Abstract
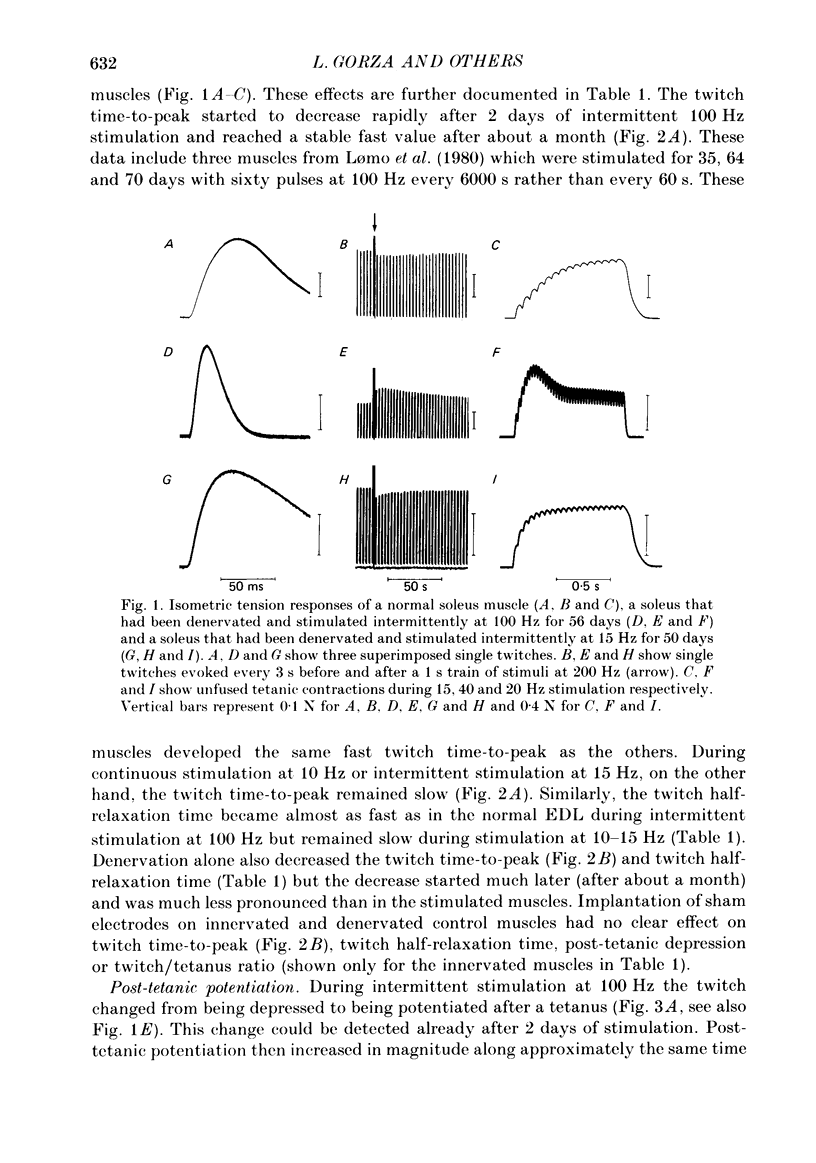
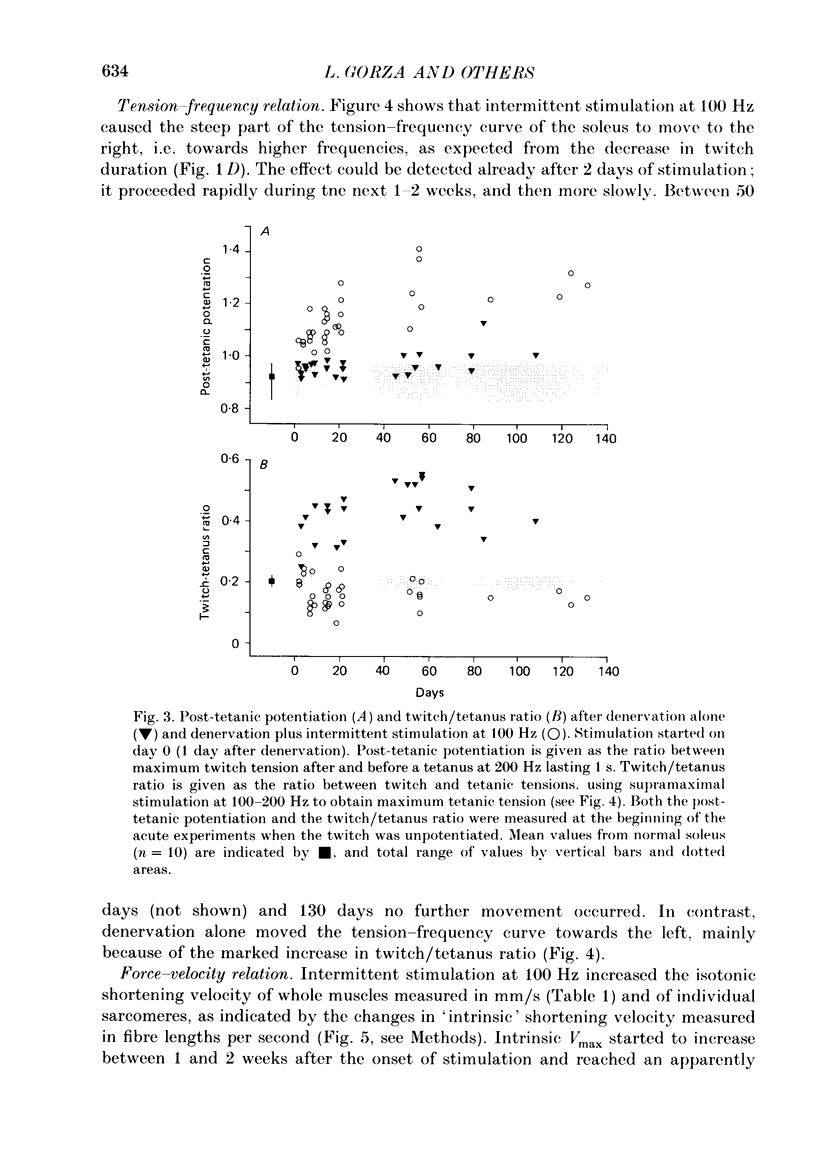
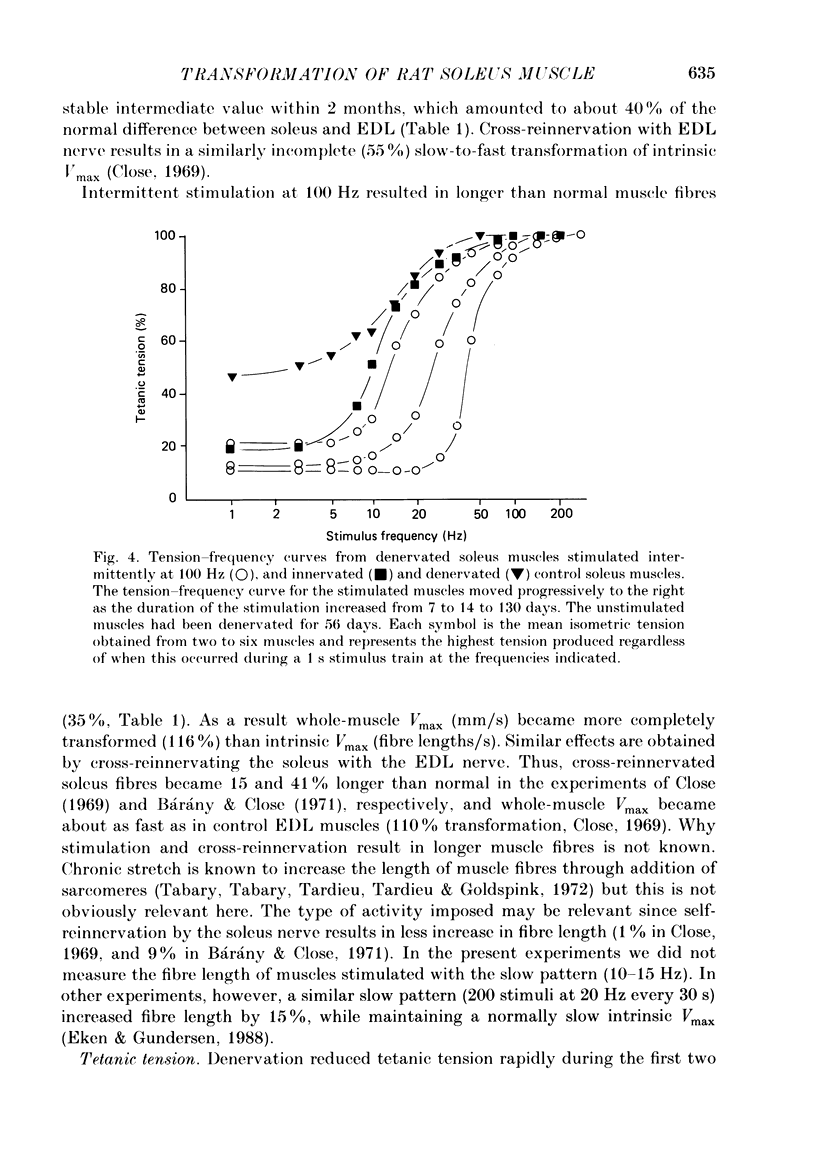
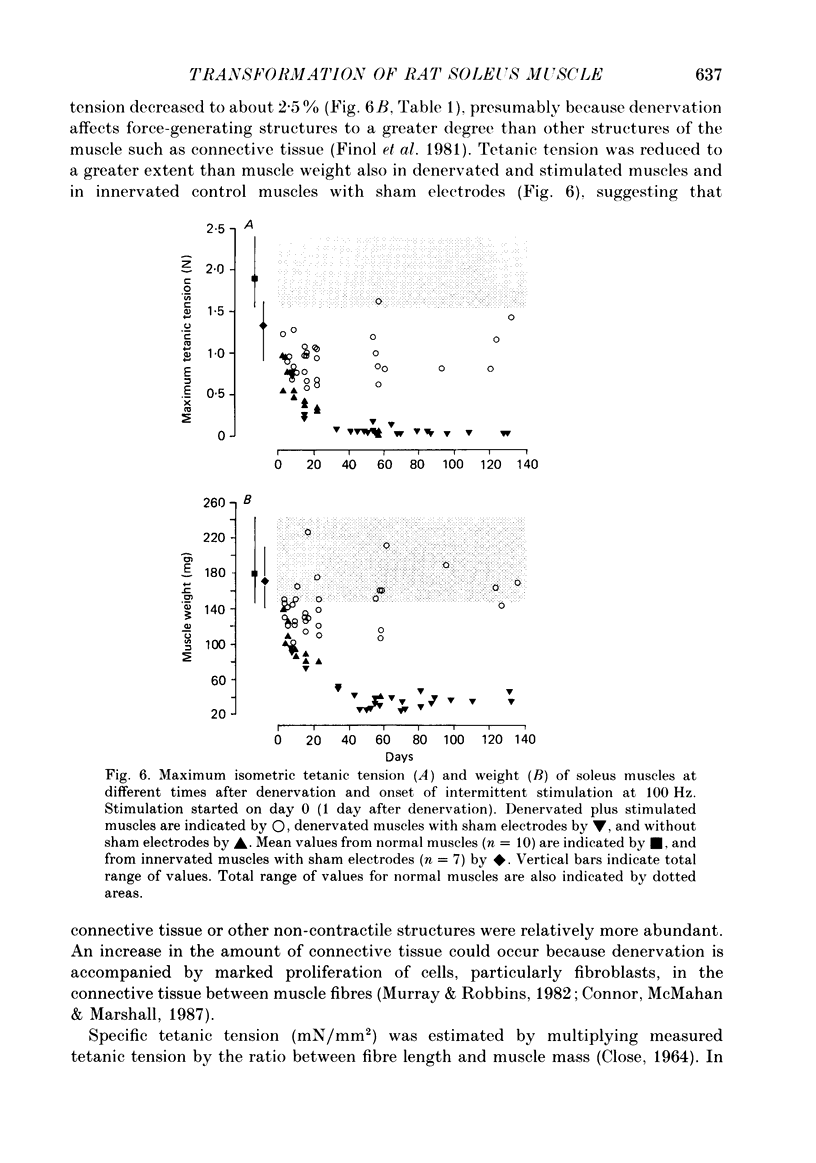
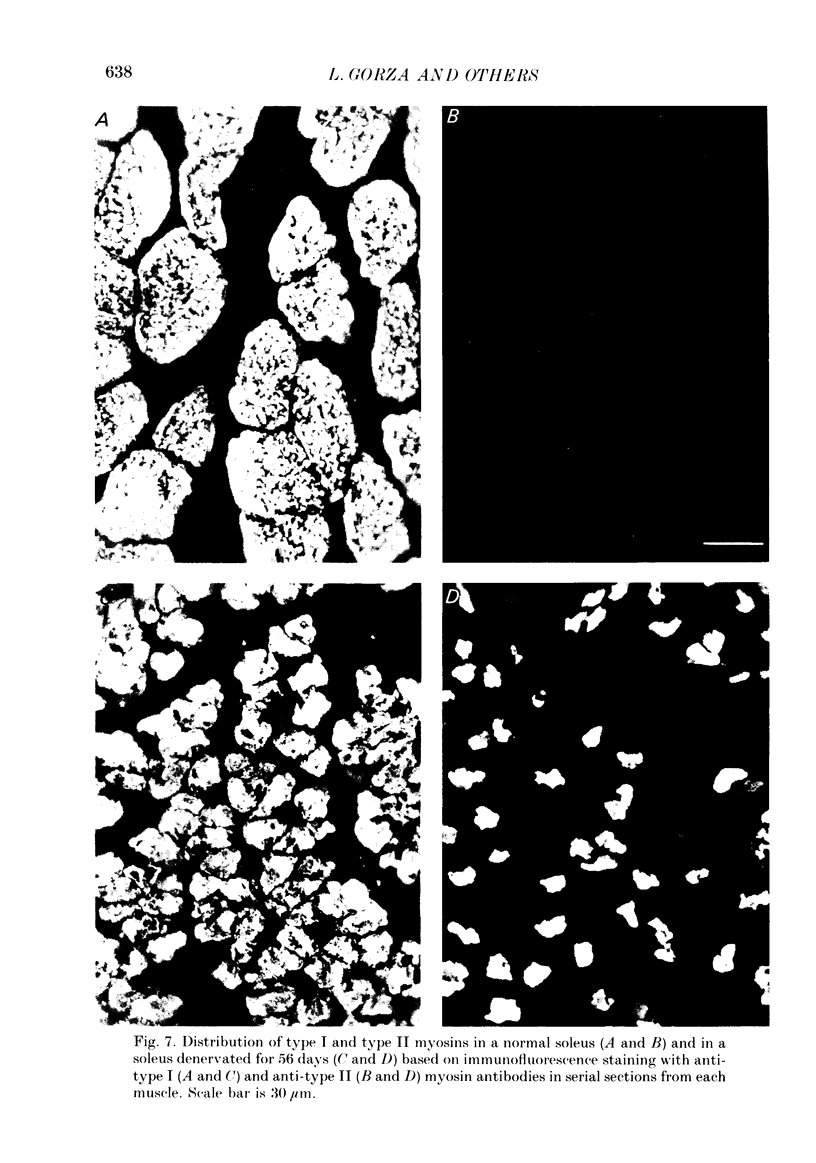
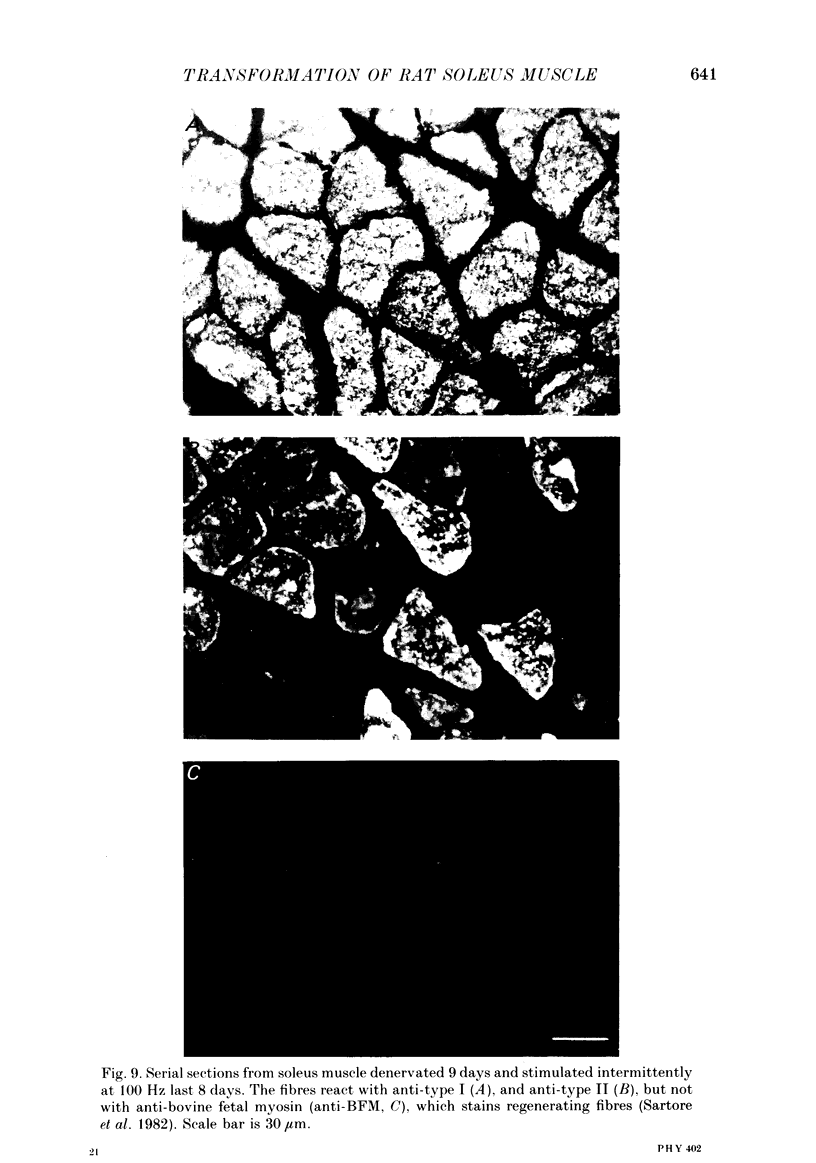
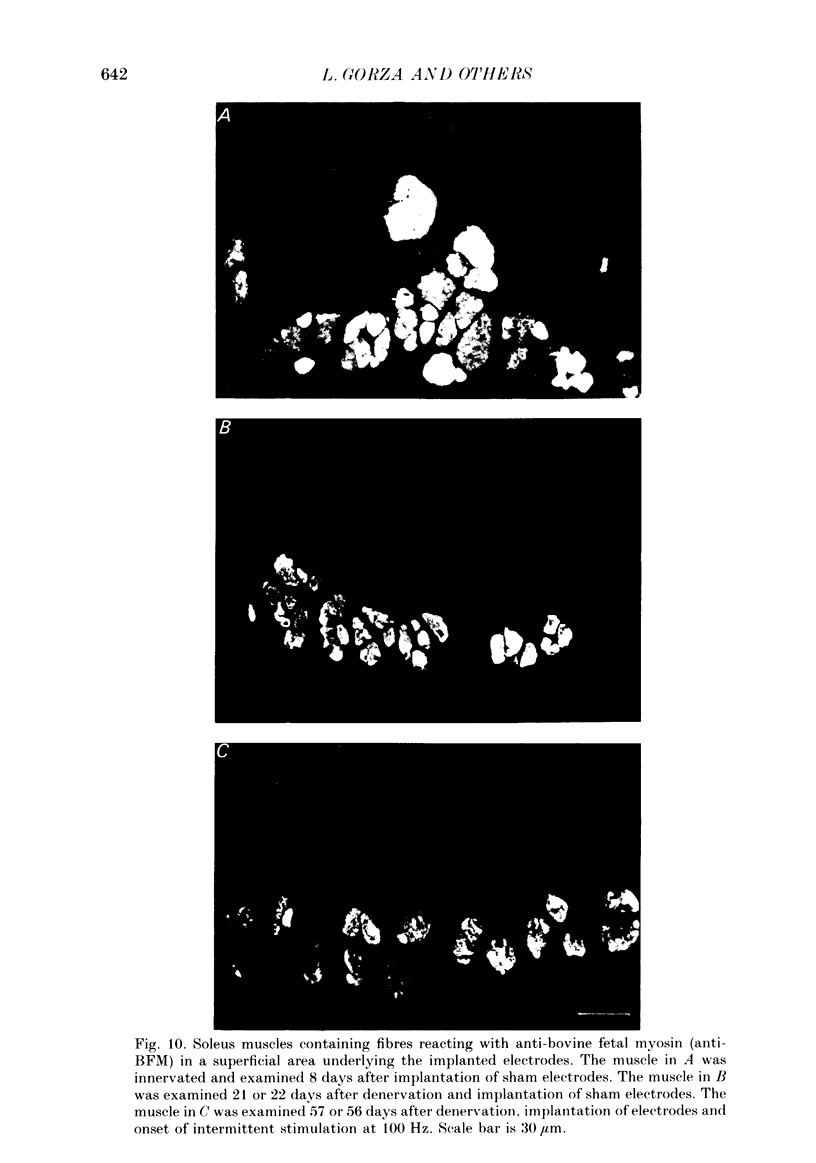
1. Adult soleus muscles were denervated and stimulated directly for 2-130 days with 'fast' (short pulse trains at 100 Hz) or 'slow' (continuously at 10 Hz, or long pulse trains at 15 Hz) stimulus patterns. 2. At the end of the period of stimulation isometric twitches and tetani and isotonic shortening velocities were measured. Frozen cross-sections were later examined with antibodies against myosin heavy chains specific for adult fast, adult slow and fetal myosin. 3. Isometric twitch duration (twitch time-to-peak and half-relaxation time) decreased during intermittent 100 Hz stimulation to values that were almost as fast as in the normal extensor digitorum longus (EDL) (95 and 94% transformation). The major part of the decrease occurred between 2 and 21 days after the onset of stimulation, and was accompanied by post-tetanic potentiation of the twitch, 'sag' in tension during an unfused tetanus, lower twitch/tetanus