Abstract
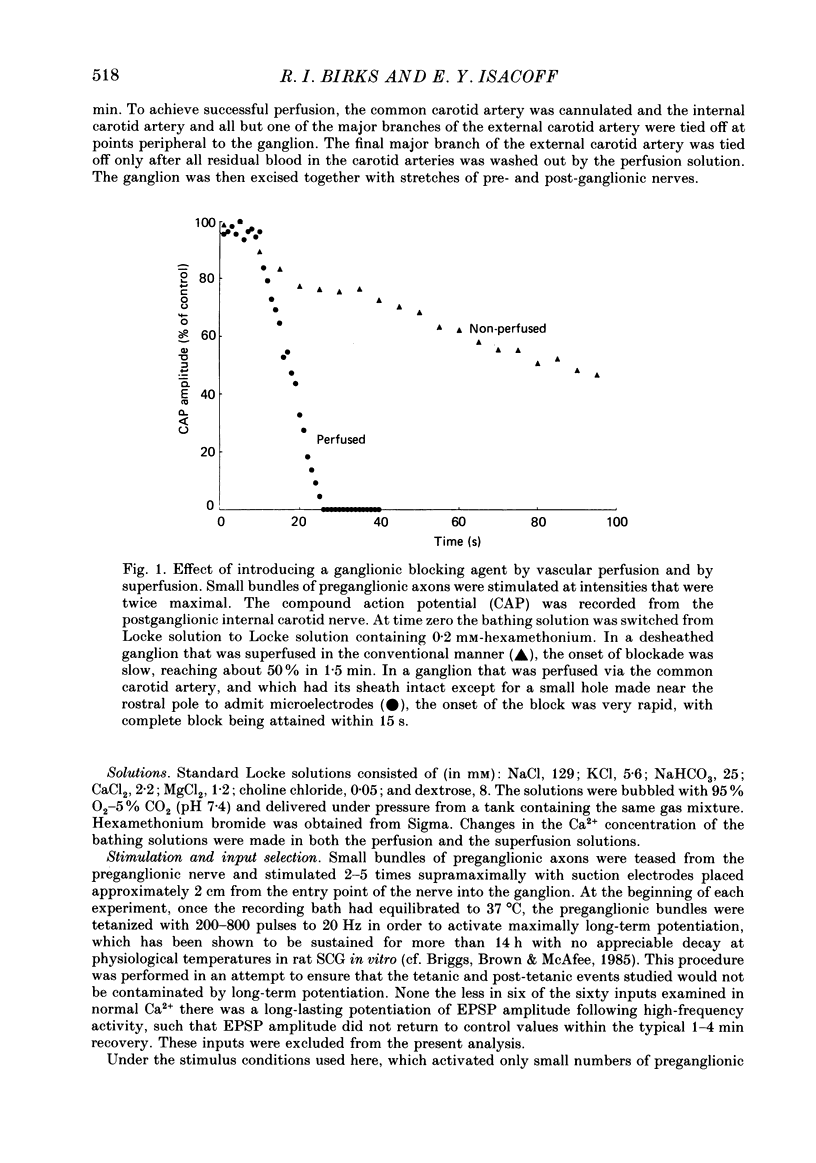
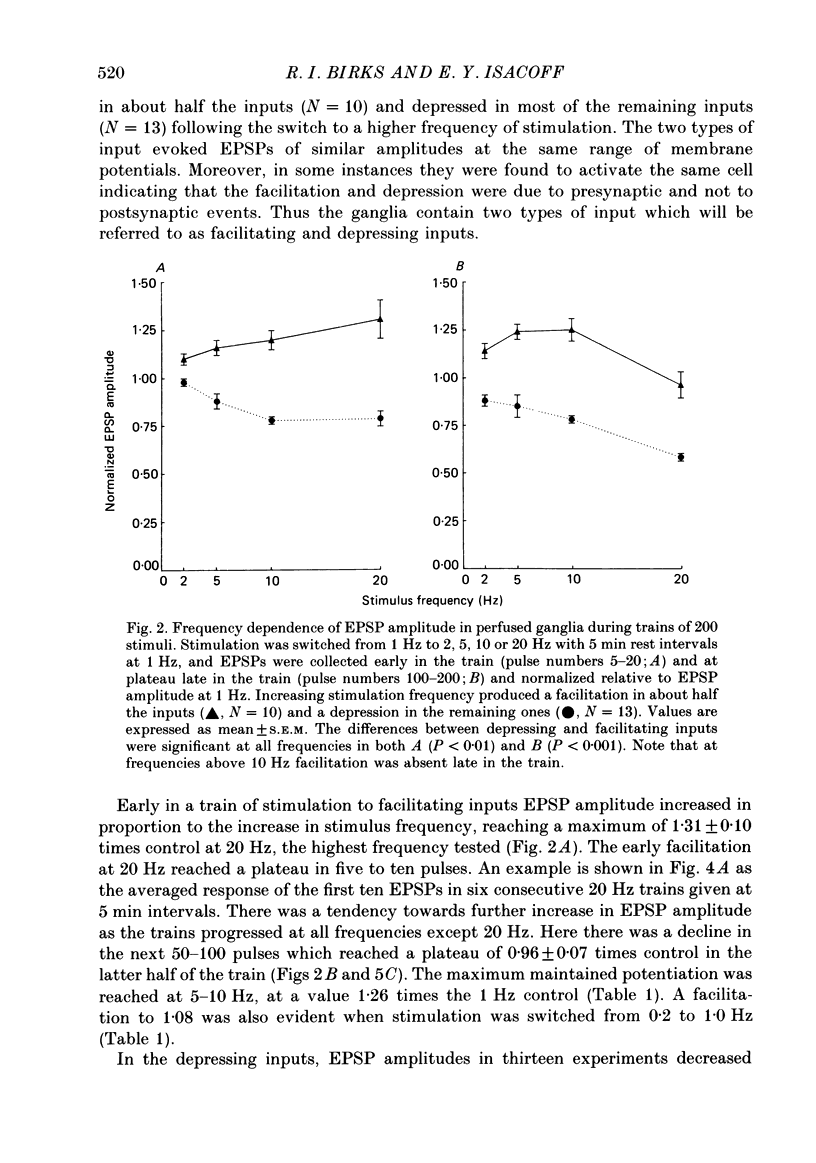
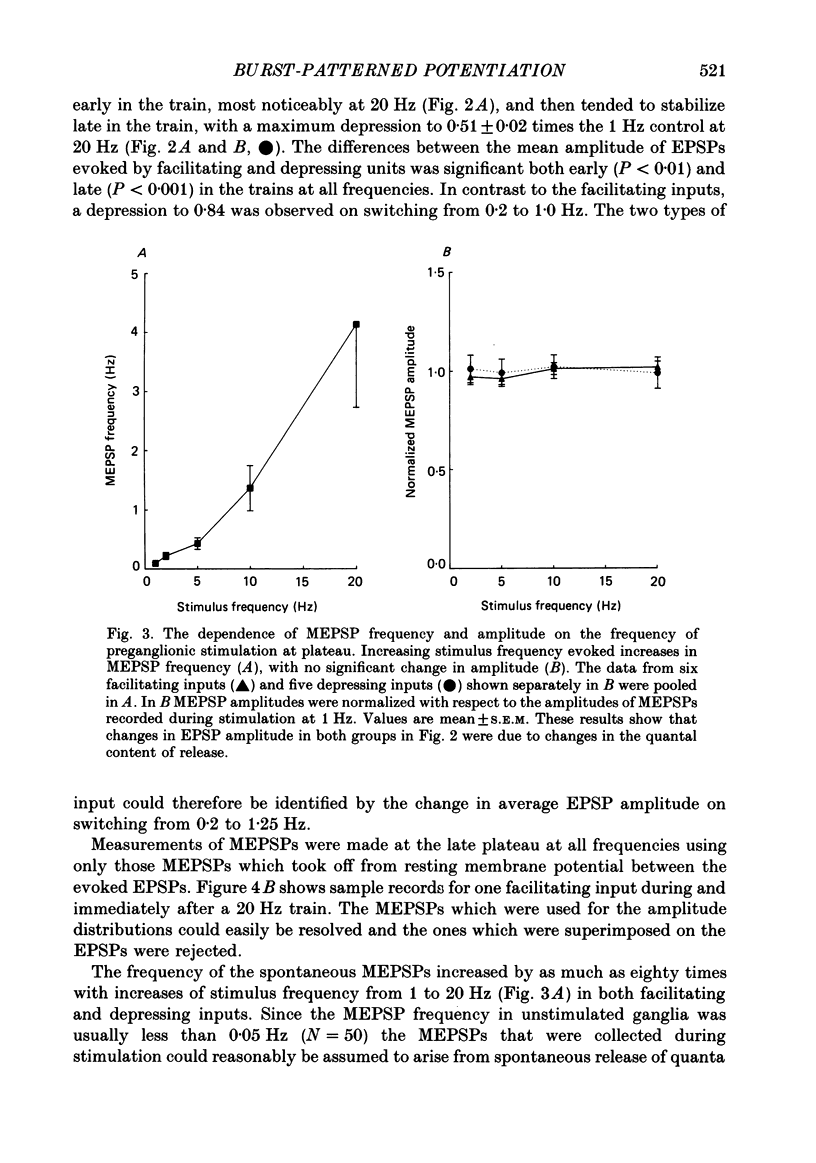
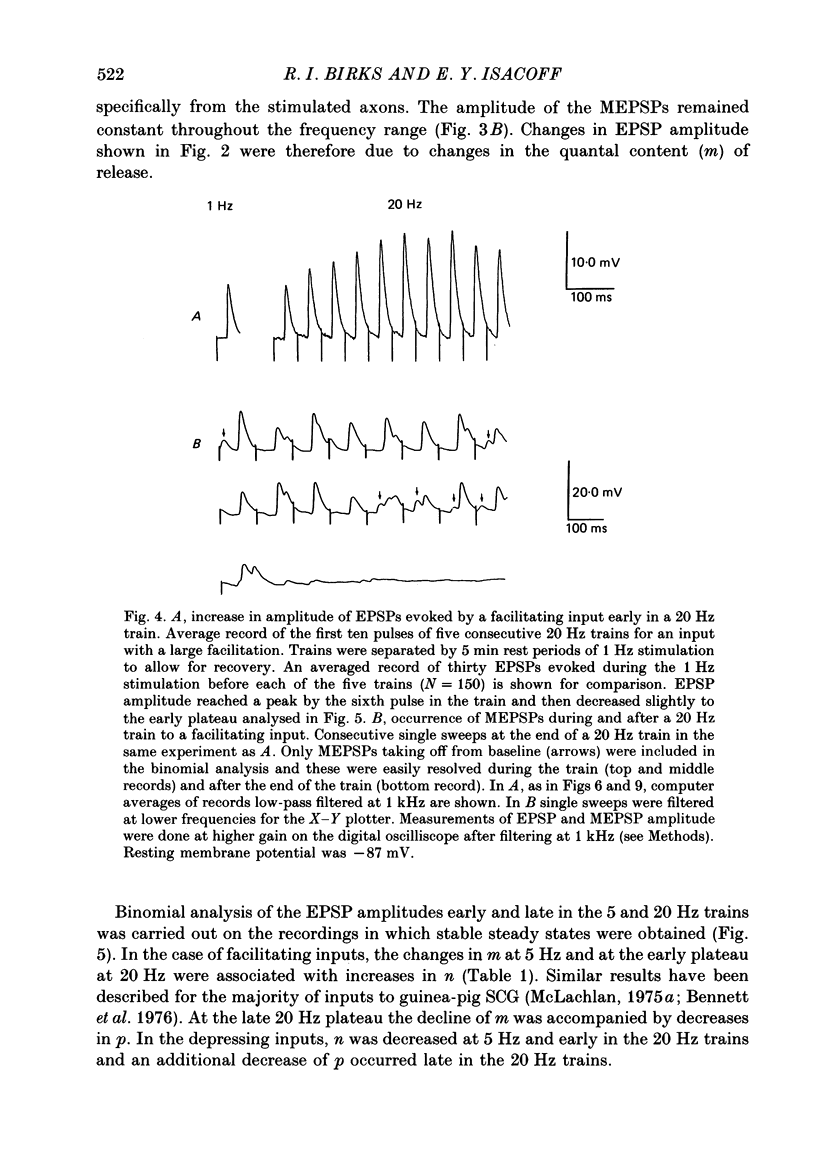
1. Intracellular recordings of small nicotinic excitatory postsynaptic potentials (EPSPs) were made from rostral cells in superior cervical ganglia (SCG) of rats during and after test stimulation of small preganglionic fibre bundles, while perfusing the isolated ganglia via their arterial vasculature. Perfusion, in contrast to superfusion of desheathed ganglia, (a) produced much more rapid and complete equilibration of drugs and ions at synaptic sites, (b) greatly reduced depression of EPSPs during high-frequency stimulation, and (c) largely prevented slowing of conduction, presumably by minimizing accumulation of K+ in the intercellular spaces surrounding these sites. 2. Preganglionic inputs were found to fall into two major groups: those in which the EPSP amplitude during 200 pulse trains was facilitated and others in which it was depressed as stimulation frequency in the train was increased from 2 to 20 Hz or from 0.2 to 1.25 Hz. Both the facilitation and the depression were presynaptic, since they occurred without changes in miniature EPSP amplitude. 