Abstract
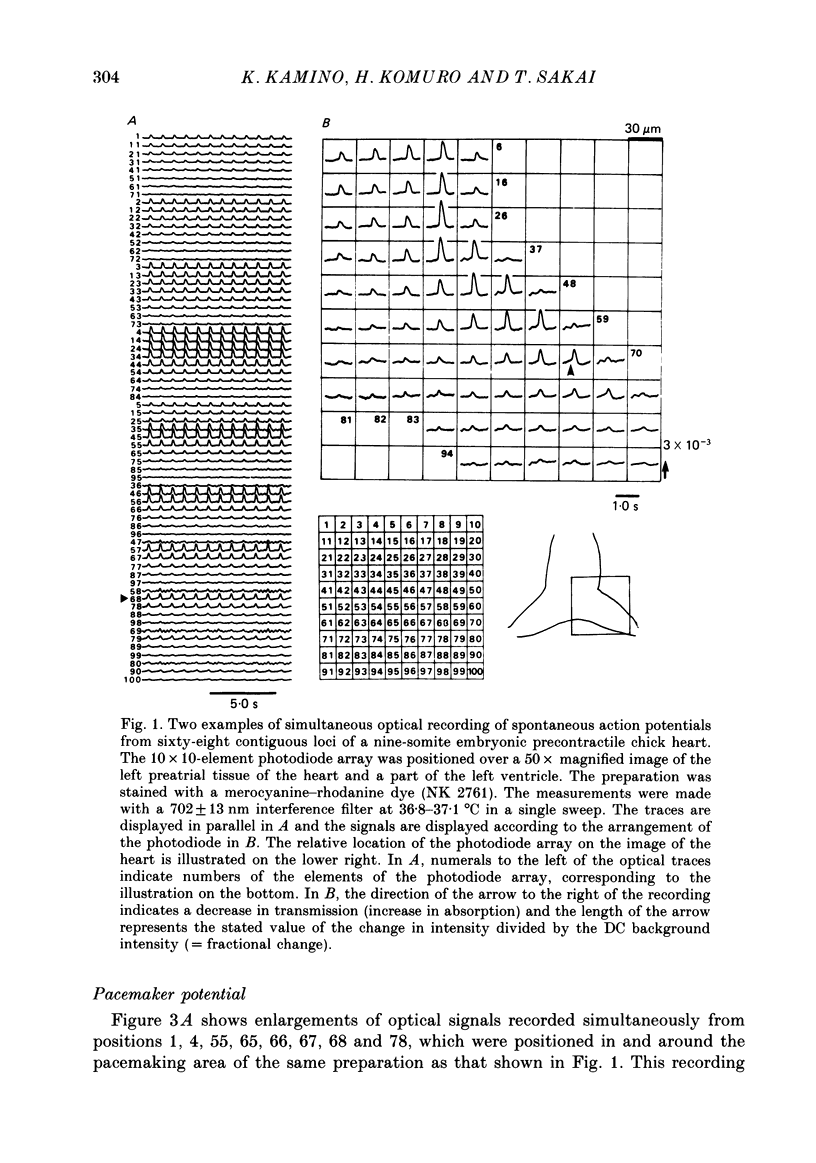
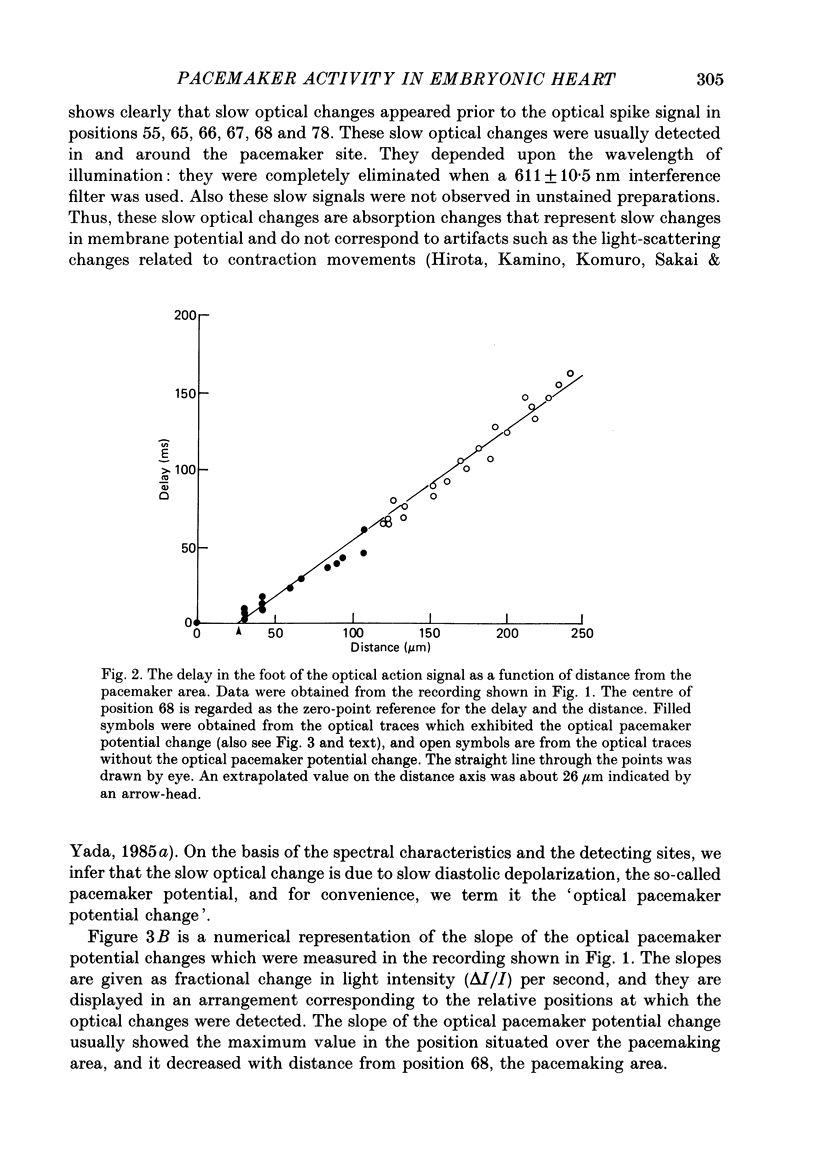
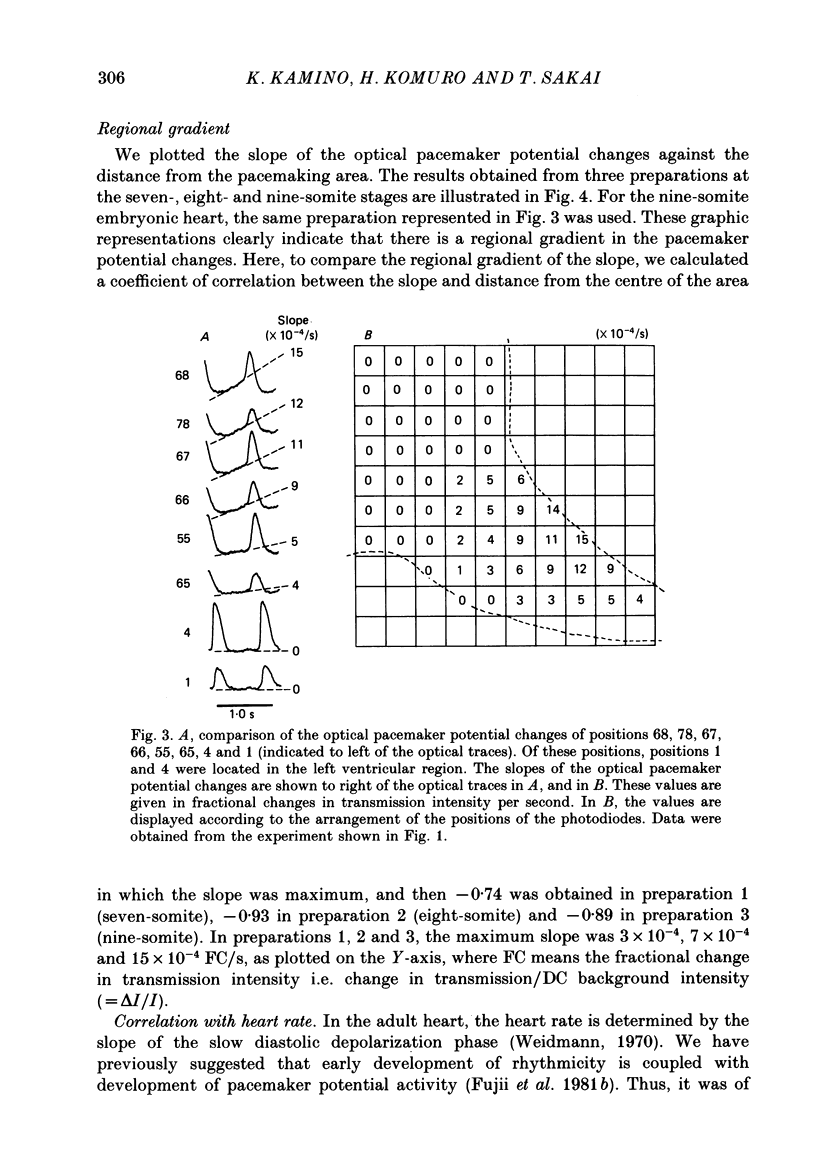
1. Regional gradient of pacemaker activity in the early embryonic precontractile chick heart was quantitatively assessed by means of simultaneous multiple-site optical recordings of changes in membrane potential, using a measuring system with a 10 X 10-element photodiode array which had a spatial resolution of 30 microns. 2. Absorption changes related to spontaneous electrical activity were recorded simultaneously from many contiguous regions in the area in which the pacemaker site was located in seven- to nine-somite embryonic hearts stained with a voltage-sensitive merocyanine-rhodanine dye (NK 2761). 3. The absorption changes related to slow diastolic depolarization were detected, and they were concentrated in and near the pacemaking area. The area in which the absorption changes related to slow diastolic depolarization were detected increased in size as development proceeded. 