Abstract
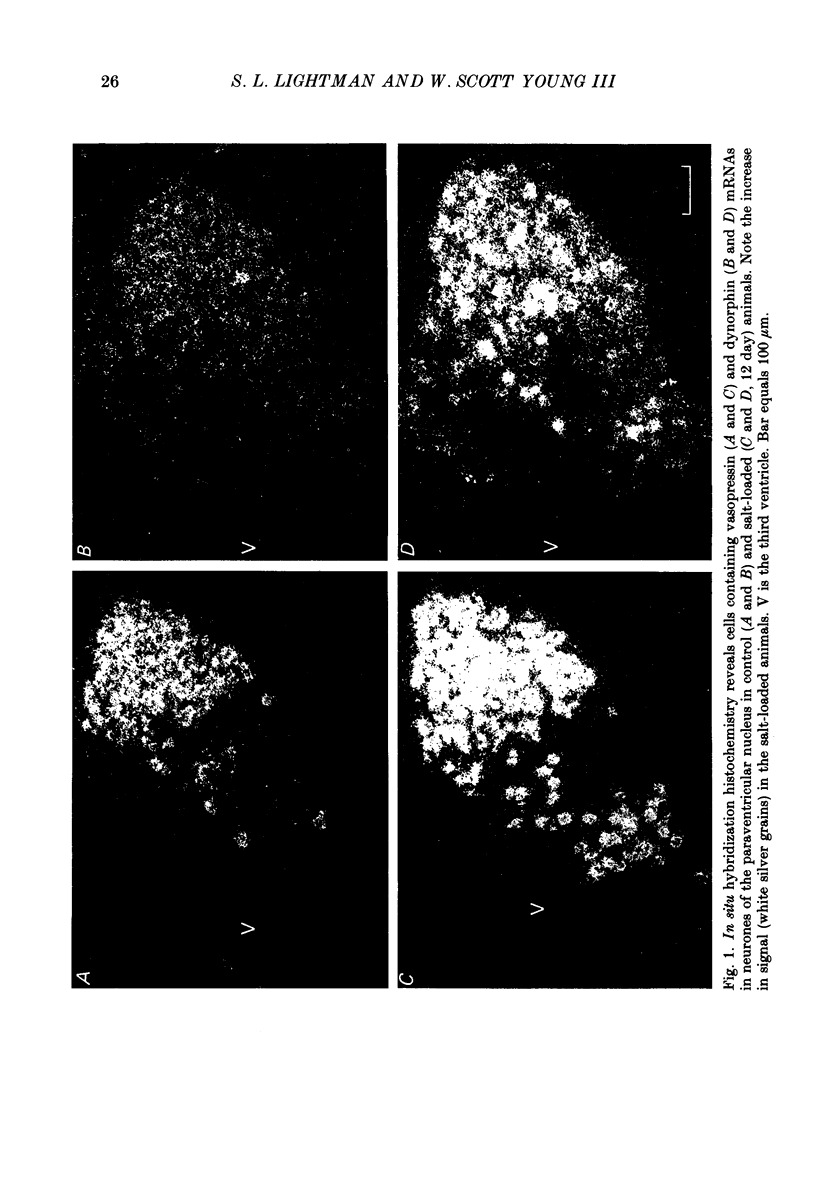
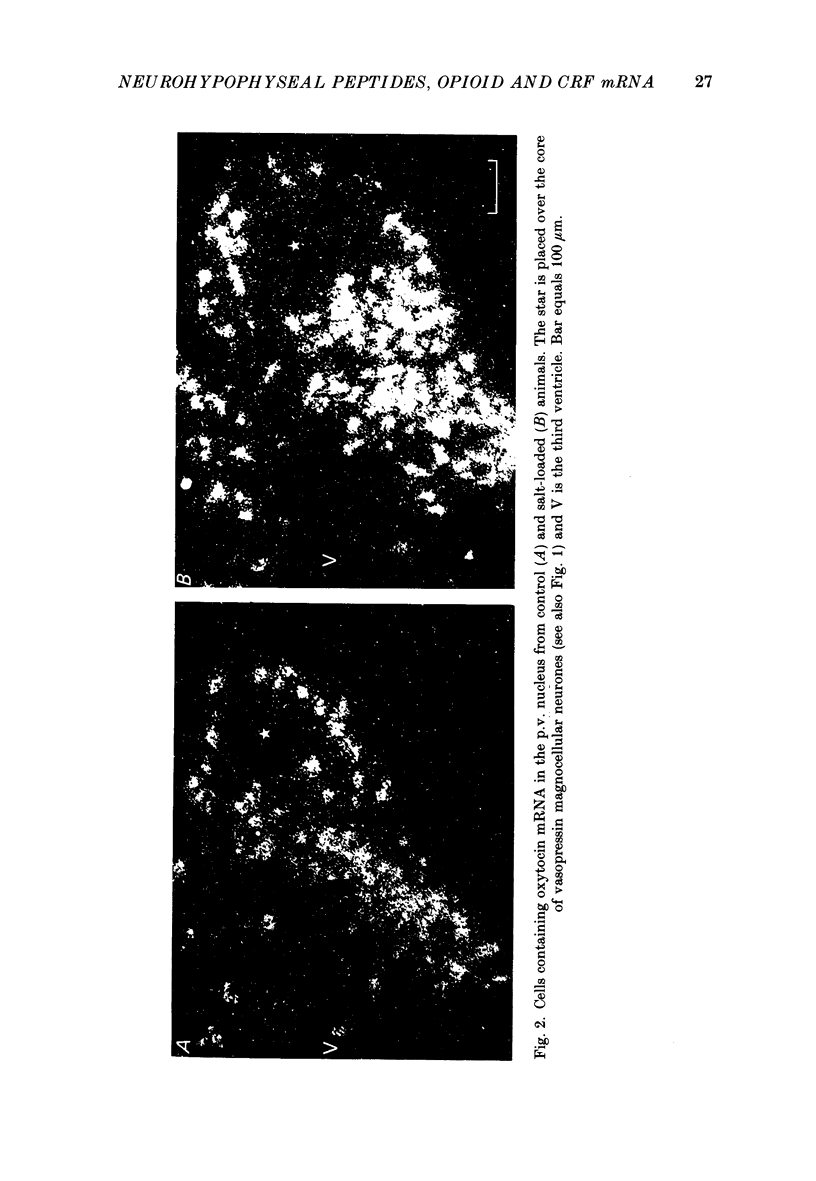
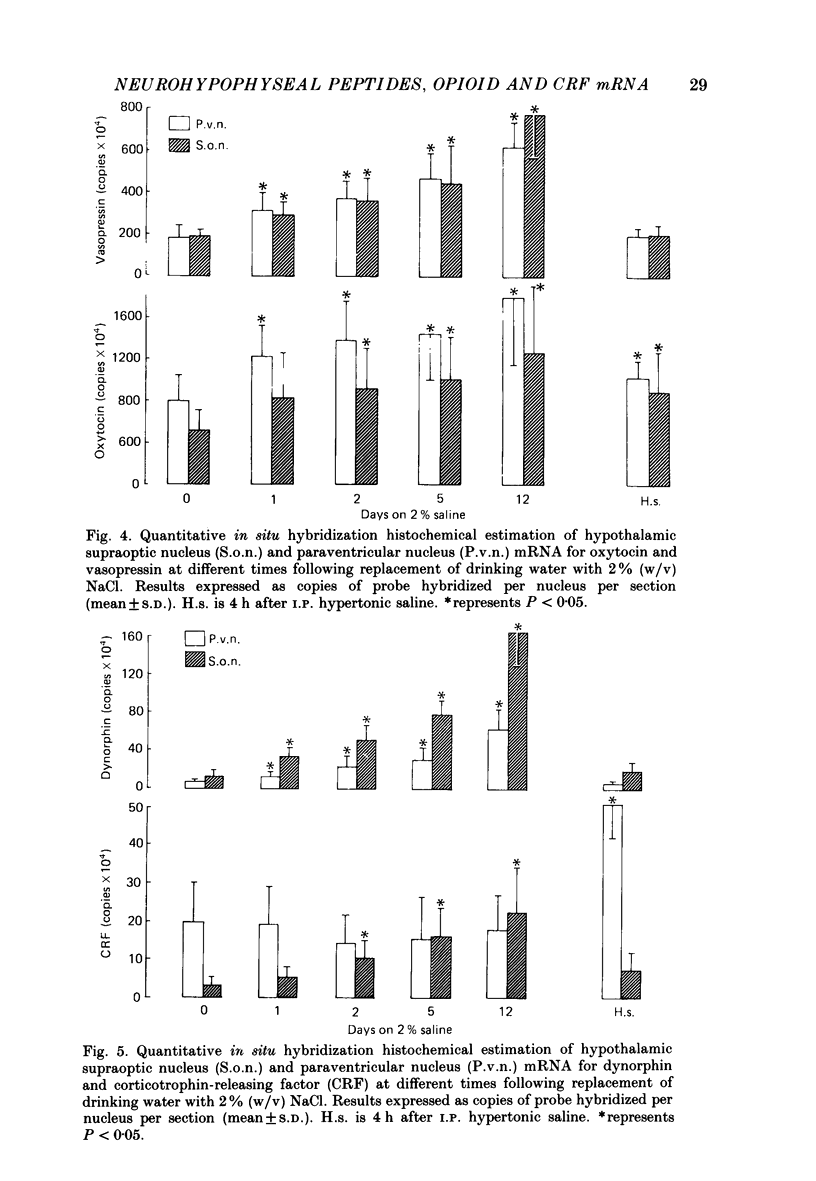
1. Cryostat sections were cut through the hypothalamus of rats which had been given a 2% (w/v) NaCl solution to drink for up to 12 days. 2. In situ hybridization histochemistry was performed on these sections using synthetic oligonucleotide probes against part of the precursor sequence for vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor (CRF). 3. Drinking 2% NaCl solution resulted in a progressive increase of vasopressin, oxytocin and dynorphin mRNAs hybridized in the magnocellular neurones of the supraoptic (s.o.) and paraventricular (p.v.) nuclei. No enkephalin mRNA was detected in the magnocellular areas of the control animals although small quantities of probe did hybridize after 12 days of salt loading and after the stress of I.P. hypertonic saline. 4. Ten-day-lactating female rats were also studied. They had a very marked increase in oxytocin mRNA with smaller increases of vasopressin and dynorphin mRNAs. No detectable enkephalin mRNA was hybridized in the magnocellular s.o. or p.v. nuclei and CRF mRNA was unchanged in both the s.o. nucleus and the p.v. nucleus.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnauld E., Cirino M., Layton B. S., Renaud L. P. Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. A microiontophoretic study in the rat. Neuroendocrinology. 1983;36(3):187–196. doi: 10.1159/000123455. [DOI] [PubMed] [Google Scholar]
- Atweh S. F., Kuhar M. J. Distribution and physiological significance of opioid receptors in the brain. Br Med Bull. 1983 Jan;39(1):47–52. doi: 10.1093/oxfordjournals.bmb.a071789. [DOI] [PubMed] [Google Scholar]
- Balment R. J., Brimble M. J., Forsling M. L., Kelly L. P., Musabayane C. T. A synergistic effect of oxytocin and vasopressin on sodium excretion in the neurohypophysectomized rat. J Physiol. 1986 Dec;381:453–464. doi: 10.1113/jphysiol.1986.sp016338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balment R. J., Brimble M. J., Forsling M. L. Release of oxytocin induced by salt loading and its influence on renal excretion in the male rat. J Physiol. 1980 Nov;308:439–449. doi: 10.1113/jphysiol.1980.sp013481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R. J., Leng G. Endogenous opiates regulate oxytocin but not vasopressin secretion from the neurohypophysis. Nature. 1982 Jul 8;298(5870):161–162. doi: 10.1038/298161a0. [DOI] [PubMed] [Google Scholar]