3. The maximum maintained facilitation was reached at 5-10 Hz with a value 1.26 times the 1.0 Hz control. This was associated with an increase in the binomial parameter n. While long 20 Hz trains produced a similar facilitation to an early plateau, and an increase in n, EPSP amplitude declined as the train progressed. This was associated with a decrease in the binomial parameter p. 4. Unlike the 20 Hz trains, stimulation with 0.5 s long, 20 Hz bursts given every 8 s produced a marked potentiation in facilitating units and this was maintained for as long as the stimulation was continued (3-11 min). Burst-patterned potentiation was 1.66 times larger than the facilitation evoked by tonic stimulation at the same average frequency (1.25 Hz), and more than twice that achieved with long, 200 pulse trains. The potentiation was associated with increases in both n and p in the first EPSP of the burst and mainly with an increase in n at the end of the burst. Potentiation persisted unchanged for about 30 s following the return to control 0.2 Hz stimulation, before declining to control levels over the next 2-3 min. Depressing units on average showed neither burst-patterned potentiation nor post-burst-patterned potentiation. 5. All inputs tested in Locke solution in which Ca2+ was reduced to 0.5 mM with addition of 1.2 mM-Mn2+ or 3.8 mM-MgCl2 exhibited a pronounced facilitation within each burst but no extension of potentiation into ensuing bursts. Both burst-patterned potentiation and the post-burst-patterned potentiation were abolished.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



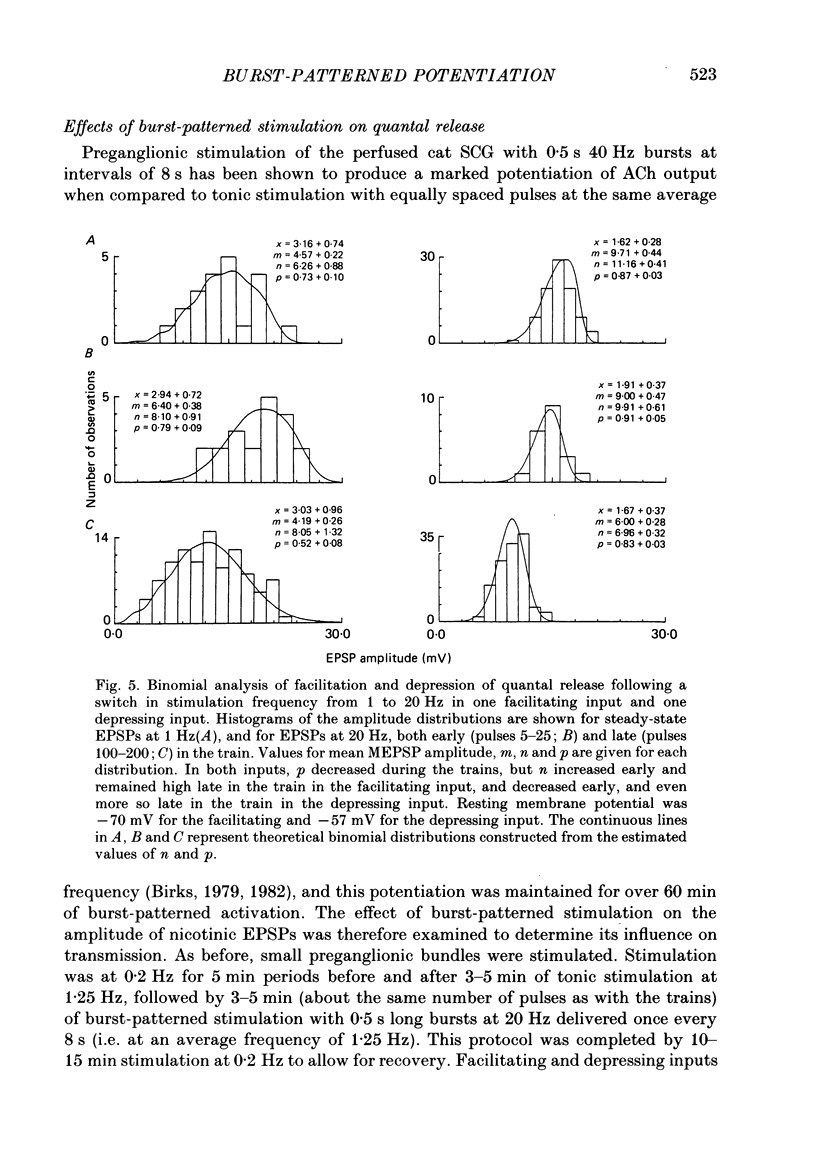
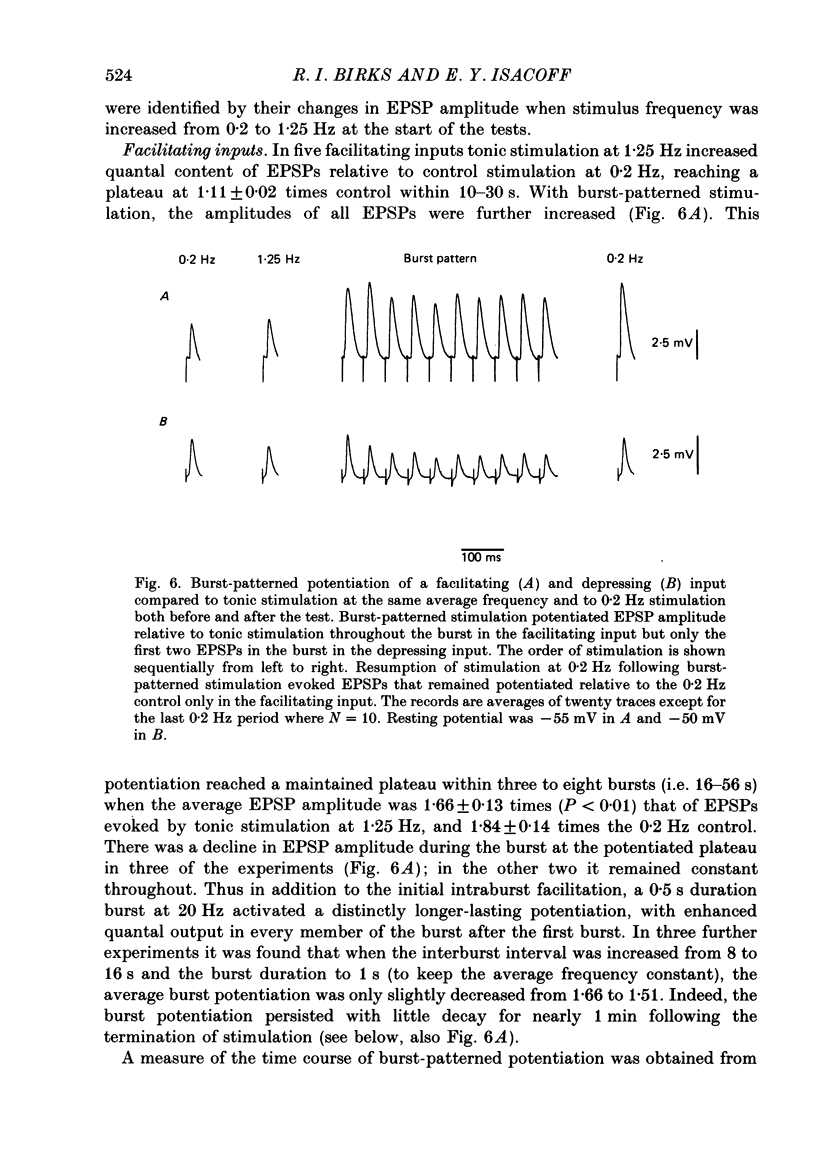
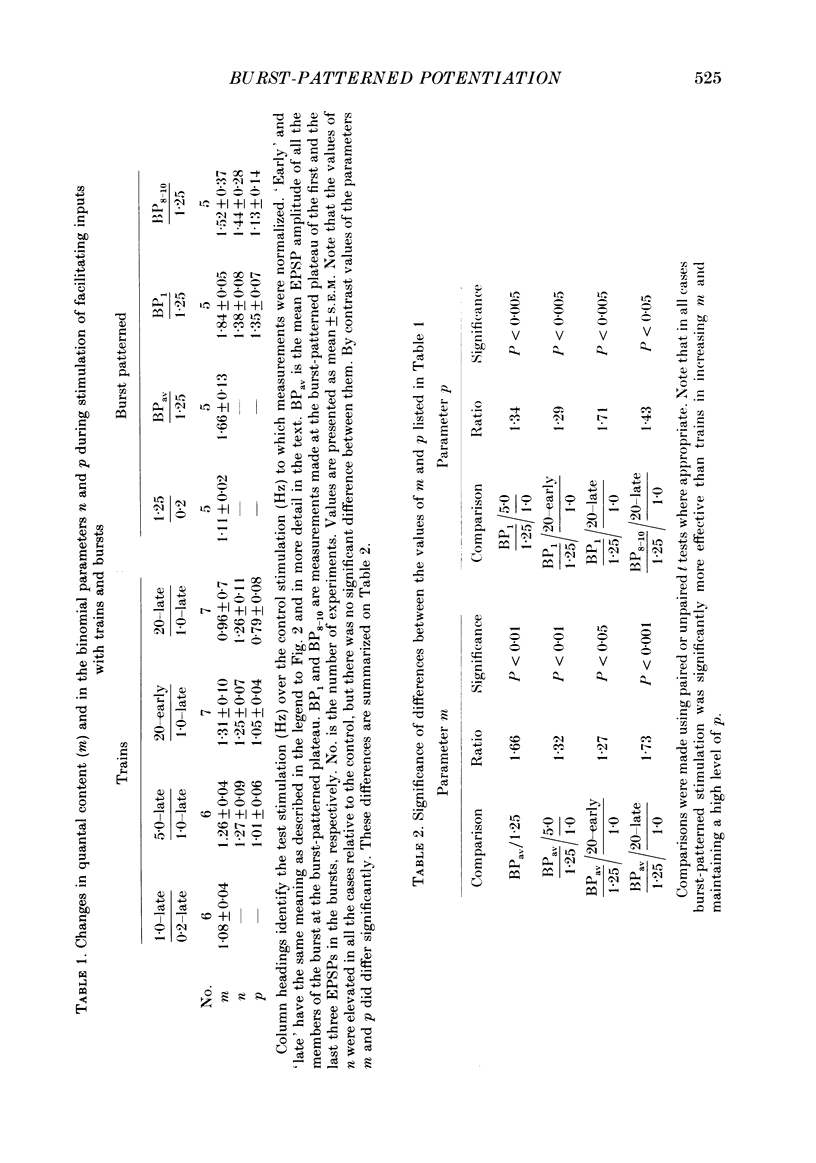
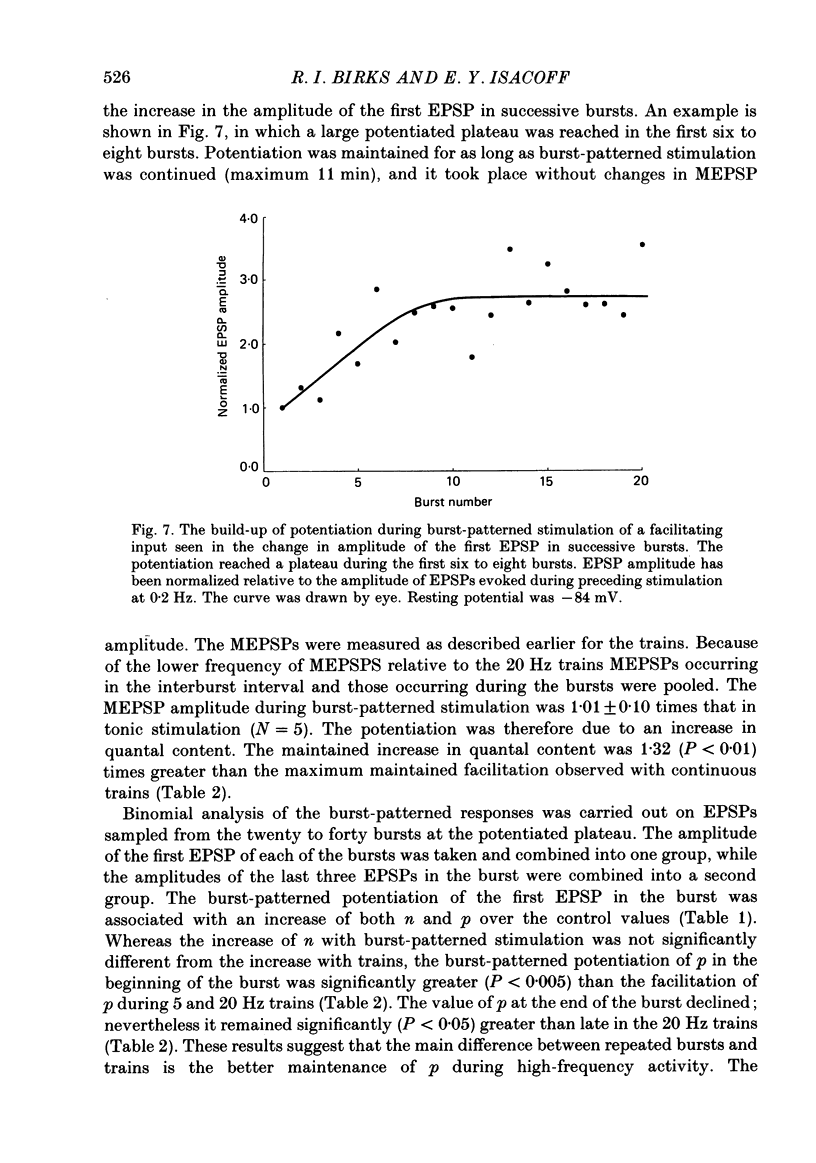

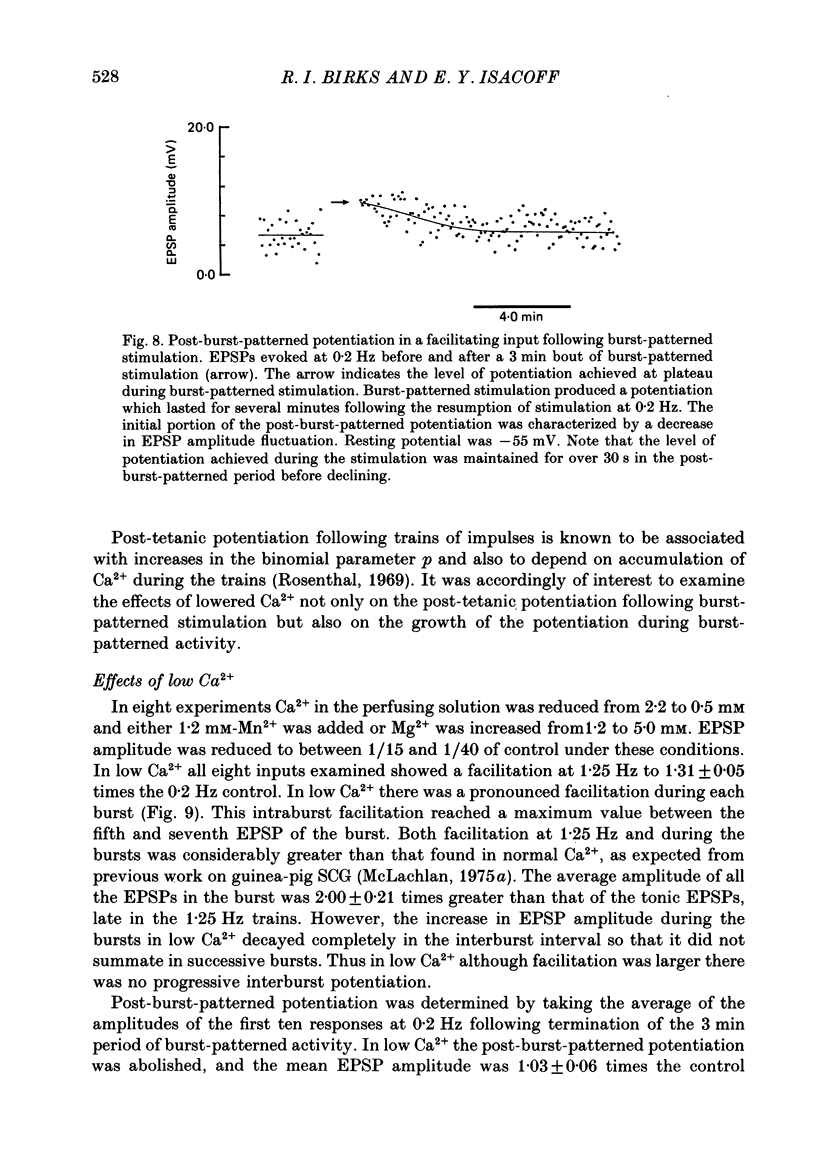



Selected References
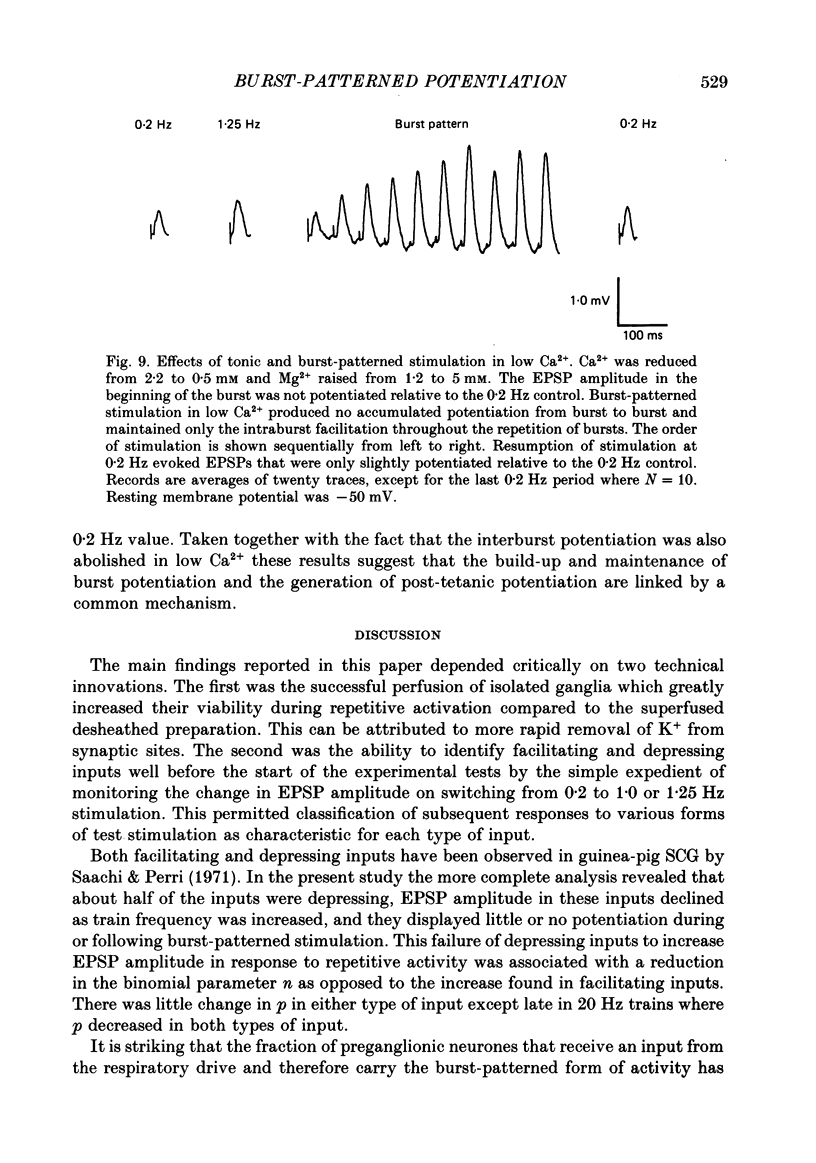
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson P. O., Bloom S. R., Edwards A. V., Järhult J. Effects of stimulation of the chorda tympani in bursts on submaxillary responses in the cat. J Physiol. 1982 Jan;322:469–483. doi: 10.1113/jphysiol.1982.sp014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKS R. I. The effects of a cardiac glycoside on subcellular structures within nerve cells and their processes in sympathetic ganglia and skeletal muscle. Can J Biochem Physiol. 