Abstract
Colorectal cancer (CRC) is ranked as the second leading cause of cancer-related deaths globally, necessitating urgent advancements in therapeutic approaches. The emergence of groundbreaking therapies, including chimeric antigen receptor-T (CAR-T) cell therapies, oncolytic viruses, and immune checkpoint inhibitors, marks a transformative era in oncology. These innovative modalities, tailored to individual genetic and molecular profiles, hold the promise of significantly enhancing patient outcomes. This comprehensive review explores the latest clinical trials and advancements, encompassing targeted molecular therapies, immunomodulatory agents, and cell-based therapies. By evaluating the strengths, limitations, and potential synergies of these approaches, this research aims to reshape the treatment landscape and improve clinical outcomes for CRC patients, offering new found hope for those who have exhausted conventional options. The culmination of this work is anticipated to pave the way for transformative clinical trials, ushering in a new era of personalized and effective CRC therapy.
Keywords: Colorectal cancer, Targeted molecular therapies, CAR-T-cell therapies, Oncolytic viruses, Immune checkpoint inhibitors
Introduction
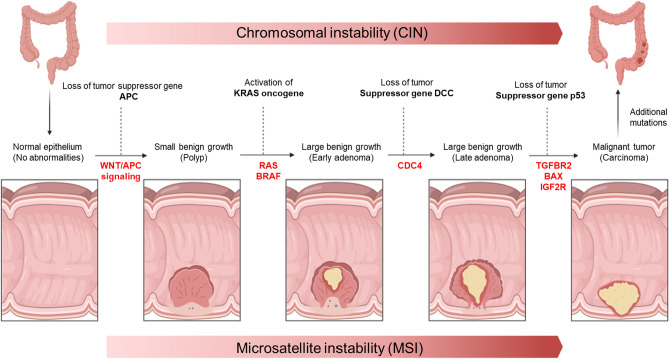
Colorectal cancer (CRC) is the second most common cause of cancer-related deaths globally [1]. The incidence is slightly higher in men and it peaks around the age of 50. Genetics, environmental, and lifestyle risk factors, all have a role in the development and progression of CRC [2]. The progression of tumors from benign early adenomas and polyps to malignant late-stage tumors is a complex and multifaceted process governed by intricate molecular signaling pathways. Two critical factors influencing this progression are microsatellite instability and chromosomal instability (CIN). Each of these factors plays a distinct role in tumor development [3]. Additionally, various gene mutations contribute to the transformation of normal cells into cancerous ones. Some of the most crucial pathways implicated in tumor progression include the MAPK/ERK, PI3K/AKT/mTOR, and Wnt/β-catenin pathways [4]. Mutations in genes involved in these pathways, such as KRAS, BRAF, and APC, can trigger aberrant signaling, driving the transition from early adenomas to malignant tumors (Fig. 1) [5].
Fig. 1.
Visualizing the Tumorigenesis Cascade. Beginning with normal epithelium, the Wnt/APC signaling pathway is disrupted, leading to the formation of polyps due to the loss of tumor suppressor genes. Subsequently, activation of the oncogene KRAS in the RAS/BRAF pathway transforms these small polyps into large benign adenomas. As the journey continues, defects in the P53 gene, coupled with additional mutations and escalating microsatellite instability and chromosomal instability, culminate in the transition from large benign polyps to malignant tumors, providing a vivid portrayal of the intricate molecular evolution underlying tumorigenesis
In recent years, the discourse surrounding novel treatments for CRC has garnered unprecedented attention and significance within the medical community. This surge in interest is propelled by a confluence of factors. Firstly, the escalating incidence of CRC worldwide has underscored the urgency to develop and optimize therapies that can effectively combat this prevalent malignancy. Furthermore, the emergence of groundbreaking techniques like natural killer (NK) cell therapy, chimeric antigen Receptor-T (CAR-T) cell therapy, oncolytic virus therapy, and immune checkpoint inhibitors represents a paradigm shift in oncology, presenting promising avenues for targeted, less toxic, and more efficacious interventions (Fig. 2). As these innovative treatments progress through rigorous clinical trials, their potential to significantly enhance patient outcomes and quality of life cannot be overstated. Moreover, the individualized nature of these therapies holds the promise of tailoring treatments to specific genetic and molecular profiles, offering a level of precision medicine previously unimaginable. Therefore, delving into these approaches is paramount, as it signifies not only a quantum leap in the fight against CRC, but also a beacon of hope for patients and clinicians alike in the pursuit of more effective and personalized treatment modalities. With an in-depth exploration of the latest clinical trials and advancements in cell-based therapies, immunomodulatory agents, and innovative approaches, this article aims to provide a comprehensive overview of the strategies revolutionizing CRC treatment, paving the way for more effective and personalized therapeutic interventions. By examining the strengths, limitations, and potential synergistic effects of these various methodologies, it is anticipated that this research will offer valuable insights that can revolutionize the treatment perspective and improve clinical outcomes for CRC patients. Ultimately, the final aim is to bring about a paradigm shift in CRC therapy, offering new hope and avenues for patients who have exhausted conventional treatment options.
