Abstract
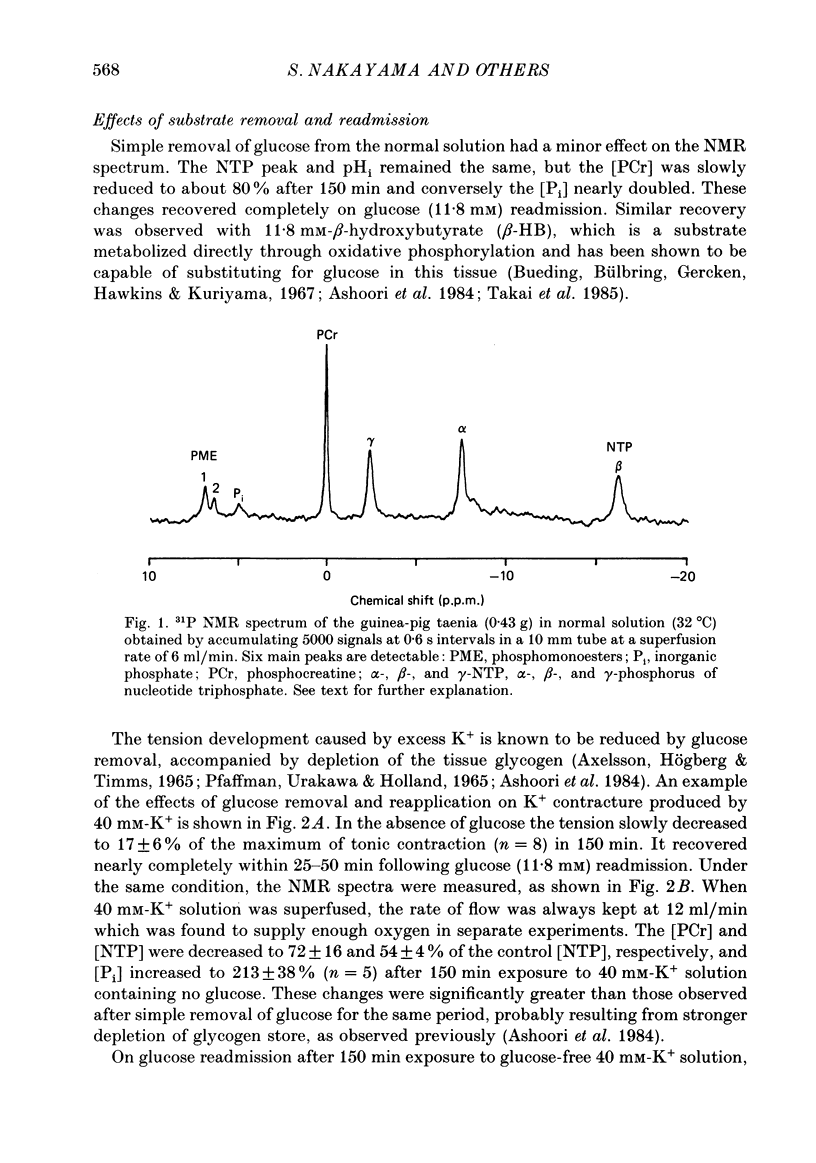
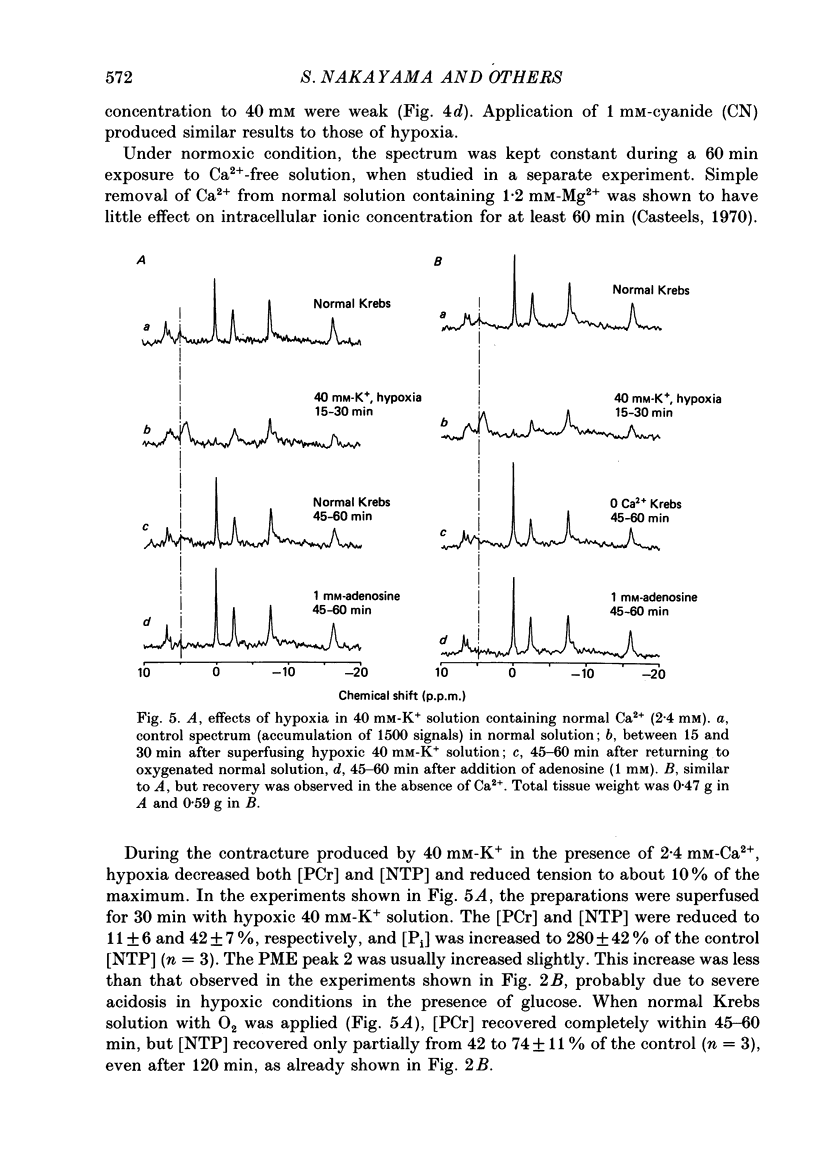
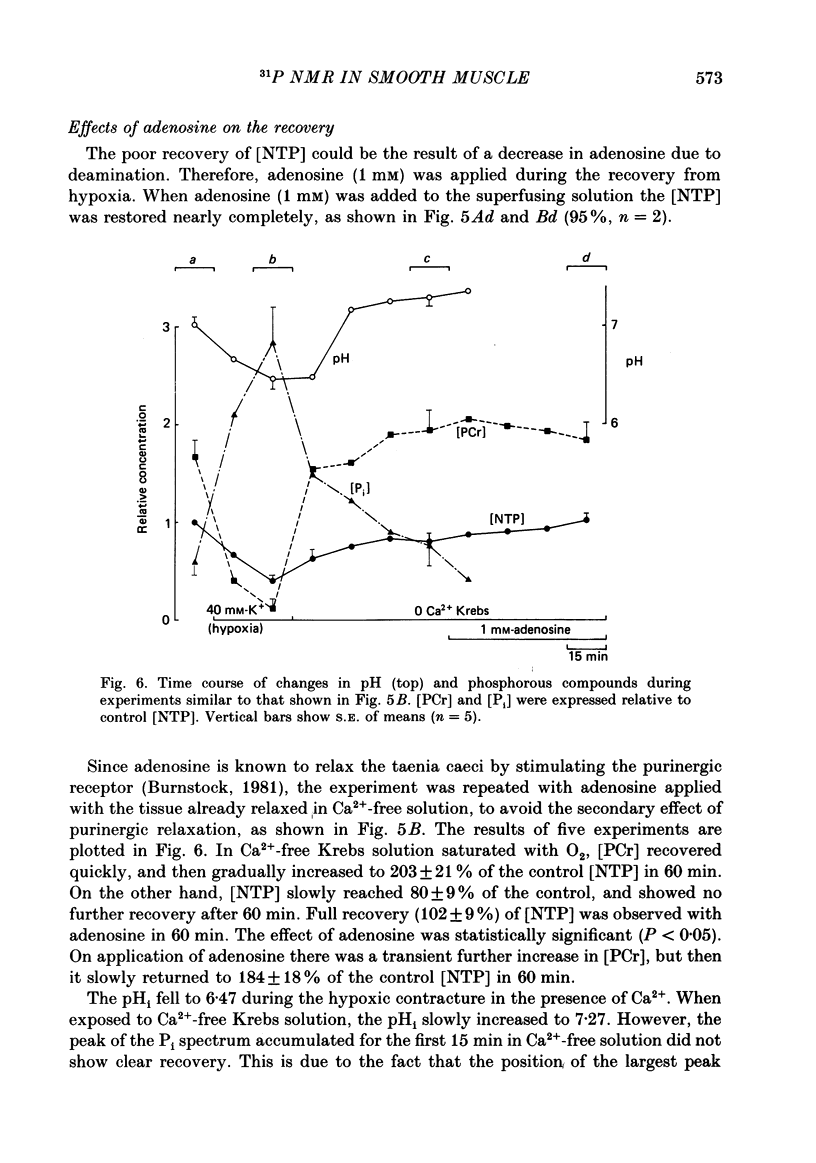
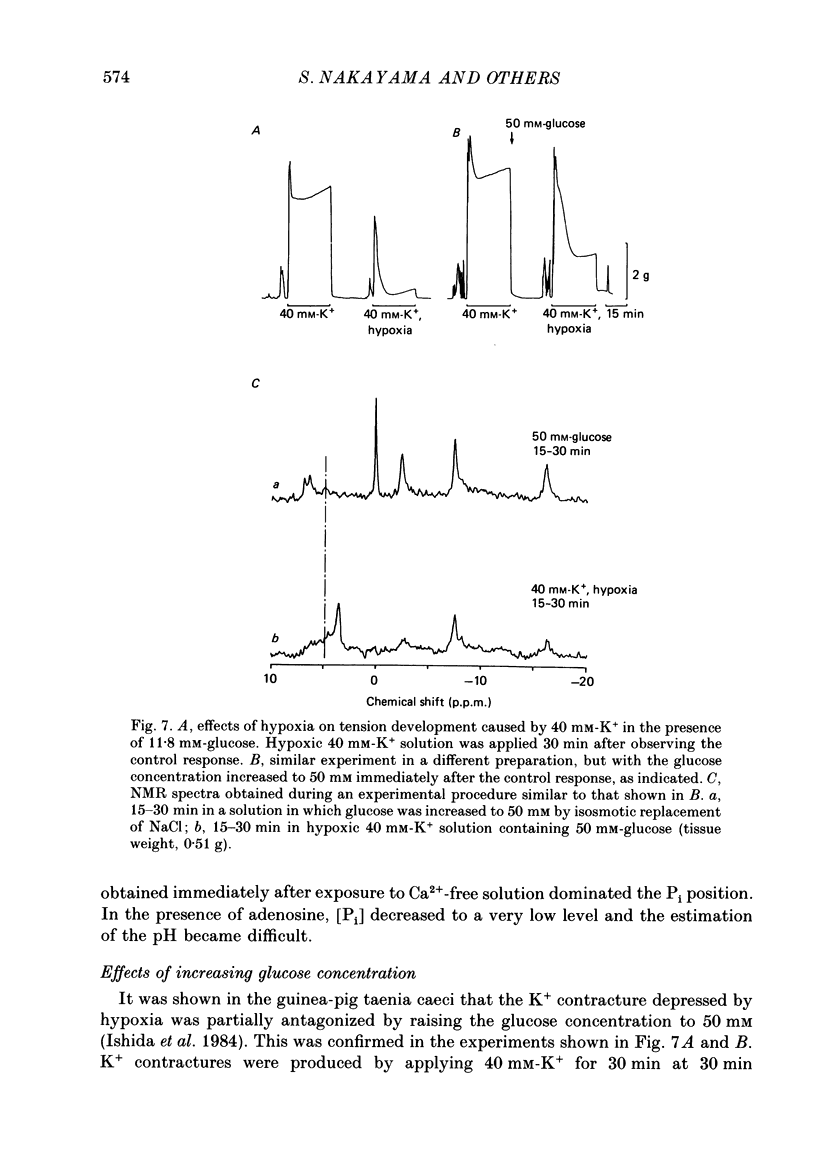
1. In the isolated taenia (0.4-0.6 g) of guinea-pig caecum, the intracellular phosphorous compounds and pH were investigated using 31P nuclear magnetic resonance (NMR) under various metabolic conditions. 2. The ratios of the intracellular concentration of phosphocreatine ([ PCr]) and inorganic phosphate [( Pi]) to nucleotide triphosphate ([ NTP]) were 1.71 +/- 0.14 and 