4. The slope of the absorption change related to diastolic depolarization was measured as an indicator of the pacemaker activity. It was largest in the pacemaking area, and gradually decreased towards the periphery. 5. The maximum slope of the optical change related to slow diastolic depolarization also increased as development proceeded and was related to early development of the heart rate. Thus, these results suggest that formation of a regional gradient of pacemaker activity results in the functional architecture of the pacemaking area in the early phases of cardiogenesis.
Full text
PDF


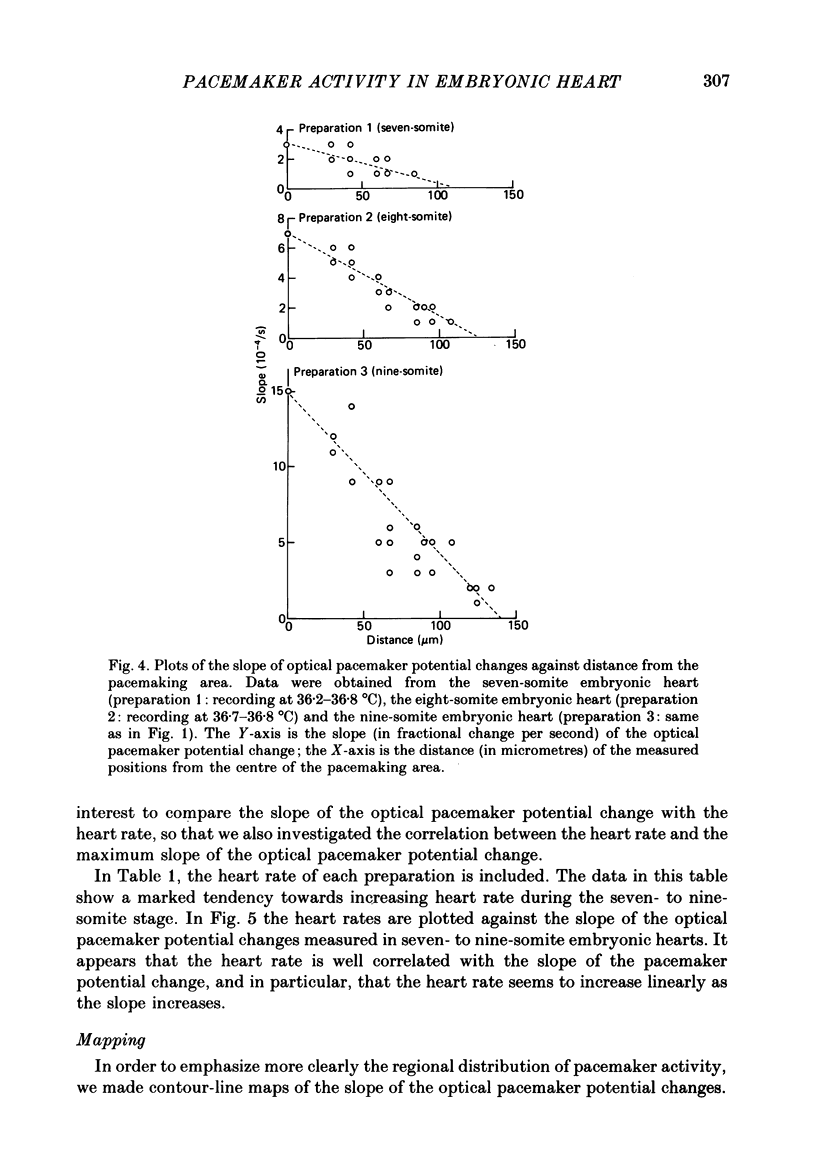
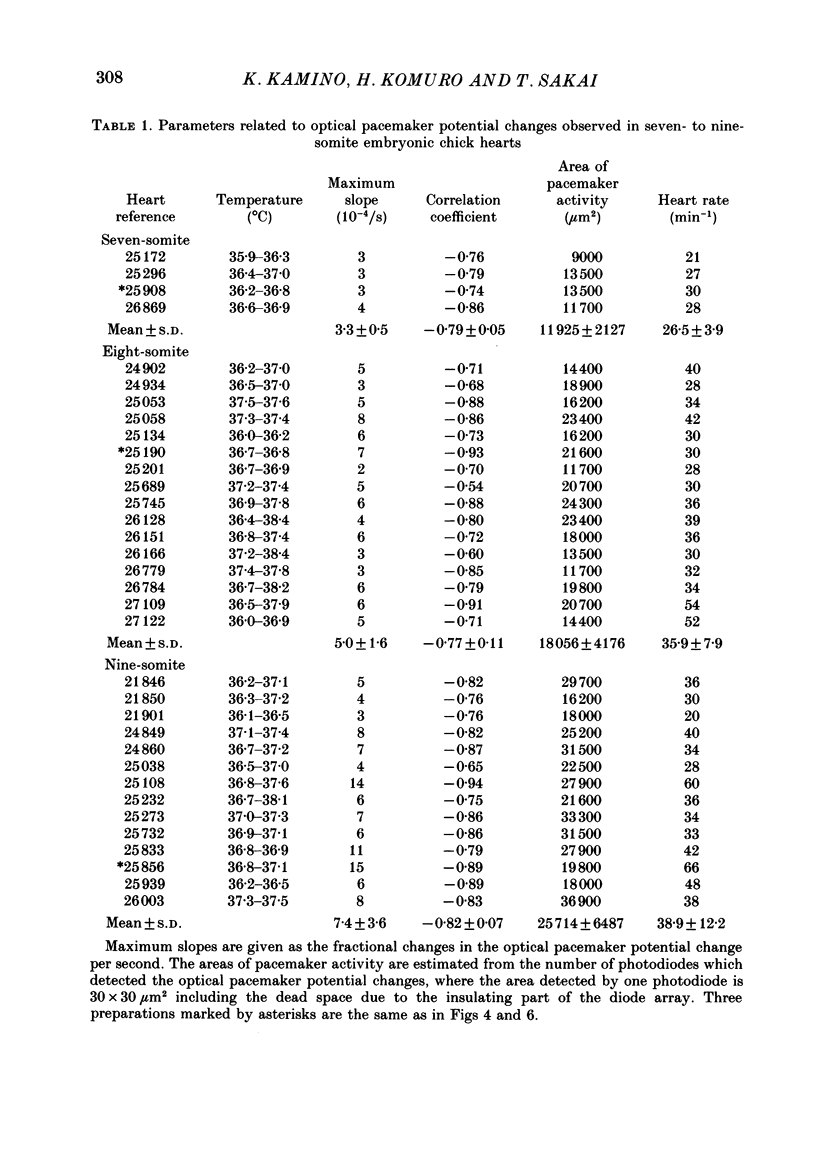
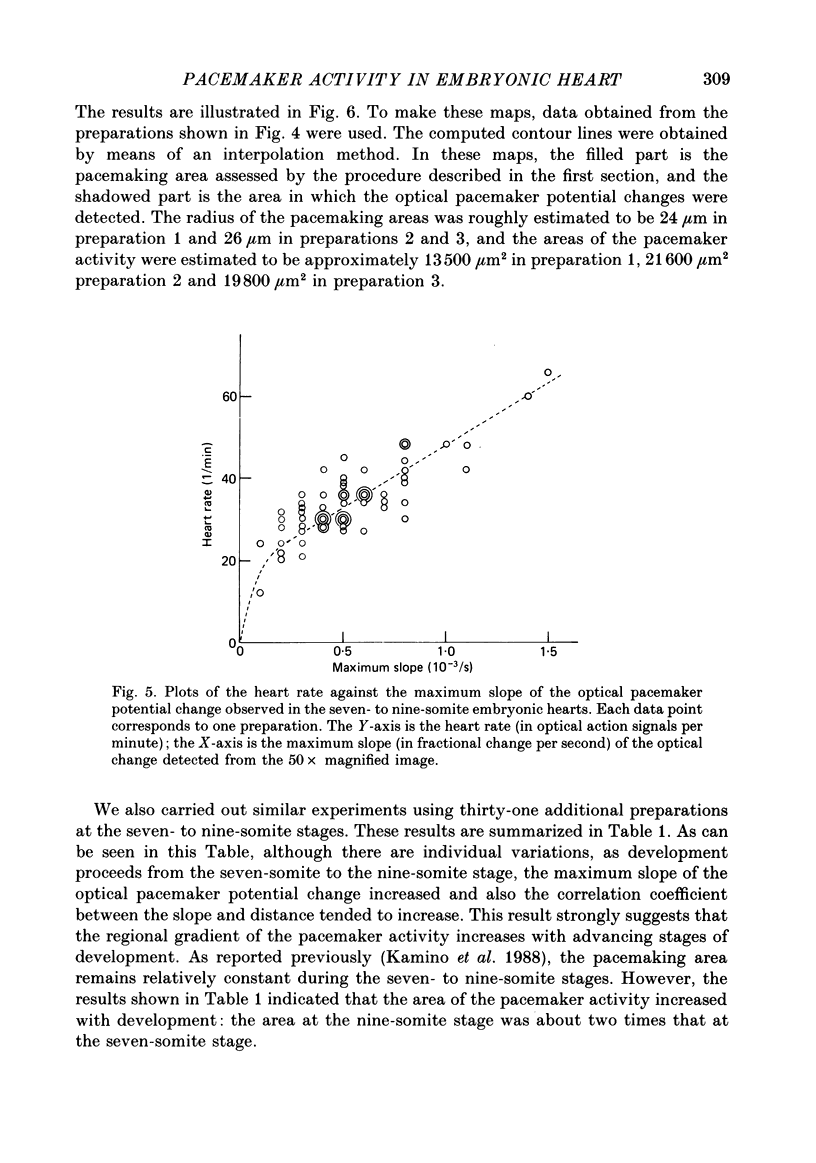
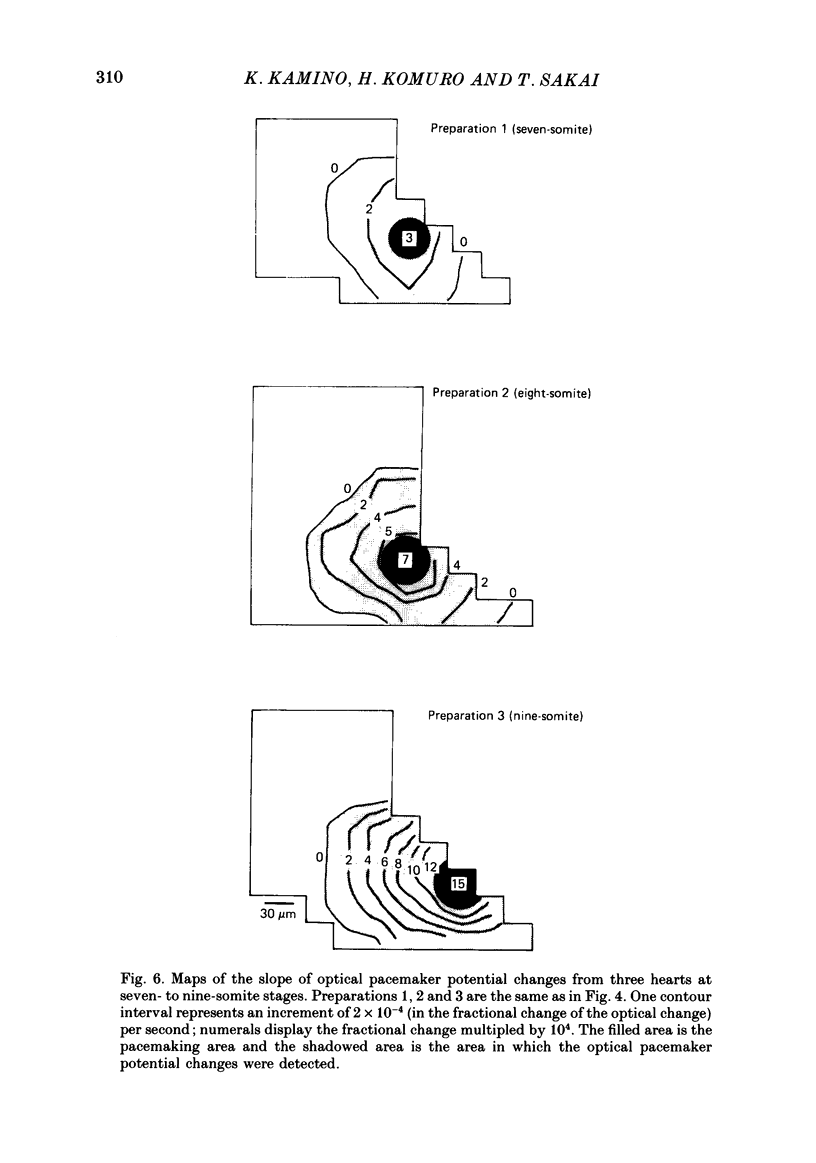




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett D., Boyse E. A. Sex ratio in progeny of mice inseminated with sperm treated with H-Y antiserum. Nature. 1973 Nov 30;246(5431):308–309. doi: 10.1038/246308a0. [DOI] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Action potential synchrony in embryonic precontractile chick heart: optical monitoring with potentiometric dyes. J Physiol. 