ratio and marked shifts to the right (higher frequencies) of the tension-frequency curve of the muscle. In contrast, during 10 or 15 Hz stimulation the isometric twitch duration remained slow, the twitch continued to show post-tetanic depression and absence of 'sag', while the twitch/tetanus ratio increased. 4. Denervation per se led to a slight increase and, then, after about a month, to a moderate and gradual decrease in twitch duration. The twitch/tetanus ratio increased markedly and post-tetanic depression became less pronounced or disappeared. Muscle weight and particularly tetanic tension were markedly reduced and these reductions were to a large extent counteracted by electrical stimulation. 5. Implantation of sham electrodes had no effect on twitch duration of denervated or innervated control muscles, but reduced tetanic tension in the innervated control muscles. 6. Maximum isotonic shortening velocity of the whole muscle (mm/s) increased during intermittent 100 Hz stimulation to a value as fast as in the normal EDL (110% transformation). Since the muscle fibres also increased in length (35%) maximum intrinsic shortening velocity (fibre lengths/s) was only incompletely transformed (55%). The increase in Vmax occurred between 7 and 14 days after the onset of stimulation. 7. All the fibres stimulated intermittently at 100 Hz were strongly labelled with anti-fast myosin and more than 90% were in addition weakly labelled by anti-slow myosin. Weak and variable labelling with anti-fast myosin was first detected 7 days after the onset of stimulation. In contrast, essentially all the fibres stimulated at 10 or 15 Hz showed no binding of anti-fast but strong binding of anti-slow myosin.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Amood W. S., Finol H. J., Lewis D. M. Chronic stimulation modifies the isotonic shortening velocity of denervated rat slow-twitch muscle. Proc R Soc Lond B Biol Sci. 1986 Jun 23;228(1250):43–58. doi: 10.1098/rspb.1986.0039. [DOI] [PubMed] [Google Scholar]
- Al-Amood W. S., Lewis D. M. The role of frequency in the effects of long-term intermittent stimulation of denervated slow-twitch muscle in the rat. J Physiol. 1987 Nov;392:377–395. doi: 10.1113/jphysiol.1987.sp016786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER A. J., ECCLES J. C., ECCLES R. M. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960 Feb;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M., Close R. I. The transformation of myosin in cross-innervated rat muscles. J Physiol. 