- Bie P. Osmoreceptors, vasopressin, and control of renal water excretion. Physiol Rev. 1980 Oct;60(4):961–1048. doi: 10.1152/physrev.1980.60.4.961. [DOI] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol. 1977 Sep;271(1):253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn T. O., Sutton S. W., Plotsky P. M., Vale W. W. Central administration of corticotropin-releasing factor modulates oxytocin secretion in the rat. Endocrinology. 1986 Oct;119(4):1558–1563. doi: 10.1210/endo-119-4-1558. [DOI] [PubMed] [Google Scholar]
- Bunn S. J., Hanley M. R., Wilkin G. P. Evidence for a kappa-opioid receptor on pituitary astrocytes: an autoradiographic study. Neurosci Lett. 1985 Apr 19;55(3):317–323. doi: 10.1016/0304-3940(85)90455-0. [DOI] [PubMed] [Google Scholar]
- Burbach J. P., De Hoop M. J., Schmale H., Richter D., De Kloet E. R., Ten Haaf J. A., De Wied D. Differential responses to osmotic stress of vasopressin-neurophysin mRNA in hypothalamic nuclei. Neuroendocrinology. 1984 Dec;39(6):582–584. doi: 10.1159/000124040. [DOI] [PubMed] [Google Scholar]
- Burbach J. P., Van Tol H. H., Bakkus M. H., Schmale H., Ivell R. Quantitation of vasopressin mRNA and oxytocin mRNA in hypothalamic nuclei by solution hybridization assays. J Neurochem. 1986 Dec;47(6):1814–1821. doi: 10.1111/j.1471-4159.1986.tb13093.x. [DOI] [PubMed] [Google Scholar]
- Burlet A., Tonon M. C., Tankosic P., Coy D., Vaudry H. Comparative immunocytochemical localization of corticotropin releasing factor (CRF-41) and neurohypophysial peptides in the brain of Brattleboro and Long-Evans rats. Neuroendocrinology. 1983 Jul;37(1):64–72. doi: 10.1159/000123517. [DOI] [PubMed] [Google Scholar]
- Carter D. A., Williams T. D., Lightman S. L. A sex difference in endogenous opioid regulation of the posterior pituitary response to stress in the rat. J Endocrinol. 1986 Nov;111(2):239–244. doi: 10.1677/joe.0.1110239. [DOI] [PubMed] [Google Scholar]
- Cheng S. W., North W. G. Responsiveness of oxytocin-producing neurons to acute salt loading in rats: comparisons with vasopressin-producing neurons. Neuroendocrinology. 1986;42(2):174–180. doi: 10.1159/000124270. [DOI] [PubMed] [Google Scholar]