1981;319:529–541. doi: 10.1113/jphysiol.1981.sp013924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical indications of pace-maker potential and rhythm generation in early embryonic chick heart. J Physiol. 1981 Mar;312:253–263. doi: 10.1113/jphysiol.1981.sp013627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical recording of development of electrical activity in embryonic chick heart during early phases of cardiogenesis. J Physiol. 1981 Feb;311:147–160. doi: 10.1113/jphysiol.1981.sp013578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical signals from early embryonic chick heart stained with potential sensitive dyes: evidence for electrical activity. J Physiol. 1980 Jul;304:503–518. doi: 10.1113/jphysiol.1980.sp013339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Salzberg B. M., Grinvald A., Cohen L. B., Kamino K., Lesher S., Boyle M. B., Waggoner A. S., Wang C. H. Improvements in optical methods for measuring rapid changes in membrane potential. J Membr Biol. 1981 Feb 15;58(2):123–137. doi: 10.1007/BF01870975. [DOI] [PubMed] [Google Scholar]
- Hirota A., Fujii S., Sakai T., Kamino K. Temperature dependence of spontaneous electrical activity in early embryonic heart monitored optically with a potential-sensitive dye. Jpn J Physiol. 1983;33(1):85–100. doi: 10.2170/jjphysiol.33.85. [DOI] [PubMed] [Google Scholar]
- Hirota A., Kamino K., Komuro H., Sakai T. Mapping of early development of electrical activity in the embryonic chick heart using multiple-site optical recording. J Physiol. 1987 Feb;383:711–728. doi: 10.1113/jphysiol.1987.sp016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A., Kamino K., Komuro H., Sakai T., Yada T. Early events in development of electrical activity and contraction in embryonic rat heart assessed by optical recording. J Physiol. 1985 Dec;369:209–227. doi: 10.1113/jphysiol.1985.sp015897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A., Kamino K., Komuro H., Sakai T., Yada T. Optical studies of excitation-contraction coupling in the early embryonic chick heart. J Physiol. 