1962 Feb;40:303–315. [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Spontaneous synaptic activity in sympathetic ganglion cells of the frog. J Physiol. 1963 Jul;167:389–401. doi: 10.1113/jphysiol.1963.sp007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoo M., Polosa C. Properties of the inspiration-related activity of sympathetic preganglionic neurones of the cervical trunk in the cat. J Physiol. 1987 Apr;385:545–564. doi: 10.1113/jphysiol.1987.sp016507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Pettigrew A. G. The effect of calcium ions on the binomial statistic parameters that control acetylcholine release at preganglionic nerve terminals. J Physiol. 1976 Jun;257(3):597–620. doi: 10.1113/jphysiol.1976.sp011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I. Acetylcholine release during burst-patterned stimulation of a sympathetic ganglion: its relation to transmission efficiency. J Physiol (Paris) 1982;78(4):417–419. [PubMed] [Google Scholar]
- Birks R. I., Laskey W., Polosa C. The effect of burst patterning of preganglionic input on the efficacy of transmission at the cat stellate ganglion. J Physiol. 1981 Sep;318:531–539. doi: 10.1113/jphysiol.1981.sp013882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I. Modulation of transmitter turnover by sodium and the sodium pump [proceedings]. J Physiol. 1979 Oct;295:51P–52P. [PubMed] [Google Scholar]
- Birks R. I. Regulation by patterned preganglionic neural activity of transmitter stores in a sympathetic ganglion. J Physiol. 1978 Jul;280:559–572. doi: 10.1113/jphysiol.1978.sp012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Ghatei M. A. Neuroendocrine responses to stimulation of the splanchnic nerves in bursts in the conscious adrenalectomized calf. J Physiol. 1984 Jan;346:519–531. doi: 10.1113/jphysiol.1984.sp015038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., Brown T. H., McAfee D. A. Neurophysiology and pharmacology of long-term potentiation in the rat sympathetic ganglion. J Physiol. 1985 Feb;359:503–521. doi: 10.1113/jphysiol.1985.sp015599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble M. J., Wallis D. I., Woodward B. Facilitation and inhibition of cell groups within the superior cervical ganglion of the rabbit. J Physiol. 1972 Nov;226(3):629–652. doi: 10.1113/jphysiol.1972.sp010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Selyanko A. A. Membrane currents underlying the cholinergic slow excitatory post-synaptic potential in the rat sympathetic ganglion. J Physiol. 