- Civelli O., Douglass J., Goldstein A., Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogterom J., Van Wimersma Greidanus T. B., Swabb D. F. Evidence for the release of vasopressin and oxytocin into cerebrospinal fluid: measurements in plasma and CSF of intact and hypophysectomized rats. Neuroendocrinology. 1977;24(2):108–118. doi: 10.1159/000122702. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Leslie F. M. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986 Jul 15;249(3):293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- Finley J. C., Maderdrut J. L., Petrusz P. The immunocytochemical localization of enkephalin in the central nervous system of the rat. J Comp Neurol. 1981 Jun 1;198(4):541–565. doi: 10.1002/cne.901980402. [DOI] [PubMed] [Google Scholar]
- Gainer H., Sarne Y., Brownstein M. J. Biosynthesis and axonal transport of rat neurohypophysial proteins and peptides. J Cell Biol. 1977 May;73(2):366–381. doi: 10.1083/jcb.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstberger R., Barden N. Dynorphin 1-8 binds to opiate kappa receptors in the neurohypophysis. Neuroendocrinology. 1986;42(5):376–382. doi: 10.1159/000124475. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Rice K. C., Jacobson A. E., Rothman R. B. Opiate receptors in rat pituitary are confined to the neural lobe and are exclusively kappa. Brain Res. 1986 Sep 24;382(2):365–371. doi: 10.1016/0006-8993(86)91346-6. [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jingami H., Mizuno N., Takahashi H., Shibahara S., Furutani Y., Imura H., Numa S. Cloning and sequence analysis of cDNA for rat corticotropin-releasing factor precursor. FEBS Lett. 1985 Oct 21;191(1):63–66. doi: 10.1016/0014-5793(85)80994-7. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Pickering B. T. Comparison of the effects of water deprivation and sodium chloride imbibition on the hormone content of the neurohypophysis of the rat. J Physiol. 1969 Aug;203(2):449–458. doi: 10.1113/jphysiol.1969.sp008874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian H., Lewis M. E., Watson S. J. Enkephalin systems in diencephalon and brainstem of the rat. J Comp Neurol. 1983 Nov 1;220(3):310–320. doi: 10.1002/cne.902200305. [DOI] [PubMed] [Google Scholar]