0.58 +/- 0.11 (n = 25), respectively, in normal solution (32 degrees C). The intracellular pH estimated from the chemical shift of Pi was 7.05 +/- 0.06 (n = 25), agreeing well with those previously obtained. 3. In the absence of glucose, the [PCr] and [NTP] were decreased to almost a half after 150 min exposure to 40 mM-K+ solution, while [Pi] was increased 3-fold. These changes were much faster than the rate of decline in tension. When glucose was readmitted, the contractile response to K+ fully recovered in 50 min. However, this was accompanied with only a partial recovery of [PCr] and [Pi], but no recovery of [NTP]. The intracellular pH was lowered by about 0.2 of a unit, suggesting an increase in glycolysis. 4. In Ca2+-free solution, respiratory inhibition with hypoxia or CN (1 mM) only decreased [PCr], leaving [NTP] nearly unchanged. On the other hand, respiratory inhibition in excess-K+ solution containing Ca2+ (2.4 mM) severely depleted PCr and decreased [NTP] to 40%. Increasing glucose to 50 mM did not prevent these changes, although it increased tension development. 5. The simultaneous decrease of [NTP] and [PCr] during K+ contracture suggests that the activity of creatine phosphokinase is low. The recovery from respiratory inhibition was much better for [PCr] than for [NTP]. Slow, but perfect, recovery of all NTP peaks was produced by adding 1 mM-adenosine to normal solution. 6. It was suggested that tension development is closely related to the turnover rate of ATP, and not to its concentration, and that deamination of adenosine is a limiting factor in the recovery of ATP after excessive consumption.