1985 Aug;365:365–387. doi: 10.1113/jphysiol.1985.sp015777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y., Dolivo M. Plasticity of synaptic functions in the exised sympathetic ganglion of the rat. Brain Res. 1968 Aug 26;10(2):271–273. doi: 10.1016/0006-8993(68)90134-0. [DOI] [PubMed] [Google Scholar]
- Dutton A., Dyball R. E. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979 May;290(2):433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V. Adrenal catecholamine output in response to stimulation of the splanchnic nerve in bursts in the conscious calf. J Physiol. 1982 Jun;327:409–419. doi: 10.1113/jphysiol.1982.sp014239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Weight F. F. Extracellular potassium and trasmitter release at the giant synapse of squid. J Physiol. 1977 Apr;266(2):209–218. doi: 10.1113/jphysiol.1977.sp011764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Bruggencate G. T., Senekowitsch R. The effects of neuronal stimulation and ouabain upon extracellular K+ and Ca2+ levels in rat isolated sympathetic ganglia. Brain Res. 1979 Jan 19;160(3):544–548. doi: 10.1016/0006-8993(79)91084-9. [DOI] [PubMed] [Google Scholar]
- Grundy D., Scratcherd T. Effect of stimulation of the vagus nerve in bursts on gastric acid secretion and motility in the anaesthetized ferret. J Physiol. 1982 Dec;333:451–461. doi: 10.1113/jphysiol.1982.sp014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt H., Smith D. O. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976 Jul;259(2):367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. MECHANSIM OF FACILITATION AND DEPRESSION OF THE EXCITATORY SYNAPTIC POTENTIAL IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:100–112. doi: 10.1113/jphysiol.1964.sp007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. PRESYNAPTIC AND POST-SYNAPTIC EVENTS DURING POST-TETANIC POTENTIATION AND FACILITATION IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Dec;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. An analysis of the release of acetylcholine from preganglionic nerve terminals. J Physiol. 1975 Feb;245(2):447–466. doi: 10.1113/jphysiol.1975.sp010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. Changes in statistical release parameters during prolonged stimulation of preganglionic nerve terminals. J Physiol. 