- Kiss J. Z., Mezey E., Skirboll L. Corticotropin-releasing factor-immunoreactive neurons of the paraventricular nucleus become vasopressin positive after adrenalectomy. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1854–1858. doi: 10.1073/pnas.81.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. E., Heil J. W., Ganten D., Hermann K., Unger T., Rascher W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology. 1983 Oct;37(4):314–316. doi: 10.1159/000123566. [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Iversen L. L., Forsling M. L. Dopamine and [D-ALA2, D-Leu5]enkephalin inhibit the electrically stimulated neurohypophyseal release of vasopressin in vitro: evidence for calcium-dependent opiate action. J Neurosci. 1982 Jan;2(1):78–81. doi: 10.1523/JNEUROSCI.02-01-00078.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman S. L., Ninkovic M., Hunt S. P., Iversen L. L. Evidence for opiate receptors on pituicytes. Nature. 1983 Sep 15;305(5931):235–237. doi: 10.1038/305235a0. [DOI] [PubMed] [Google Scholar]
- Liposits Z., Paull W. K., Sétáló G., Vigh S. Evidence for local corticotropin releasing factor (CRF)-immunoreactive neuronal circuits in the paraventricular nucleus of the rat hypothalamus. An electron microscopic immunohistochemical analysis. Histochemistry. 1985;83(1):5–16. doi: 10.1007/BF00495294. [DOI] [PubMed] [Google Scholar]
- Majzoub J. A., Rich A., van Boom J., Habener J. F. Vasopressin and oxytocin mRNA regulation in the rat assessed by hybridization with synthetic oligonucleotides. J Biol Chem. 1983 Dec 10;258(23):14061–14064. [PubMed] [Google Scholar]
- Martin R., Geis R., Holl R., Schäfer M., Voigt K. H. Co-existence of unrelated peptides in oxytocin and vasopressin terminals of rat neurohypophyses: immunoreactive methionine-enkephalin-, leucine-enkephalin- and cholecystokinin-like substances. Neuroscience. 1983;8(2):213–227. doi: 10.1016/0306-4522(83)90061-1. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I., Maderdrut J. L., Altschuler R. A., Petrusz P. Immunocytochemical localization of proenkephalin-derived peptides in the central nervous system of the rat. Neuroscience. 1986 Feb;17(2):325–348. doi: 10.1016/0306-4522(86)90250-2. [DOI] [PubMed] [Google Scholar]