Fig. 2.
Novel therapies for CRC. This diagram shows the various types of cancer vaccines and targeted therapies that are being investigated for the treatment of CRC. The vaccines target different molecular targets and signaling pathways, including RAS, RAF, HER2, PIK3CA, BRAF, NTRK, and mismatch repair. The targeted therapies include monoclonal antibodies (MABs), cytokines, and viral vector vaccines
Molecular Targets and Signaling Pathways
The primary signaling pathways involved in regulating key cellular processes such as proliferation, differentiation, apoptosis, and survival in CRC include Sonic Hedgehog (SHH), Wnt/β-catenin, and transforming growth factor-β (TGF-β)/SMAD. These pathways provided promising targets during past decades for precision therapy in CRC [6]. The Food and Drug Administration (FDA) approved cetuximab (Erbitux™) as the first targeted drug for CRC in February 2004 [7]. Several targeted drugs for colorectal cancer (CRC), such as the EGFR-specific antibody cetuximab, have been approved by the FDA and introduced to the market. Table 1 summarizes the molecular subtypes of colorectal cancer (CRC) along with associated therapeutic approaches and key clinical findings. However, challenges in completely inhibiting specific biologic interactions and managing complex downstream signaling have limited the scope of existing data.
Table 1.
Advances in colorectal cancer: molecular subtypes, targeted therapies, and immunotherapy strategies
| Molecular subtype | Key characteristics | Therapeutic approach | Outcome/challenges | References |
|---|---|---|---|---|
| RAS/RAF Wild-Type, MSS | Found in ~ 30–40% of CRC cases. Loss of APC leads to dependence on EGFR signaling | Cetuximab and panitumumab combined with chemotherapy; VEGF-targeting bevacizumab tested in CALGB/SWOG 80405 trial | Median OS: 31.2 months (CALGB/SWOG 80405). No significant OS difference between bevacizumab and cetuximab. Up to 60% of patients develop resistance to EGFR inhibitors | [8–15] |
| KRAS/NRAS Mutant, MSS | KRAS mutations in > 40% of CRC cases, NRAS in 2–5%; activates RAS/MAPK pathway, causing resistance to EGFR inhibitors | Cobimetinib (MEK inhibitor) + atezolizumab (anti-PDL1) tested in phase I–III trials; regorafenib as comparator | Phase III trial showed no OS improvement. Efficacy for KRAS mutant-MSS still under investigation | [16–19] |
| MSI-High/dMMR | ~ 4–5% of mCRC cases; characterized by mismatch repair deficiency or high microsatellite instability | Anti-PD1 therapies (pembrolizumab, nivolumab). CheckMate 142 trial tested nivolumab + ipilimumab | Pembrolizumab: 78% PFS (20 weeks), Nivolumab: 69% PFS (> 12 weeks). Combination therapy: 60% ORR, 83% OS (12 months). FDA-approved pembrolizumab and nivolumab as second-line treatments | [20–25] |
| BRAF Mutant | ~ 10% of CRC cases, 80% are V600E mutations; associated with poor prognosis (median OS: 11 months) | BEACON trial: Encorafenib (RAF inhibitor) + cetuximab, ± binimetinib (MEK inhibitor). FDA-approved encorafenib-cetuximab | Triplet vs. doublet therapy showed no significant PFS/OS difference but better toxicity profile. Combined therapies with ICIs showed promise. Molecular heterogeneity and resistance remain challenges | [26–33] |
| PIK3CA Mutant | ~ 18% of CRC cases. Mutations in PI3K/mTOR pathway lead to potential drug resistance | PI3K inhibitors (GDC-0941, GDC-0980, MEN1611) evaluated in phase I trials; mTORC1/2 inhibitors tested in preclinical models | Moderate anti-tumor activity observed; resistance noted in patient-derived spheroid cultures. MEN1611 in combination with cetuximab under evaluation (expected completion July 2023) | [34–37] |