1975 Dec;253(2):477–491. doi: 10.1113/jphysiol.1975.sp011201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I., Hochstein S., Parnas H. Theoretical analysis of parameters leading to frequency modulation along an inhomogeneous axon. J Neurophysiol. 1976 Jul;39(4):909–923. doi: 10.1152/jn.1976.39.4.909. [DOI] [PubMed] [Google Scholar]
- Preiss G., Kirchner F., Polosa C. Patterning of sympathetic preganglionic neuron firing by the central respiratory drive. Brain Res. 1975 Apr 11;87(2-3):363–374. doi: 10.1016/0006-8993(75)90434-5. [DOI] [PubMed] [Google Scholar]
- Rosenthal J. Post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1969 Jul;203(1):121–133. doi: 10.1113/jphysiol.1969.sp008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi O., Perri V. Quantal release of acetylcholine from the nerve endings of the guinea-pig superior cervical ganglion. Pflugers Arch. 1971;329(3):207–219. doi: 10.1007/BF00586615. [DOI] [PubMed] [Google Scholar]
- THIES R. E. NEUROMUSCULAR DEPRESSION AND THE APPARENT DEPLETION OF TRANSMITTER IN MAMMALIAN MUSCLE. J Neurophysiol. 1965 May;28:428–442. doi: 10.1152/jn.1965.28.3.427. [DOI] [PubMed] [Google Scholar]