- Muehlethaler M., Gaehwiler B. H., Dreifuss J. J. Enkephalin-induced inhibition of hypothalmaic paraventricular neurons. Brain Res. 1980 Sep 15;197(1):264–268. doi: 10.1016/0006-8993(80)90457-6. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B., Dyball R. E. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):367–384. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- Sar M., Stumpf W. E., Miller R. J., Chang K. J., Cuatrecasas P. Immunohistochemical localization of enkephalin in rat brain and spinal cord. J Comp Neurol. 1978 Nov 1;182(1):17–37. doi: 10.1002/cne.901820103. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Vale W. W. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1883–1887. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Vale W. W. Corticotropin-releasing factor: co-expression within distinct subsets of oxytocin-, vasopressin-, and neurotensin-immunoreactive neurons in the hypothalamus of the male rat. J Neurosci. 1984 Apr;4(4):1118–1129. doi: 10.1523/JNEUROSCI.04-04-01118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman T. G., Civelli O., Douglass J., Herbert E., Watson S. J. Coordinate expression of hypothalamic pro-dynorphin and pro-vasopressin mRNAs with osmotic stimulation. Neuroendocrinology. 1986;44(2):222–228. doi: 10.1159/000124649. [DOI] [PubMed] [Google Scholar]
- Sherman T. G., McKelvy J. F., Watson S. J. Vasopressin mRNA regulation in individual hypothalamic nuclei: a northern and in situ hybridization analysis. J Neurosci. 1986 Jun;6(6):1685–1694. doi: 10.1523/JNEUROSCI.06-06-01685.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKABATAKE Y., SACHS H. VASOPRESSIN BIOSYNTHESIS. 3. IN VITRO STUDIES. Endocrinology. 1964 Dec;75:934–942. doi: 10.1210/endo-75-6-934. [DOI] [PubMed] [Google Scholar]
- Tramu G., Croix C., Pillez A. Ability of the CRF immunoreactive neurons of the paraventricular nucleus to produce a vasopressin-like material. Immunohistochemical demonstration in adrenalectomized guinea pigs and rats. Neuroendocrinology. 1983 Dec;37(6):467–469. doi: 10.1159/000123595. [DOI] [PubMed] [Google Scholar]
- Uhl G. R., Zingg H. H., Habener J. F. Vasopressin mRNA in situ hybridization: localization and regulation studied with oligonucleotide cDNA probes in normal and Brattleboro rat hypothalamus. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5555–5559. doi: 10.1073/pnas.82.16.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tol H. H., Voorhuis D. T., Burbach J. P. Oxytocin gene expression in discrete hypothalamic magnocellular cell groups is stimulated by prolonged salt loading. Endocrinology. 