Abstract
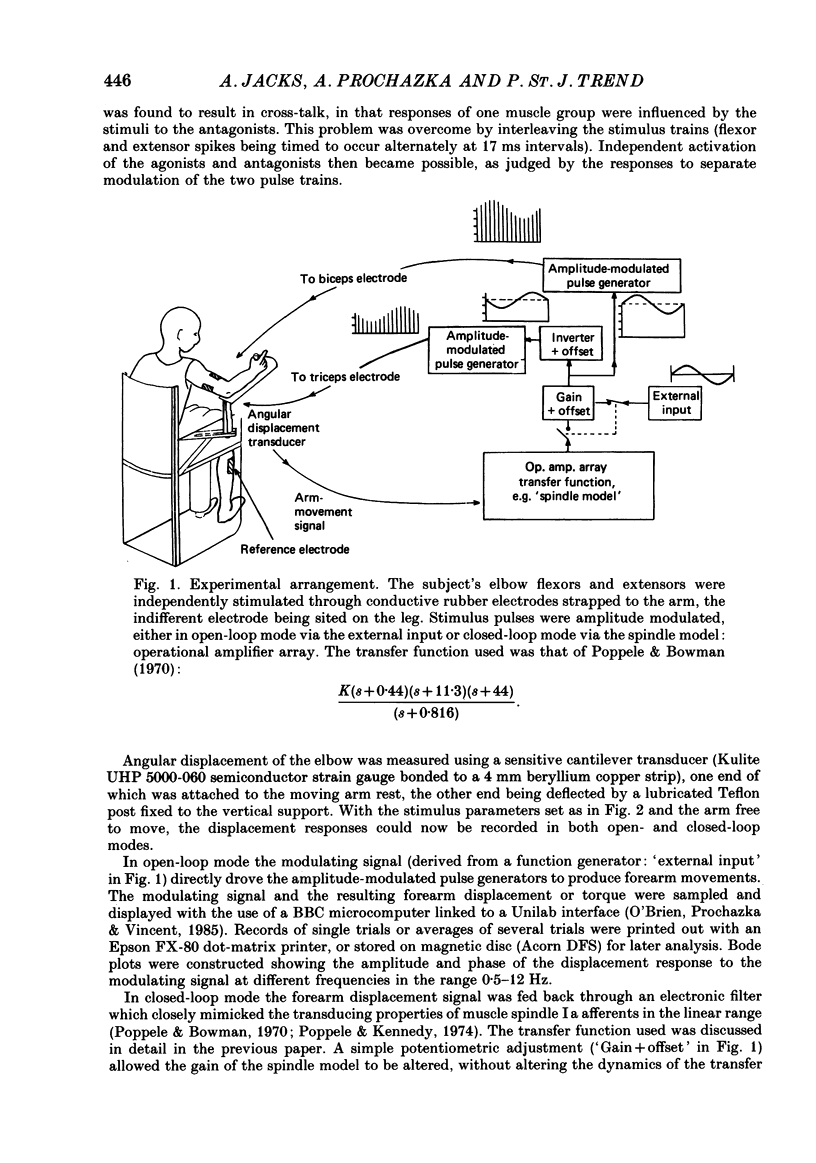
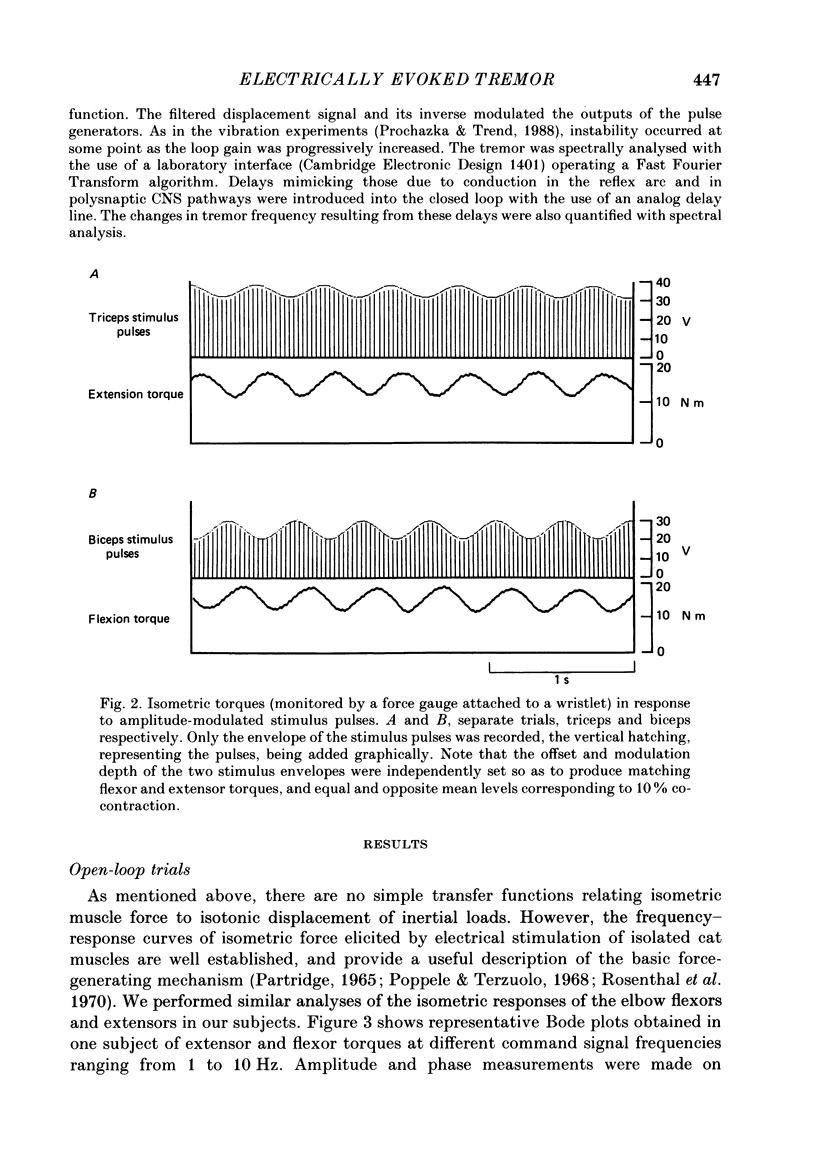
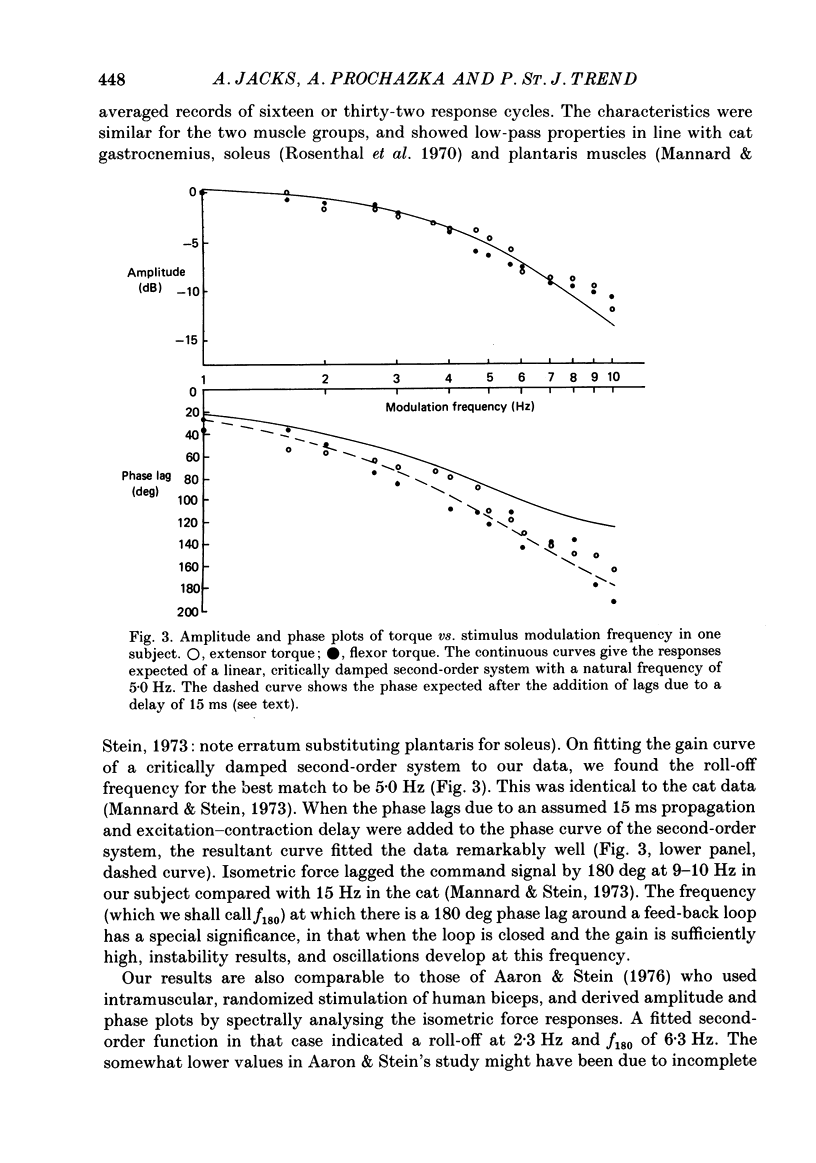
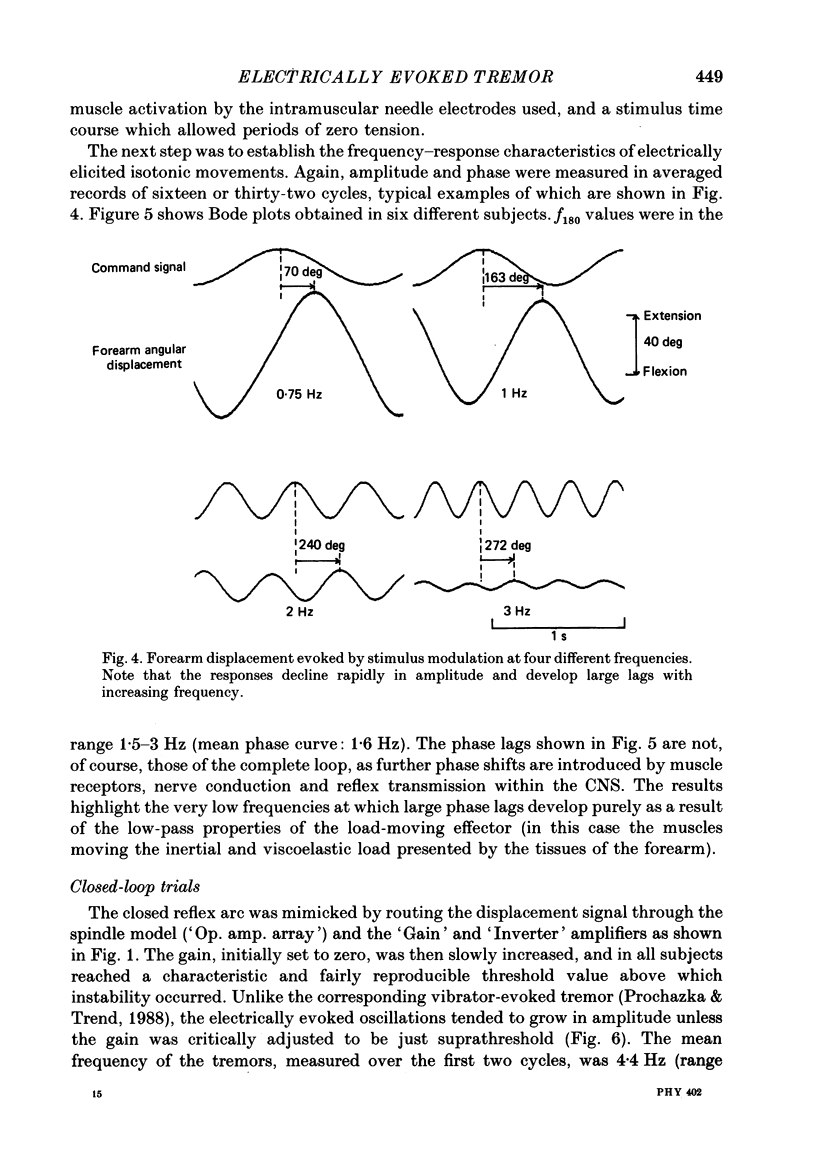
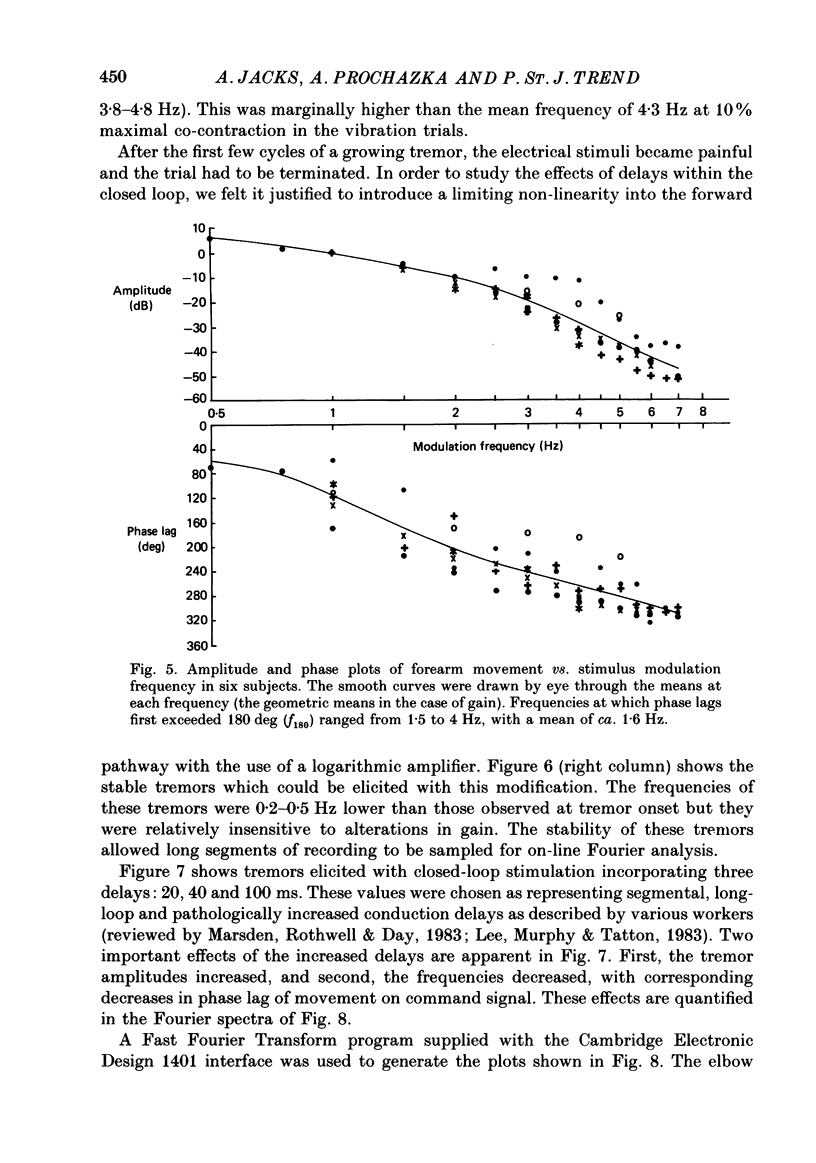
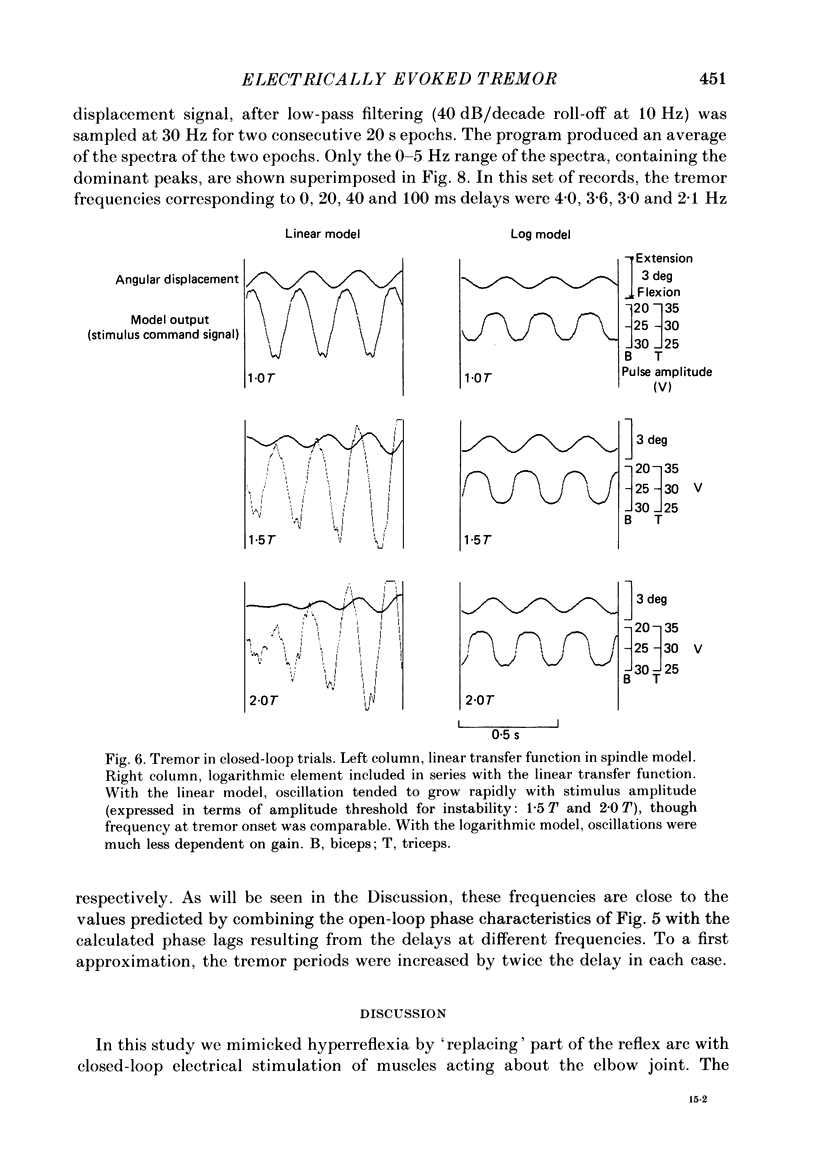
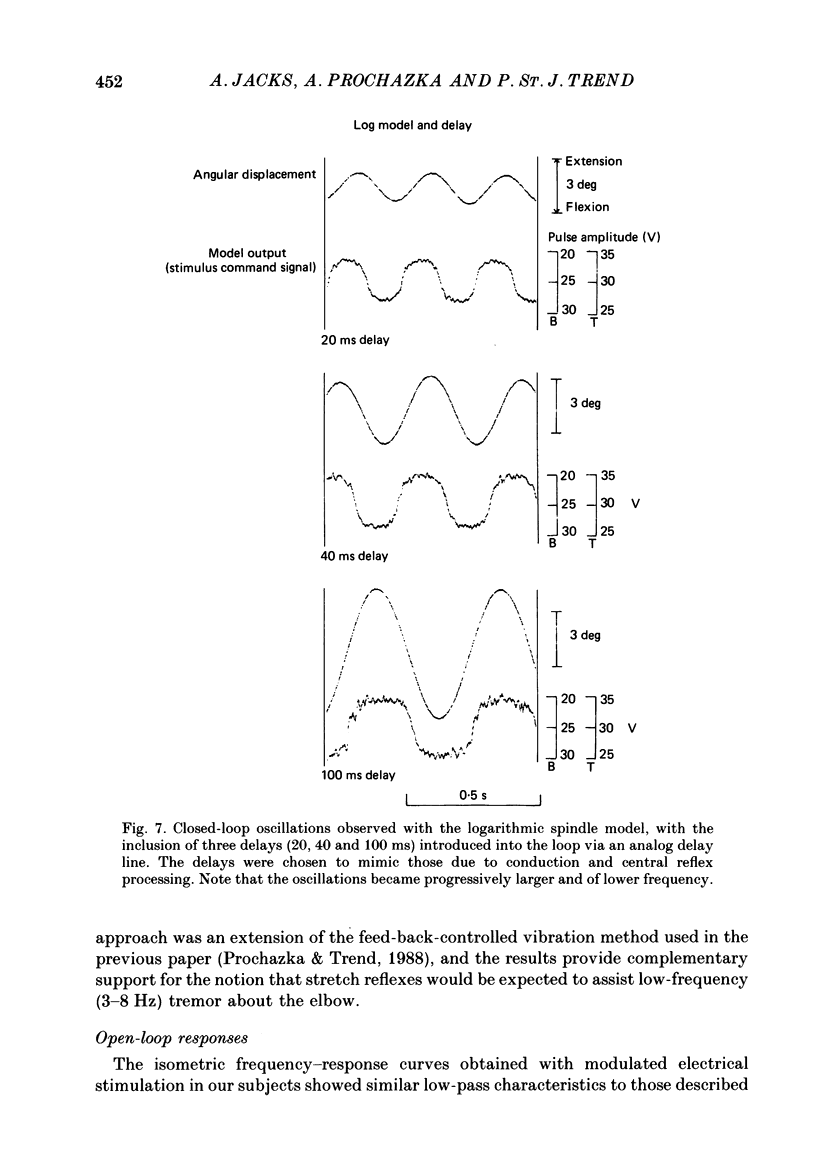
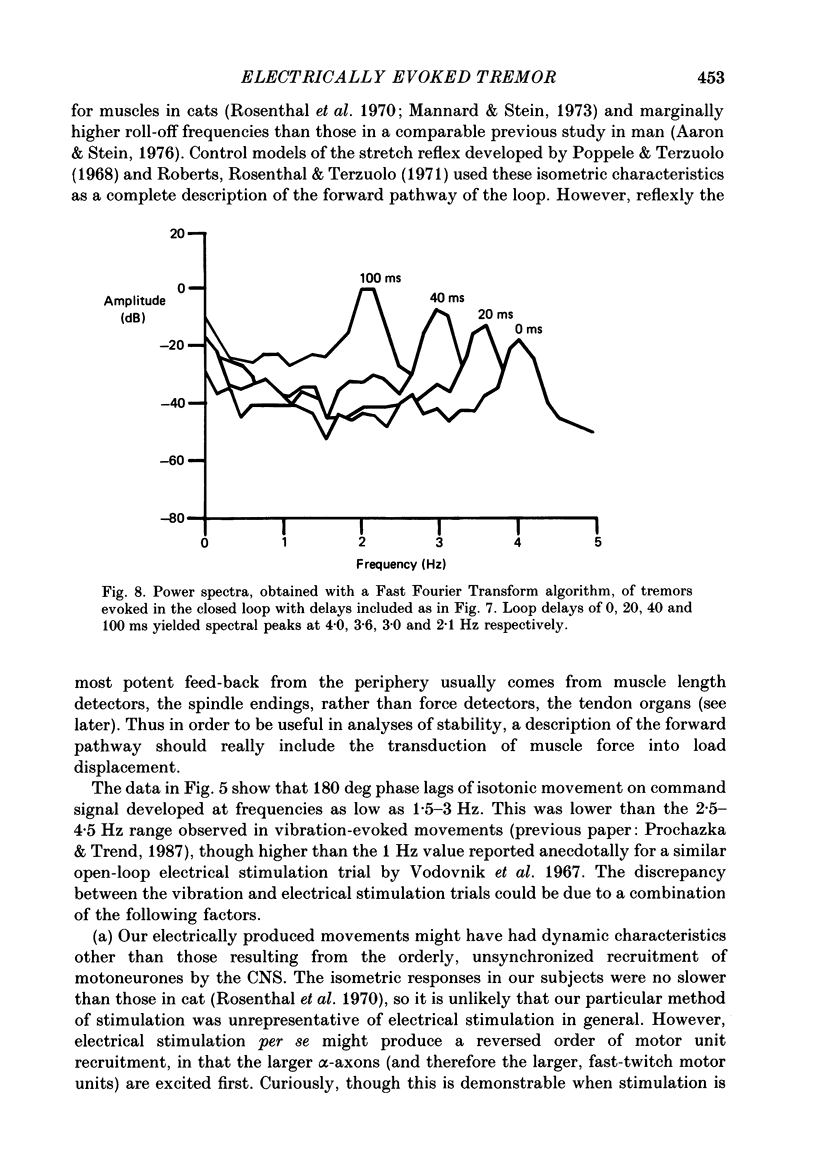
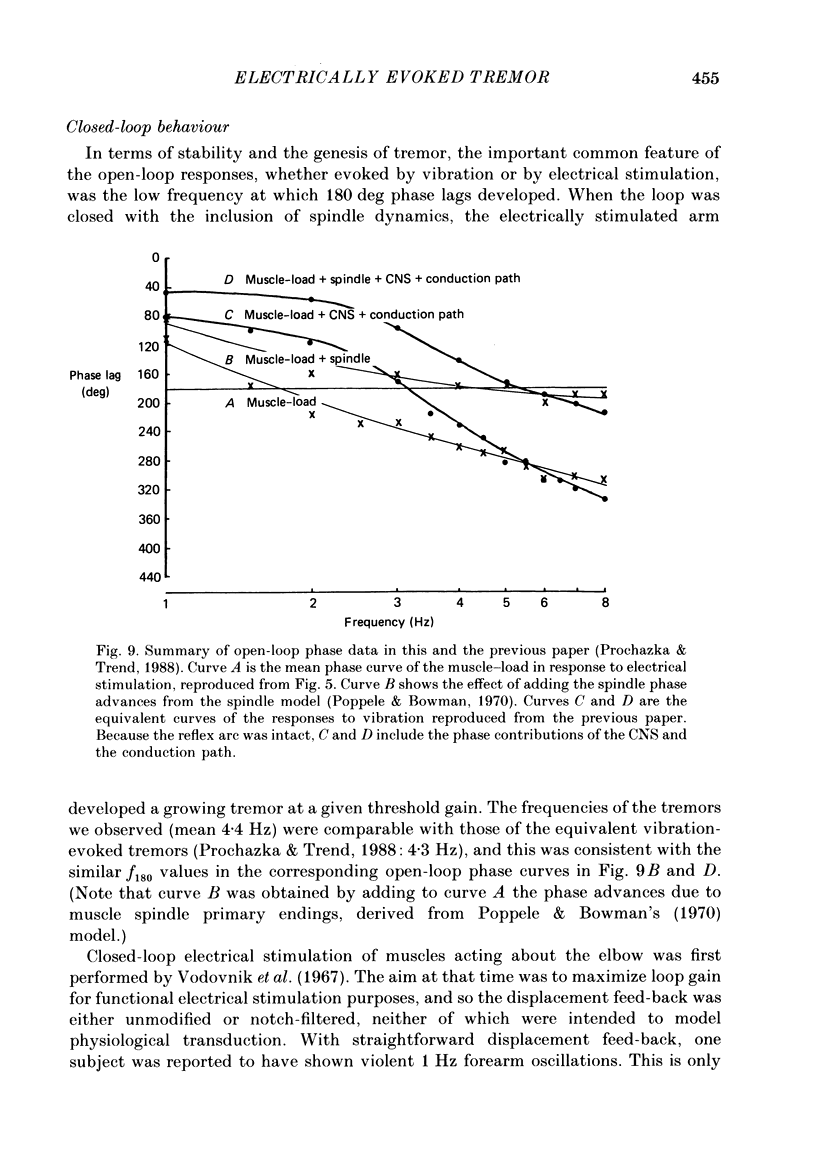
1. Amplitude-modulated electrical stimulation was applied to the elbow flexors and extensors to produce movements of the forearm in normal subjects. The parameters of the modulating (command) signal were set in isometric trials so as to produce equal and opposite background torques, and equal and supportive torque modulations. 2. Bode plots relating forearm movement to command signal (modulating) frequency showed the muscle-load to have a low-pass characteristic similar to that previously described in the cat, and a slightly larger bandwidth than described previously in man. 3. The transduced forearm signals were fed back to provide the command signal to the stimulators via a filter which mimicked the transfer function of muscle spindle primary endings. In effect this replaced the neural part of the reflex arc with an accessible model, but left the muscle-load effector intact. 