| HER2-Amplification | Found in 3–5% of CRC cases; activates RAS/MAPK and PI3K/mTOR pathways, causing resistance to anti-EGFR therapies | HERACLES trial: Trastuzumab + lapatinib. MyPathway trial: Pertuzumab + trastuzumab. FDA-approved tucatinib + trastuzumab for HER2-positive mCRC | HERACLES: 30% ORR. MyPathway: 38% ORR. Tucatinib approved for cases resistant to prior chemotherapy | [38, 39] |
| NTRK Fusions | Fusion of NTRK genes leads to overexpression or activation of Trk kinases. Rare in CRC | FDA-approved larotrectinib and entrectinib for CRC with NTRK fusions | Resistance to NTRK fusion blockers common; need for new molecules to overcome resistance | [40–42] |
| Immunotherapy | Focuses on enhancing immune system’s ability to target tumor-specific antigens. Complementary to traditional therapies | Immune checkpoint inhibitors, combination therapies with chemotherapy or biologic treatments | Promising clinical trial results, but resistance remains an issue in some patients. Ongoing research into combination therapies to improve response rates | [43] |
Immunotherapy
Every person’s immune system is the first defense line to attack cancer cells, thus potentially representing an effective assistance in cancer treatment and diagnosis. About a century ago, the idea of activating the host’s immune system to eradicate cancer was proposed [8]. The limited efficacy of traditional treatments like chemotherapy and radiotherapy in colorectal cancer (CRC) has highlighted the need for immune-based approaches as complementary or alternative therapies. Tumor-specific antigens, resulting from mutations and structural changes during tumor formation, are recognized by the immune system as foreign, triggering immune responses. Recent advances in CRC immunotherapy have demonstrated its potential to enhance the body's natural defense against malignant cells, as supported by clinical trials.
Monoclonal Antibodies (mABs)
Monoclonal antibodies are synthetic proteins that mimic the role of human antibodies in the immune system. They are considered a form of immunotherapy as they enhance the immune response, helping the body to identify and combat cancer cells with greater efficiency [9]. CRC expresses several receptors that can be targeted by monoclonal antibodies [10]. Table 2 shows the target of common mABs. Bevacizumab, cetuximab, panitumumab, and ramucirumab are among the most commonly used monoclonal antibodies for treating colorectal tumors. Of these, bevacizumab specifically targets VEGF [11] and cetuximab and panitumumab act on EGFR [12]. Clinical studies have shown that the combination of bevacizumab and chemotherapy, i.e., shown by Hurwitz et al., significantly increases PFS in CRC patients. Hurwitz et al. combined bevacizumab with irinotecan leading to the approval of bevacizumab for use in combination with chemotherapy as a first-line treatment for mCRCs [13]. There is evidence to support the use of cetuximab as a preferred drug in the initial treatment of mCRCs that have wild-type RAS and BRAF genes and proficient mismatch repair [14, 15]. The SWOG 1406 trial demonstrated that the combination of vemurafenib, irinotecan, and cetuximab improved PFS. This suggests that when the activity of either BRAF or EGFR inhibition alone is minimal, the combination of BRAF and EGFR inhibition can provide clinical benefit [16]. Furthermore, in a randomized clinical trial (NCT02394795) involving 802 patients with CRC, it was found that adding panitumumab to first-line chemotherapy improved OS compared to bevacizumab. This improvement was observed in patients with left-sided tumors and in the overall population [17]. Among mABs, only cetuximab, bevacizumab, and panitumumab have been approved by the FDA and broadly used in the USA. Figure 3 indicates FDA-approved mABs.