Full text
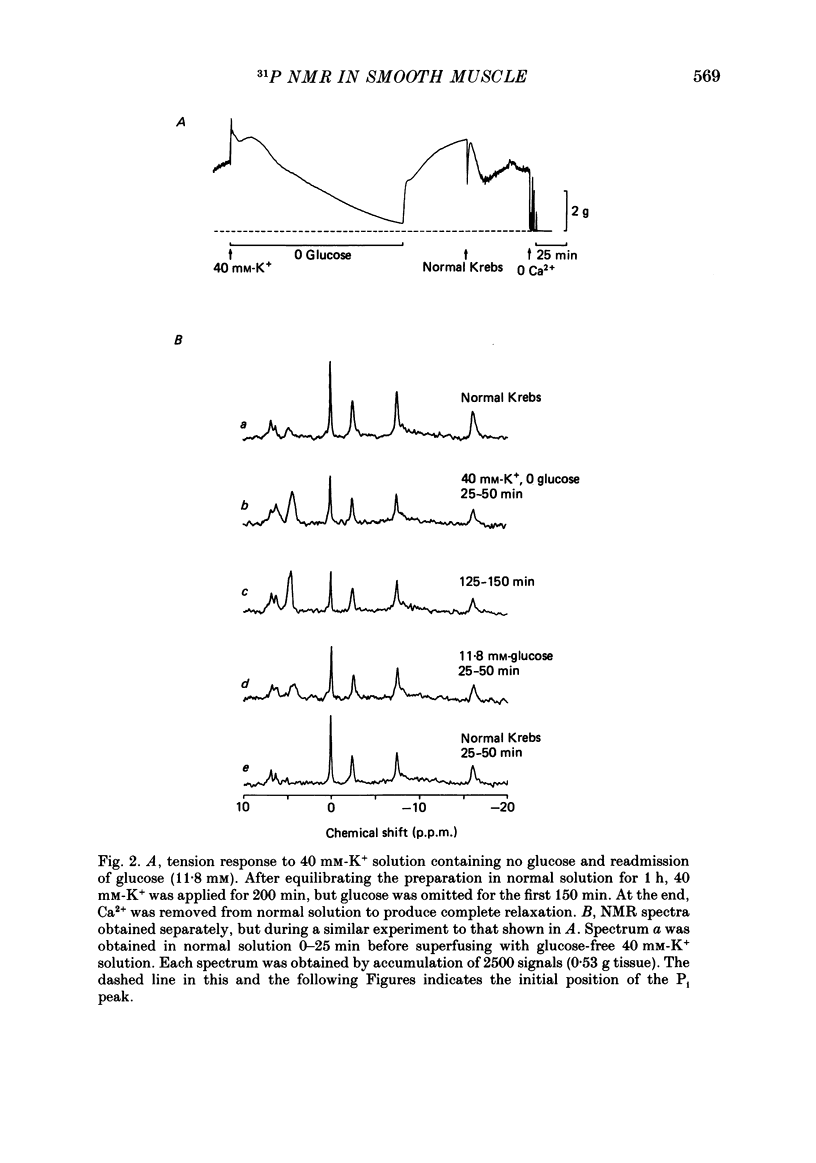
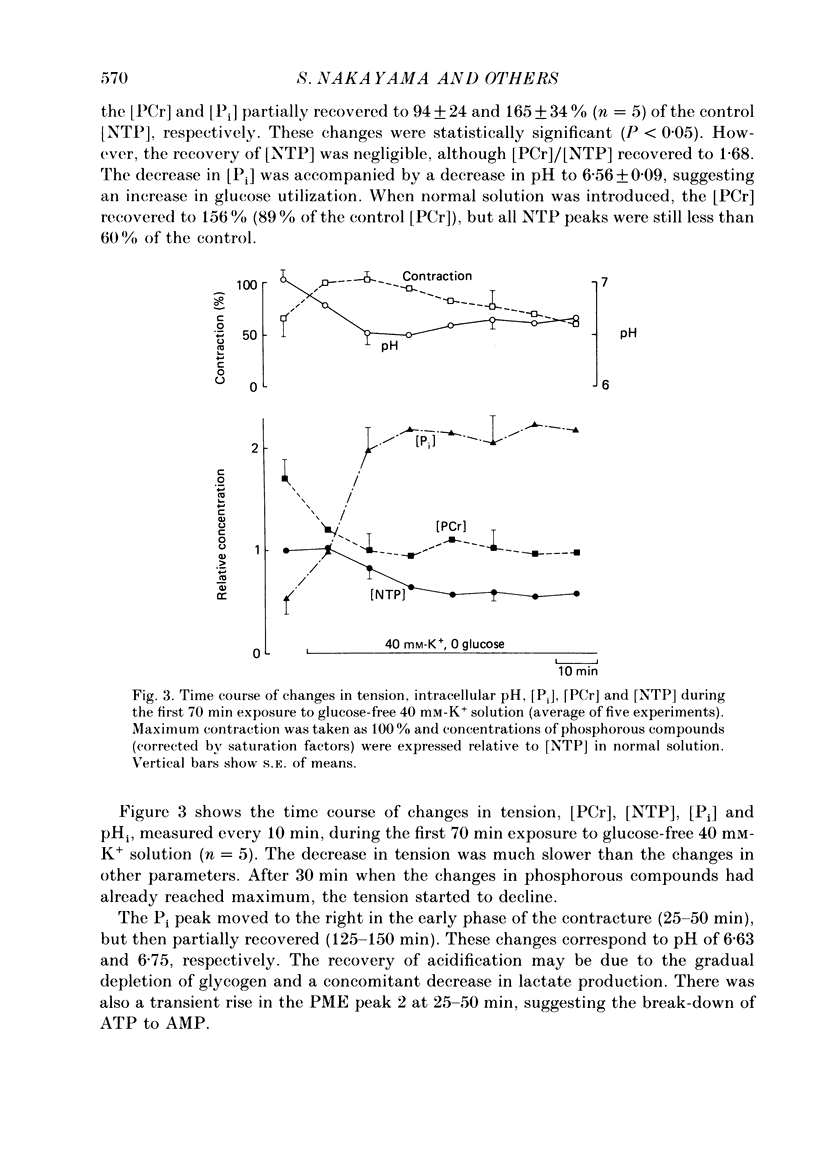
PDF


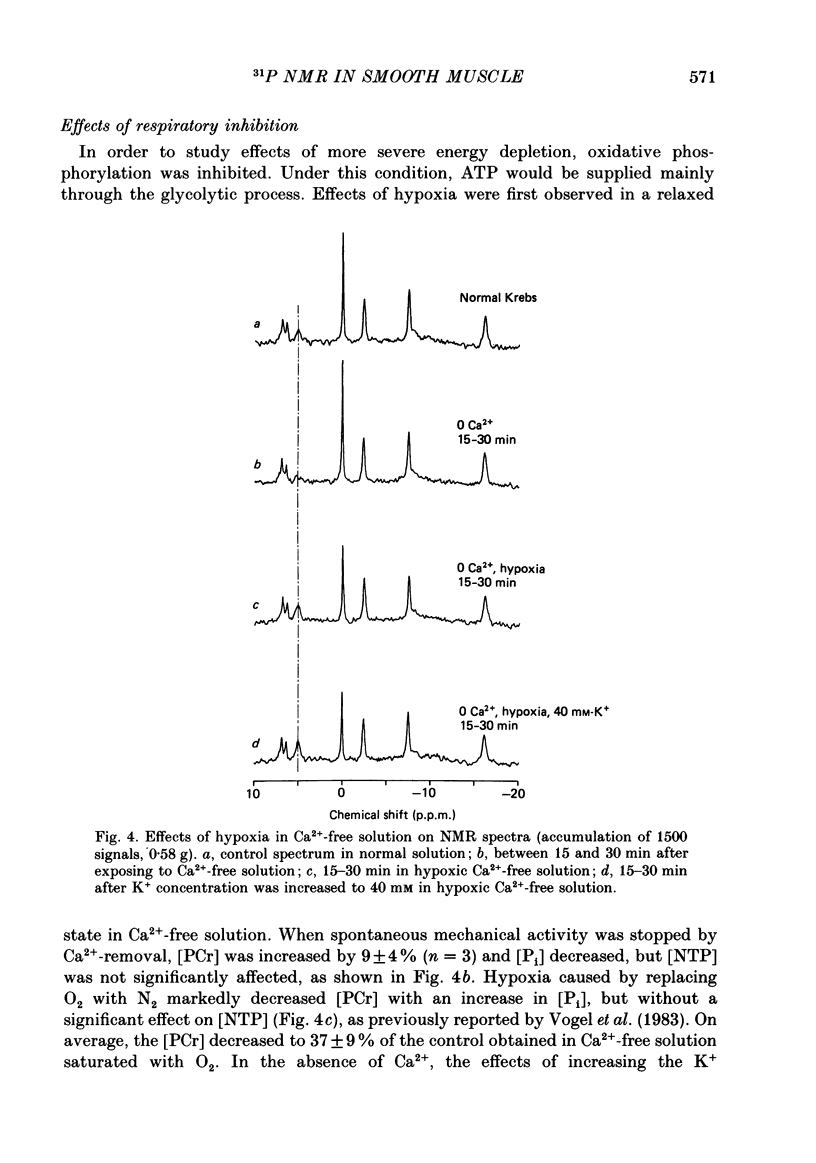




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., HOEGBERG S. G., TIMMS A. R. THE EFFECT OF REMOVING AND READMITTING GLUCOSE ON THE ELECTRICAL AND MECHANICAL ACTIVITY AND GLUCOSE AND GLYCOGEN CONTENT OF INTESTINAL SMOOTH MUSCLE FROM THE TAENIA COLI OF THE GUINEA PIG. Acta Physiol Scand. 1965 May-Jun;64:28–42. doi: 10.1111/j.1748-1716.1965.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Aickin C. C. Direct measurement of intracellular pH and buffering power in smooth muscle cells of guinea-pig vas deferens. J Physiol. 1984 Apr;349:571–585. doi: 10.1113/jphysiol.1984.sp015174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashoori F., Takai A., Tokuno H., Tomita T. Effects of glucose removal and readmission on potassium contracture in the guinea-pig taenia coli. J Physiol. 1984 Nov;356:33–48. doi: 10.1113/jphysiol.1984.sp015451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avison M. J., Hetherington H. P., Shulman R. G. Applications of NMR to studies of tissue metabolism. Annu Rev Biophys Biophys Chem. 1986;15:377–402. doi: 10.1146/annurev.bb.15.060186.002113. [DOI] [PubMed] [Google Scholar]
- Bueding E., Bülbring E., Gercken G., Hawkins J. T., Kuriyama H. The effect of adrenaline on the adenosine otriphosphate and creatine phosphate content of intestinal smooth muscle. J Physiol. 1967 Nov;193(1):187–212. doi: 10.1113/jphysiol.1967.sp008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T. M., Siegman M. J. High-energy phosphate metabolism in vascular smooth muscle. Annu Rev Physiol. 1985;47:629–643. doi: 10.1146/annurev.ph.47.030185.003213. [DOI] [PubMed] [Google Scholar]