4. All six subjects developed forearm oscillations (tremor) when the loop gain exceeded a threshold value. The mean tremor frequency at onset was 4.4 Hz, which was similar to that of the equivalent vibration-evoked tremor (previous paper, Prochazka & Trend, 1988). 5. With the linear spindle model, oscillations tended to grow rapidly in amplitude, and the stimuli became painful. The inclusion of a logarithmic limiting element resulted in stable oscillations, without significant alterations in frequency. This allowed us to study the effect on tremor of including analog delays in the loop, mimicking those associated with peripheral nerve transmission and central reflexes. In one subject, loop delays of 0, 20, 40 and 100 ms resulted in tremor at 4.0, 3.6, 3.0 and 2.1 Hz respectively, as quantified by spectral analysis. 6. By considering separately the phase contributions of the different elements of the reflex arc, including delays, it became clear that muscle-load properties were important in setting the upper limit of tremor frequencies which could conceivably be supported by reflexes. 7. The results support the conclusion of the related vibration study (Prochazka & Trend, 1988), that for moderate levels of background co-contraction, the contribution of stretch reflexes to tremor at the elbow should be sought in the 3-5 Hz range. Exaggerated long-latency reflexes would be expected to reduce these baseline frequencies by 1 or 2 Hz.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaron S. L., Stein R. B. Comparison of an EMG-controlled prosthesis and the normal human biceps brachii muscle. Am J Phys Med. 1976 Feb;55(1):1–14. [PubMed] [Google Scholar]
- Amis A., Prochazka A., Short D., Trend P. S., Ward A. Relative displacements in muscle and tendon during human arm movements. J Physiol. 1987 Aug;389:37–44. doi: 10.1113/jphysiol.1987.sp016645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. N., Sears T. A. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol. 1970 Aug;209(3):711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler R., Stein R. B., Thomas C. K. Axonal conduction velocity and force of single human motor units. Muscle Nerve. 1988 Feb;11(2):136–145. doi: 10.1002/mus.880110209. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957 Sep 30;138(2):227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows S. J., Rack P. M. Changes in the length of the human biceps brachii muscle during elbow movements. J Physiol. 1987 Feb;383:405–412. doi: 10.1113/jphysiol.1987.sp016416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Jankowska E., Johannisson T., Lipski J. Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. J Physiol. 