Table 2.
Monoclonal antibodies and their receptors for CRC targeting
| Monoclonal antibody | Trade name | Target | Function | References |
|---|---|---|---|---|
| Pembrolizumab | Keytruda® | PD1 | Inhibition of PD1 | [133] |
| Cetuximab | ErbituxTM | EGFR | Treatment of K-Ras mutated CRC | [134] |
| Bevacizumab | Avastin® | VEGF | Antiangiogenic agents | [135] |
| Conatumumab | _ | DR5 | Agonist against (tumor necrosis factor-related apoptosis-inducing ligand) | [136] |
| Trastuzumab | Herceptin | HER2/neu | Treatment of HER-2-overexpressing metastatic cancer | [137] |
| Adecatumumab | _ | EpCAM | Agonist against the tumor-associated antigen | [138] |
| Ensituximab | _ | MUC5AC | A novel chimeric mAb targeting a glycosylated variant of MUC5AC | [139] |
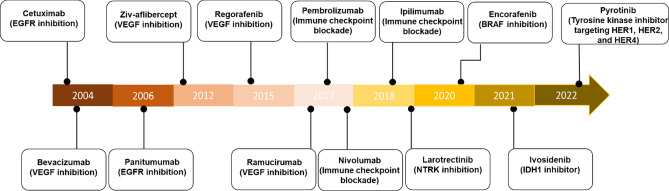
Fig. 3.
Timeline of FDA-approved targeted therapy for colorectal cancer. VEGF vascular endothelial growth factor, EGFR epidermal growth factor receptor, NTRK neurotrophic tropomyosin receptor kinase, IDH1 isocitrate dehydrogenase 1
Immune Checkpoint Inhibitors (ICIs)
ICIs prevent checkpoint proteins from binding to receptor proteins, enabling T cells to attack cancer cells effectively. CTLA4, PD1, and PDL1 are the molecules that are currently approved checkpoint inhibitor targets [18]. Figure 4 indicates the ligands and receptors which can be inhibited by ICIs.
Fig. 4.
Unlocking Immune Potential: in the first frame, PD1 engages with PDL1, stifling T-cell activation through immune checkpoint inhibition. The second frame depicts the pivotal role of immune checkpoint inhibitors, releasing this suppressive interaction and reinvigorating T-cell activation, showcasing the promise of immunotherapy in unleashing the body’s innate defenses against cancer
ICIs enhance the activity of T cells by preventing the negative regulators of T-cell function from binding. However, this can also result in uncontrolled immune responses leading to autoimmune effects on normal organs known as immune-related side effects [10]. CTLA4 inhibitors have a higher incidence of immune-related adverse events (irAEs) than programmed cell death 1 (PD1) and its ligand 1 (PDL1) inhibitors. Combining CTLA4 and PD1 inhibitors further increases the risk of irAEs [19]. While ICIs have shown efficacy in treating various cancers, mCRC has been less responsive. Nonetheless, the FDA has approved certain PD1 inhibitors as first-line therapy for unresectable or metastatic MSI-H or dMMR CRC [20].