- Carlier P. G., Grandjean J., Michel P., D'Orio V., Rorive G. L. Arterial metabolism as studied in vitro by NMR: preliminary results in normotensive and hypertensive aortas. Arch Int Physiol Biochim. 1985 Dec;93(5):107–118. doi: 10.3109/13813458509080631. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977 Jun;267(3):703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Wray S. The effects of pregnancy and parturition on phosphorus metabolites in rat uterus studied by 31P nuclear magnetic resonance. J Physiol. 1985 Nov;368:19–31. doi: 10.1113/jphysiol.1985.sp015844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degani H., Shaer A., Victor T. A., Kaye A. M. Estrogen-induced changes in high-energy phosphate metabolism in rat uterus: 31P NMR studies. Biochemistry. 1984 Jun 5;23(12):2572–2577. doi: 10.1021/bi00307a006. [DOI] [PubMed] [Google Scholar]
- Gadian D. G. Whole organ metabolism studied by NMR. Annu Rev Biophys Bioeng. 1983;12:69–89. doi: 10.1146/annurev.bb.12.060183.000441. [DOI] [PubMed] [Google Scholar]
- Gagelmann M., Güth K. Effect of inorganic phosphate on the Ca2+ sensitivity in skinned Taenia coli smooth muscle fibers. Comparison of tension, ATPase activity, and phosphorylation of the regulatory myosin light chains. Biophys J. 1987 Mar;51(3):457–463. doi: 10.1016/S0006-3495(87)83367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Gupta P., Moore R. D. NMR studies of intracellular metal ions in intact cells and tissues. Annu Rev Biophys Bioeng. 