1985 Sep;366:89–106. doi: 10.1113/jphysiol.1985.sp015786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma T., Hirakow R. An ultrastructural topographical study on myofibrillogenesis in the heart of the chick embryo during pulsation onset period. Anat Embryol (Berl) 1985;172(3):325–329. doi: 10.1007/BF00318980. [DOI] [PubMed] [Google Scholar]
- Kamino K., Hirota A., Fujii S. Localization of pacemaking activity in early embryonic heart monitored using voltage-sensitive dye. Nature. 1981 Apr 16;290(5807):595–597. doi: 10.1038/290595a0. [DOI] [PubMed] [Google Scholar]
- London J. A., Zecević D., Cohen L. B. Simultaneous optical recording of activity from many neurons during feeding in Navanax. J Neurosci. 1987 Mar;7(3):649–661. doi: 10.1523/JNEUROSCI.07-03-00649.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasek F. J. Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J Morphol. 1968 Jul;125(3):329–365. doi: 10.1002/jmor.1051250306. [DOI] [PubMed] [Google Scholar]
- Obaid A. L., Orkand R. K., Gainer H., Salzberg B. M. Active calcium responses recorded optically from nerve terminals of the frog neurohypophysis. J Gen Physiol. 1985 Apr;85(4):481–489. doi: 10.1085/jgp.85.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach H. S., Cohen L. B., Grinvald A. Optical mapping of electrical activity in rat somatosensory and visual cortex. J Neurosci. 1985 Jul;5(7):1886–1895. doi: 10.1523/JNEUROSCI.05-07-01886.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach H. S., Cohen L. B. Optical monitoring of activity from many areas of the in vitro and in vivo salamander olfactory bulb: a new method for studying functional organization in the vertebrate central nervous system. J Neurosci. 1983 Nov;3(11):2251–2262. doi: 10.1523/JNEUROSCI.03-11-02251.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Grinvald A., Davila H. V., Waggoner A. S., Wang C. H. Changes in absorption, fluorescence, dichroism, and Birefringence in stained giant axons: : optical measurement of membrane potential. J Membr Biol. 1977 May 6;33(1-2):141–183. doi: 10.1007/BF01869514. [DOI] [PubMed] [Google Scholar]
- Sakai T., Hirota A., Fujii S., Kamino K. Flexibility of regional pacemaking priority in early embryonic heart monitored by simultaneous optical recording of action potentials from multiple sites. Jpn J Physiol. 1983;33(3):337–350. doi: 10.2170/jjphysiol.33.337. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Obaid A. L., Senseman D. M., Gainer H. Optical recording of action potentials from vertebrate nerve terminals using potentiometric probes provides evidence for sodium and calcium components. Nature. 1983 Nov 3;306(5938):36–40. doi: 10.1038/306036a0. [DOI] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada T., Sakai T., Komuro H., Hirota A., Kamino K. Development of electrical rhythmic activity in early embryonic cultured chick double-heart monitored optically with a voltage-sensitive dye. Dev Biol. 1985 Aug;110(2):455–466. doi: 10.1016/0012-1606(85)90103-4. [DOI] [PubMed] [Google Scholar]