1987 Jan;120(1):71–76. doi: 10.1210/endo-120-1-71. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Noble R., Clarke G. Effects of morphine and D-Ala, D-Leu enkephalin on the electrical activity of supraoptic neurosecretory cells in vitro. Neuroscience. 1983 Sep;10(1):73–81. doi: 10.1016/0306-4522(83)90081-7. [DOI] [PubMed] [Google Scholar]
- Wamsley J. K., Zarbin M. A., Young W. S., 3rd, Kuhar M. J. Distribution of opiate receptors in the monkey brain: an autoradiographic study. Neuroscience. 1982 Mar;7(3):595–613. doi: 10.1016/0306-4522(82)90066-5. [DOI] [PubMed] [Google Scholar]
- Watson S. J., Akil H., Fischli W., Goldstein A., Zimmerman E., Nilaver G., van wimersma Griedanus T. B. Dynorphin and vasopressin: common localization in magnocellular neurons. Science. 1982 Apr 2;216(4541):85–87. doi: 10.1126/science.6121376. [DOI] [PubMed] [Google Scholar]
- Whitnall M. H., Gainer H., Cox B. M., Molineaux C. J. Dynorphin-A-(1-8) is contained within vasopressin neurosecretory vesicles in rat pituitary. Science. 1983 Dec 9;222(4628):1137–1139. doi: 10.1126/science.6648526. [DOI] [PubMed] [Google Scholar]
- Williams T. D., Carter D. A., Lightman S. L. Sexual dimorphism in the posterior pituitary response to stress in the rat. Endocrinology. 1985 Feb;116(2):738–740. doi: 10.1210/endo-116-2-738. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Williams C., Sabol S. L. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984 Nov 25;259(22):14301–14308. [PubMed] [Google Scholar]
- Young W. S., 3rd, Bonner T. I., Brann M. R. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9827–9831. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. S., 3rd Corticotropin-releasing factor mRNA in the hypothalamus is affected differently by drinking saline and by dehydration. FEBS Lett. 1986 Nov 10;208(1):158–162. doi: 10.1016/0014-5793(86)81553-8. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Mezey E., Siegel R. E. Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett. 1986 Oct 8;70(2):198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]
- Zingg H. H., Lefebvre D., Almazan G. Regulation of vasopressin gene expression in rat hypothalamic neurons. Response to osmotic stimulation. J Biol Chem. 1986 Oct 5;261(28):12956–12959. [PubMed] [Google Scholar]