1984;13:221–246. doi: 10.1146/annurev.bb.13.060184.001253. [DOI] [PubMed] [Google Scholar]
- Hellstrand P., Vogel H. J. Phosphagens and intracellular pH in intact rabbit smooth muscle studied by 31P-NMR. Am J Physiol. 1985 Mar;248(3 Pt 1):C320–C329. doi: 10.1152/ajpcell.1985.248.3.C320. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Takagi K., Urakawa N. Tension maintenance, calcium content and energy production of the taenia of the guinea-pig caecum under hypoxia. J Physiol. 1984 Feb;347:149–159. doi: 10.1113/jphysiol.1984.sp015058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. Inorganic phosphate regulates the contraction-relaxation cycle in skinned muscles of the rabbit mesenteric artery. J Physiol. 1986 Jul;376:231–252. doi: 10.1113/jphysiol.1986.sp016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Dillon P. F., Meyer R. A., Brown T. R., Krisanda J. M., Sweeney H. L. 31P NMR spectroscopy, chemical analysis, and free Mg2+ of rabbit bladder and uterine smooth muscle. J Biol Chem. 1986 Nov 5;261(31):14420–14429. [PubMed] [Google Scholar]
- Meyer R. A., Brown T. R., Kushmerick M. J. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am J Physiol. 1985 Mar;248(3 Pt 1):C279–C287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Terjung R. L. AMP deamination and IMP reamination in working skeletal muscle. Am J Physiol. 1980 Jul;239(1):C32–C38. doi: 10.1152/ajpcell.1980.239.1.C32. [DOI] [PubMed] [Google Scholar]