Neoadjuvant Immunotherapy
Adjuvants refer to a variety of substances, such as organic or inorganic molecules, colloids, and polymers, that are employed alone or in combination with other agents to stimulate the immune system. Their purpose is to enhance the immune response [21]. They can activate antigen-presenting cells, which help to present epitopes on the major histocompatibility complex class I (MHC-I), thereby promoting the activity of cytotoxic T lymphocytes. This process can lead to the destruction of cancer cells [22]. Ipilimumab is a neoadjuvant that stimulates the immune response by targeting cCTLA-4, a protein receptor responsible for suppressing the immune system. By blocking this receptor, it eliminates the inhibitory mechanism displayed by cytotoxic T cells, enabling them to combat cancer cells better. In other words, it enhances the body’s immune response to fight cancer cells [23]. In a study published in 2020, neoadjuvant immunotherapy was suggested for the treatment of patients with early-stage CRC. In this research, patients with dMMR or pMMR tumors were given a single dose of ipilimumab and two doses of nivolumab prior to surgery. The treatment was well tolerated among all patients, and they exhibited a favorable response to the treatment [24]. Overall, it appears that neoadjuvant immunotherapy could be the standard treatment for specific groups of CRC patients. However, this assertion requires further confirmation through more extensive studies. Dostarlimab is a checkpoint inhibitor that has been shown to be effective in the treatment of a variety of cancers, including rectal cancer. In a recent phase 2 clinical trial, Cercek et al. reported that dostarlimab was well tolerated and led to the absence of residual disease, as proven by rectal magnetic resonance imaging [25], 26. In all 12 patients with locally advanced rectal cancer [27] who were ineligible for surgery. The results of this trial are very promising, as they suggest that dostarlimab may be a new and effective treatment option for patients with locally advanced rectal cancer [27]. This is the first time that a complete clinical response has been reported in a phase 2 clinical trial of a cancer immunotherapy. The long-term follow-up of these patients is still ongoing, and it is important to see if the complete clinical responses are durable or long-lasting. However, the results of this trial suggest that dostarlimab may be a promising new treatment option for patients with LARC.
Oncolytic Viruses
Oncolytic viruses are viruses that have been genetically engineered to infect and kill cancer cells. They work by infecting specifically cancer cells, replicating inside, leading to lysis and subsequent death of the cells. The use of viruses in the treatment of cancer dates back to about a century ago, but with the presence of genetic engineering methods and the ability to remove pathogenic genes from viruses, oncolytic viruses entered the field as a new and promising line of treatment. Oncolytic viruses have been shown to be effective in the treatment of a variety of tumors, including melanoma, head and neck cancer, and bladder cancer. No oncolytic virus has yet been approved for the treatment of CRC. However, promising oncolytic viruses that are currently being investigated in clinical trials for the treatment of CRC include talimogene laherparepvec, coxsackievirus, and adenovirus. T-VEC is a modified herpes simplex virus that has been engineered to infect and kill melanoma cells. T-VEC has been shown to be effective in shrinking tumors and improving survival in patients with melanoma. Coxsackievirus is a virus that is naturally found in humans and has been modified to infect and kill cancer cells. Coxsackievirus is currently being investigated in combination with chemotherapy for the treatment of pancreatic cancer. Similarly, naturally found human adenovirus has been modified to infect and kill cancer cells and is currently being investigated in combination with immunotherapy for the treatment of CRC. There are several reports from oncolytic viruses which indicates their promising role in treatment of KRAS-mutated CRC [28]. Pelareorep is an oncolytic virus containing non-enveloped dsRNA that can destroy KRAS-mutated colorectal tumor cells. It induces autophagic mechanisms and up-regulates autophagic proteins, leading to increased apoptosis and cell death in CRC cells [29]. In addition, pelareorep enhances immune efficacy by increasing the expression of MHC-I molecules and activating CD4 and CD8 T-cell populations in CRC patients with KRAS mutations [30]. The results of clinical trials that are investigating oncolytic viruses in combination therapy for CRC are still pending. However, the early results are promising, and oncolytic viruses may one day become a standard treatment for CRC. In addition, there are several different types of oncolytic viruses that are being investigated in combination therapy for CRC (Table 3).
Table 3.
Available oncolytic viruses for colorectal cancer treatment
Cytokines
Cytokines are messenger molecules that facilitate communication between cells and coordinate immune system interactions. They are produced by both immune and non-immune cells in response to stress, such as infection, inflammation, and tumorigenesis [31]. Cytokines play a vital role in enabling the efficient and rapid propagation of immune signals, which elicit a coordinated and potent immune response to target antigens [32]. The latest findings indicate that it is possible to inhibit the development of CRC or enhance the effectiveness of chemotherapy or checkpoint inhibitors in treating CRC tumors by targeting particular cytokine pathways. A common form of cytokine therapy involves the use of high doses of IL2 to induce cancer regression in patients with metastatic cancer. Along with IL2, interferon-alpha (IFN-α) is also utilized as a therapeutic cytokine in cancer treatment [33]. To increase the efficacy of cytokine therapy in CRC, one should either inhibit several cytokines together or combine this method with other treatment methods. Indeed, blocking a single cytokine is not likely to produce significant outcomes as cytokine signals frequently overlap with each other [34], thus, this strategy is not likely to be effective in CRC treatment. Therefore, it is likely to be more effective targeting multiple cytokines or combining cytokine therapy with other treatment methods.
