DOI] [PubMed] [Google Scholar]
- Pepe I. M., Panfoli I., Cugnoli C. Guanylate cyclase in rod outer segments of the toad retina. Effect of light and Ca2+. FEBS Lett. 1986 Jul 14;203(1):73–76. doi: 10.1016/0014-5793(86)81439-9. [DOI] [PubMed] [Google Scholar]
- Robinson P. R., Kawamura S., Abramson B., Bownds M. D. Control of the cyclic GMP phosphodiesterase of frog photoreceptor membranes. J Gen Physiol. 1980 Nov;76(5):631–645. doi: 10.1085/jgp.76.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Torre V., Matthews H. R., Lamb T. D. Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7109–7113. doi: 10.1073/pnas.83.18.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V., Pasino E., Capovilla M., Cervetto L. Rod photoresponses in the absence of external sodium in retinae treated with phosphodiesterase inhibitors. Exp Brain Res. 1981;44(4):427–430. doi: 10.1007/BF00238835. [DOI] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984 May 24;309(5966):352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]