1979 Aug;293:173–195. doi: 10.1113/jphysiol.1979.sp012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E., Katz R., Pierrot-Deseilligny E. Descending control of reflex pathways in the production of voluntary isolated movements in man. Brain Res. 1983 Dec 12;288(1-2):375–377. doi: 10.1016/0006-8993(83)90122-1. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C. Response to sudden torques about ankle in man. III. Suppression of stretch-evoked responses during phasic contraction. J Neurophysiol. 1980 Aug;44(2):233–246. doi: 10.1152/jn.1980.44.2.233. [DOI] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E., Johannisson T. Shared reflex pathways of group I afferents of different cat hind-limb muscles. J Physiol. 1983 May;338:113–128. doi: 10.1113/jphysiol.1983.sp014664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Exp Brain Res. 1969;7(4):365–391. doi: 10.1007/BF00237321. [DOI] [PubMed] [Google Scholar]
- Houk J. C., Singer J. J., Goldman M. R. An evaluation of length and force feedback to soleus muscles of decerebrate cats. J Neurophysiol. 1970 Nov;33(6):784–811. doi: 10.1152/jn.1970.33.6.784. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Roberts R. C. The role of muscle spindle afferents in stretch and vibration reflexes of the soleus muscle of the decerebrate cat. Brain Res. 1978 May 12;146(2):366–372. doi: 10.1016/0006-8993(78)90981-2. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., LLOYD D. P. C. Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol. 1952 Jun;169(3):609–621. doi: 10.1152/ajplegacy.1952.169.3.609. [DOI] [PubMed] [Google Scholar]
- Lee R. G., Murphy J. T., Tatton W. G. Long-latency myotatic reflexes in man: mechanisms, functional significance, and changes in patients with Parkinson's disease or hemiplegia. Adv Neurol. 1983;39:489–508. [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Role of joint afferents in motor control exemplified by effects on reflex pathways from Ib afferents. J Physiol. 1978 Nov;284:327–343. doi: 10.1113/jphysiol.1978.sp012543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannard A., Stein R. B. Determination of the frequency response of isometric soleus muscle in the cat using random nerve stimulation. J Physiol. 1973 Mar;229(2):275–296. doi: 10.1113/jphysiol.1973.sp010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Rothwell J. C., Day B. L. Long-latency automatic responses to muscle stretch in man: origin and function. Adv Neurol. 1983;39:509–539. [PubMed] [Google Scholar]
- Matthews P. B. Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol. 1984 Mar;348:383–415. doi: 10.1113/jphysiol.1984.sp015116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Observations on the time course of the electromyographic response reflexly elicited by muscle vibration in man. J Physiol. 1984 Aug;353:447–461. doi: 10.1113/jphysiol.1984.sp015346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969 Feb;200(3):723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComas A. J., Fawcett P. R., Campbell M. J., Sica R. E. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry. 1971 Apr;34(2):121–131. doi: 10.1136/jnnp.34.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARTRIDGE L. D. MODIFICATIONS OF NEURAL OUTPUT SIGNALS BY MUSCLES: A FREQUENCY RESPONSE STUDY. J Appl Physiol. 1965 Jan;20:150–156. doi: 10.1152/jappl.1965.20.1.150. [DOI] [PubMed] [Google Scholar]
- Partridge L. D. Signal-handling characteristics of load-moving skeletal muscle. Am J Physiol. 1966 May;210(5):1178–1191. doi: 10.1152/ajplegacy.1966.210.5.1178. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Bergego C., Katz R. Reversal in cutaneous of Ib pathways during human voluntary contraction. Brain Res. 1982 Feb 11;233(2):400–403. doi: 10.1016/0006-8993(82)91213-6. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Bergego C., Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42(3-4):337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Poppele R. E., Bowman R. J. Quantitative description of linear behavior of mammalian muscle spindles. J Neurophysiol. 1970 Jan;33(1):59–72. doi: 10.1152/jn.1970.33.1.59. [DOI] [PubMed] [Google Scholar]
- Poppele R. E., Kennedy W. R. Comparison between behavior of human and cat muscle spindles recorded in vitro. Brain Res. 1974 Jul 26;75(2):316–319. doi: 10.1016/0006-8993(74)90753-7. [DOI] [PubMed] [Google Scholar]
- Poppele R. E., Terzuolo C. A. Myotatic reflex: its input-output relation. Science. 1968 Feb 16;159(3816):743–745. doi: 10.1126/science.159.3816.743. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Trend P. S. Instability in human forearm movements studied with feed-back-controlled muscle vibration. J Physiol. 1988 Aug;402:421–442. doi: 10.1113/jphysiol.1988.sp017213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack P. M., Ross H. F. The role of reflexes in the resting tremor of Parkinson's disease. Brain. 1986 Feb;109(Pt 1):115–141. doi: 10.1093/brain/109.1.115. [DOI] [PubMed] [Google Scholar]
- Roberts W. J., Rosenthal N. P., Terzuolo C. A. A control model of stretch reflex. J Neurophysiol. 1971 Jul;34(4):620–634. doi: 10.1152/jn.1971.34.4.620. [DOI] [PubMed] [Google Scholar]