Cancer Vaccines
Cancer vaccines are one of the novel immunotherapy strategies to create a stronger immune response and to prevent cancer occurrence and micro-metastasis [35], 36. Recently, effective cancer vaccines have been developed with the ability to strengthen the immune system and harbor low toxicity. There are various adjuvants, such as cytokines, pathogen-associated molecular pattern molecules (PAMPs), and Toll-like receptors (TLRs), that can be combined with cancer vaccines to enhance their ability to fight cancer synergistically [37, 38]. In the following, we focus on new vaccines for cancer treatment and experimental strategies in CRC immunotherapy.
Protein/Peptide-Based Vaccines
Protein/peptide-based vaccines are a type of vaccine that uses specific proteins or peptides derived from pathogens, such as viruses or bacteria, to stimulate an immune response in the body. Unlike traditional vaccines, which may use weakened or inactivated forms of the entire pathogen, protein/peptide-based vaccines contain only selected protein fragments or peptides that are known to trigger a strong immune response [39]. These vaccines work by presenting these protein fragments or peptides to the immune system, which then recognizes them as foreign and mounts an immune response. This response involves the production of antibodies, specialized immune cells, and memory cells that “remember” the pathogen. This way, if the individual is later exposed to the actual pathogen, their immune system can quickly recognize and combat it, providing protection against infection. One of the advantages of protein/peptide-based vaccines is their precision [25]. By targeting specific proteins or peptides, scientists can design vaccines to focus on the most immunogenic parts of the pathogen, reducing the risk of unwanted side effects. Additionally, they can be produced using biotechnological methods, which can lead to more efficient and scalable vaccine production. These types of vaccines have been explored for various diseases, including certain types of cancer and infectious diseases. They are an important area of research in the development of new and innovative vaccines [40]. Immunization with proteins primarily leads to the activation of humoral immune response. However, for cancer treatment, it is essential to stimulate both humoral and cellular immune responses. Protein-based antibodies used for immunization must contain immunogenic sites that can be recognized by MHC-I/II molecules [41]. This process involves identifying and combining epitopes to stimulate anti-tumor or immune responses against tumor-associated antigens. Peptide antibodies for CRC patients are generally safe and well tolerated, but there are limitations to their application in clinical trials, such as frequent reactions at the injection site and restrictions based on patient's HLA type.
A phase II trial with 96 patients with advanced CRC demonstrated the safety of a vaccination mixture containing five HLA-A*2404-restricted peptides (RNF43, KOC1, TOMM34, VEGFR1, VEGFR2). The vaccine was administered concurrently with oxaliplatin-based chemotherapy [42]. In a phase I trial, a combination of KOC1, TTK, URLC10, DEPDC1, and MPHOSPH1 as an HLA-A*2404-restricted vaccine was found to be safe. The vaccine also demonstrated an OS of 9.4 months in comparison to untreated patients [43].
DNA Vaccines
Naked nucleic acid vaccines have the potential to be effective in treating cancer patients [44]. DNA vaccines are being studied as therapeutic gene vaccines for CRC due to their potential to enhance the growth of CD8 T cells. The efficacy of DNA vaccines in CRC has not been extensively investigated in clinical trials, despite the wealth of information derived from in vitro and in vivo studies [45].