- PFAFFMAN M., URAKAWA N., HOLLAND W. C. ROLE OF METABOLISM IN K-INDUCED TENSION CHANGES IN GUINEA PIG TAENIA COLI. Am J Physiol. 1965 Jun;208:1203–1205. doi: 10.1152/ajplegacy.1965.208.6.1203. [DOI] [PubMed] [Google Scholar]
- Seo Y., Murakami M., Watari H., Imai Y., Yoshizaki K., Nishikawa H., Morimoto T. Intracellular pH determination by a 31P-NMR technique. The second dissociation constant of phosphoric acid in a biological system. J Biochem. 1983 Sep;94(3):729–734. doi: 10.1093/oxfordjournals.jbchem.a134413. [DOI] [PubMed] [Google Scholar]
- Takai A., Tokuno H., Tomita T. Effects of readmission of substrate on the membrane potential in glycogen-depleted guinea-pig taenia coli. J Physiol. 1985 May;362:39–50. doi: 10.1113/jphysiol.1985.sp015661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermuë N. A., Nicolay K. Energetics of smooth muscle taenia caecum of guinea-pig: a 31P-NMR study. FEBS Lett. 1983 Jun 13;156(2):293–297. doi: 10.1016/0014-5793(83)80515-8. [DOI] [PubMed] [Google Scholar]
- Vogel H. J., Lilja H., Hellstrand P. Phosphorus-31 NMR studies of smooth muscle from guinea-pig taenia coli. Biosci Rep. 1983 Sep;3(9):863–870. doi: 10.1007/BF01133785. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K., Radda G. K., Inubushi T., Chance B. 1H- and 31P-NMR studies on smooth muscle of bullfrog stomach. Biochim Biophys Acta. 1987 Apr 2;928(1):36–44. doi: 10.1016/0167-4889(87)90083-8. [DOI] [PubMed] [Google Scholar]