1971 Mar;213(2):455–474. doi: 10.1113/jphysiol.1971.sp009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Dynamic properties of fast and slow skeletal muscles of the rat after nerve cross-union. J Physiol. 1969 Oct;204(2):331–346. doi: 10.1113/jphysiol.1969.sp008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor E. A., McMahan U. J. Cell accumulation in the junctional region of denervated muscle. J Cell Biol. 1987 Jan;104(1):109–120. doi: 10.1083/jcb.104.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Eerbeek O., Kernell D., Verhey B. A. Effects of fast and slow patterns of tonic long-term stimulation on contractile properties of fast muscle in the cat. J Physiol. 1984 Jul;352:73–90. doi: 10.1113/jphysiol.1984.sp015278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eken T., Gundersen K. Electrical stimulation resembling normal motor-unit activity: effects on denervated fast and slow rat muscles. J Physiol. 1988 Aug;402:651–669. doi: 10.1113/jphysiol.1988.sp017227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finol H. J., Lewis D. M., Owens R. The effects of denervation on contractile properties or rat skeletal muscle. J Physiol. 1981;319:81–92. doi: 10.1113/jphysiol.1981.sp013893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Robbins N. Changes in contractile properties of disused soleus muscles. J Physiol. 1969 Apr;201(2):305–320. doi: 10.1113/jphysiol.1969.sp008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen K., Leberer E., Lømo T., Pette D., Staron R. S. Fibre types, calcium-sequestering proteins and metabolic enzymes in denervated and chronically stimulated muscles of the rat. J Physiol. 1988 Apr;398:177–189. doi: 10.1113/jphysiol.1988.sp017037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E., Carlson B. M. Contractile and histochemical properties of regenerating cross-transplanted fast and slow muscles in the rat. Pflugers Arch. 1975;353(3):227–239. doi: 10.1007/BF00584286. [DOI] [PubMed] [Google Scholar]
- Hennig R., Lømo T. Firing patterns of motor units in normal rats. Nature. 1985 Mar 14;314(6007):164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Hudlická O., Tyler K. R., Srihari T., Heilig A., Pette D. The effect of different patterns of long-term stimulation on contractile properties and myosin light chains in rabbit fast muscles. Pflugers Arch. 1982 Apr;393(2):164–170. doi: 10.1007/BF00582940. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Jaweed M. M., Herbison G. J., Ditunno J. F. Denervation and reinnervation of fast and slow muscles. A histochemical study in rats. J Histochem Cytochem. 1975 Nov;23(11):808–827. doi: 10.1177/23.11.127809. [DOI] [PubMed] [Google Scholar]
- Jolesz F., Sreter F. A. Development, innervation, and activity-pattern induced changes in skeletal muscle. Annu Rev Physiol. 1981;43:531–552. doi: 10.1146/annurev.ph.43.030181.002531. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D., Eerbeek O., Verhey B. A., Donselaar Y. Effects of physiological amounts of high- and low-rate chronic stimulation on fast-twitch muscle of the cat hindlimb. I. Speed- and force-related properties. J Neurophysiol. 1987 Sep;58(3):598–613. doi: 10.1152/jn.1987.58.3.598. [DOI] [PubMed] [Google Scholar]
- Kernell D. Rhythmic properties of motoneurones innervating muscle fibres of different speed in m. gastrocnemius medialis of the cat. Brain Res. 1979 Jan 5;160(1):159–162. doi: 10.1016/0006-8993(79)90612-7. [DOI] [PubMed] [Google Scholar]
- Klug G. A., Leberer E., Leisner E., Simoneau J. A., Pette D. Relationship between parvalbumin content and the speed of relaxation in chronically stimulated rabbit fast-twitch muscle. Pflugers Arch. 1988 Feb;411(2):126–131. doi: 10.1007/BF00582304. [DOI] [PubMed] [Google Scholar]
- Leberer E., Härtner K. T., Pette D. Reversible inhibition of sarcoplasmic reticulum Ca-ATPase by altered neuromuscular activity in rabbit fast-twitch muscle. Eur J Biochem. 1987 Feb 2;162(3):555–561. doi: 10.1111/j.1432-1033.1987.tb10675.x. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H., Dahl H. A. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- Murray M. A., Robbins N. Cell proliferation in denervated muscle: time course, distribution and relation to disuse. Neuroscience. 1982 Jul;7(7):1817–1822. doi: 10.1016/0306-4522(82)90039-2. [DOI] [PubMed] [Google Scholar]
- Pette D., Smith M. E., Staudte H. W., Vrbová G. Effects of long-term electrical stimulation on some contractile and metabolic characteristics of fast rabbit muscles. Pflugers Arch. 1973 Feb 6;338(3):257–272. doi: 10.1007/BF00587391. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985 Aug 5;260(16):9077–9080. [PubMed] [Google Scholar]
- Salmons S., Sréter F. A. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976 Sep 2;263(5572):30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Salmons S., Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969 May;201(3):535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore S., Gorza L., Schiaffino S. Fetal myosin heavy chains in regenerating muscle. Nature. 1982 Jul 15;298(5871):294–296. doi: 10.1038/298294a0. [DOI] [PubMed] [Google Scholar]
- Spector S. A. Effects of elimination of activity on contractile and histochemical properties of rat soleus muscle. J Neurosci. 1985 Aug;5(8):2177–2188. doi: 10.1523/JNEUROSCI.05-08-02177.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreter F. A., Pinter K., Jolesz F., Mabuchi K. Fast to slow transformation of fast muscles in response to long-term phasic stimulation. Exp Neurol. 1982 Jan;75(1):95–102. doi: 10.1016/0014-4886(82)90009-7. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabary J. C., Tabary C., Tardieu C., Tardieu G., Goldspink G. Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. J Physiol. 1972 Jul;224(1):231–244. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M., Butler-Browne G. S., Schwartz K., Bouveret P., Pinset-Härstöm I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1981 Aug 27;292(5826):805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]