- Rosenthal N. P., McKean T. A., Roberts W. J., Terzuolo C. A. Frequency analysis of stretch reflex and its main subsystems in triceps surae muscles of the cat. J Neurophysiol. 1970 Nov;33(6):713–749. doi: 10.1152/jn.1970.33.6.713. [DOI] [PubMed] [Google Scholar]
- Rymer W. Z., Hasan Z. Absence of force-feedback regulation in soleus muscle of the decerebrate cat. Brain Res. 1980 Feb 17;184(1):203–209. doi: 10.1016/0006-8993(80)90599-5. [DOI] [PubMed] [Google Scholar]
- Tatton W. G., Lee R. G. Evidence for abnormal long-loop reflexes in rigid Parkinsonian patients. Brain Res. 1975 Dec 26;100(3):671–676. doi: 10.1016/0006-8993(75)90167-5. [DOI] [PubMed] [Google Scholar]
- Thompson P. D., Rothwell J. C., Day B. L., Berardelli A., Dick J. P., Kachi T., Marsden C. D. The physiology of orthostatic tremor. Arch Neurol. 1986 Jun;43(6):584–587. doi: 10.1001/archneur.1986.00520060048016. [DOI] [PubMed] [Google Scholar]
- Vodovnik L., Crochetiere W. J., Reswick J. B. Control of a skeletal joint by electrical stimulation of antagonists. Med Biol Eng. 1967 Mar;5(2):97–109. doi: 10.1007/BF02474498. [DOI] [PubMed] [Google Scholar]
- Westbury D. R. The response of alpha-motoneurones of the cat to sinusoidal movements of the muscles they innervate. Brain Res. 1971 Jan 8;25(1):75–86. doi: 10.1016/0006-8993(71)90568-3. [DOI] [PubMed] [Google Scholar]