For example, Staff et al. administered a modified plasmid DNA vaccine encoding the CEA antigen (CEA66 DNA) along with T helper cell-related epitopes to patients with CRC. The study demonstrated that the vaccine was well tolerated and did not cause any autoimmune response [46]. In addition, pVAX1-HER2-CTLA-4 is a DNA vaccine that is currently under investigation in a clinical trial [47].
mRNA Vaccines
Studies have shown that the administration of naked or vehicle-loaded mRNA vaccines that carry tumor antigens can activate antigen-presenting (APC) cells, leading to the stimulation of innate/adaptive immune responses [48]. Compared to other cancer vaccines, mRNA vaccines have several distinctive features: mRNA is a non-infectious, non-integrating platform, easier to modify for different purposes, and has a higher translation efficiency compared to DNA vaccines, leading to higher protein yields [49].
mRNA has the capability to encode multiple antigens concurrently or a complete protein containing epitopes that bind to both, MHC-I and MHC-II, enabling the facilitation of both humoral and cellular adaptive immune responses. mRNA vaccines are devoid of viral and cellular components, posing no risk of infection [48]. Clinical trials have shown that these vaccines are typically safe, with few reports of adverse reactions at the injection site [50]. Nevertheless, there are significant concerns regarding mRNA instability, innate immunogenicity, and the limited efficacy of in vivo delivery. Currently, a phase I trial (NCT03948763) is underway to investigate the efficacy of mRNA 5671, a vaccine targeting KRAS-positive cancers, in combination with Pembrolizumab for non-MSI-H patients. Another phase I trial (NCT03313778) is evaluating the clinical effectiveness of mRNA-4157, a cancer vaccine based on mRNA, in combination with Pembrolizumab in non-MSI-H patients [23].
Whole-Cell Vaccines
Whole-cell vaccines offer the advantage of being applicable to multiple individuals, as they are not personalized for each patient. This characteristic makes them easier to manufacture, saving time and money, while also reducing the risk of cancer cells evading the treatment [51]. However, developing a universally applicable vaccine for all patients is challenging. Whole-cell vaccines often exhibit low immunogenicity and generate non-specific reactions. This is primarily due to the fact that the majority of antigens in these vaccines are shared with normal cells, while only a small fraction of the proteins is specific to tumors [52]. Therefore, several combinational strategies are developed to enhance autologous vaccines efficiency. One of these strategies, involves injecting modified autologous tumor cells that have been infected with the nonlytic Newcastle disease virus (NDV) to express immune-stimulating antigens and alter the tumor cells [53]. OncoVax is a CRC cancer vaccine strategy that involves combining autologous cancer cells with the Bacillus Calmette–Guérin (BCG) vaccine. It is considered one of the extensively investigated CRC cancer vaccines and was the subject of early-phase clinical trials in the 1980s [54]. Subsequently, the combination of OncoVax with 5-fluorouracil (5FU) and leucovorin demonstrated a safe approach to minimize clinical effects in patients with stage III CRC [55]. Another vaccine called GVAX, which is an allogenic whole-cell vaccine engineered to secrete granulocyte–macrophage colony-stimulating factor (GM-CSF), exhibited an anti-tumor response in a phase II trial targeting advanced CRC patients with proficient mismatch repair. Currently, clinical trials (NCT01966289) are focused on enhancing the immunologic activity of GVAX [22]. In order to further improve GVAX, epigenetic therapy has been tried to enhance immunologic activity in both preclinical and clinical trials (NCT01966289) [56, 57]. A feasibility study of combined epigenetic and vaccine therapy in advanced CRC with pharmacodynamic endpoint [56]. Neoantigen-based EpiGVAX vaccine initiates anti-tumor immunity in CRC [57]. Unlike whole-cell vaccines, autologous vaccines do not employ the entire tumor cell in their formulation [58]. Autologous vaccines are isolated and altered to be injected back into the same patient’s body [59]. Utilizing the individual’s own cells in autologous vaccines reduces the likelihood of rejection, and these cells specifically contain the antigens that are highly efficient in triggering an immune response [58].
Dendritic Cells Vaccines
Dendritic cells (DCs) are powerful APCs utilized in cancer vaccines due to their capacity to initiate immune responses against tumors [60]. DC vaccines involve the manipulation of DCs outside the patient's body by exposing them to cancer cells or antigens. This process enhances the ability of the DCs to recognize and target cancer cells with the same antigens, leading to more effective attacks against cancer cells [61]. There are ongoing clinical studies to explore the effectiveness of DC vaccines for CRC patients. For instance, in one randomized clinical trial phase II study, autologous tumor lysate dendritic cell vaccine (ADC) combination with best supportive care (BSC) indicated that ADC induces a tumor-specific immune response but has no effect on PFS or OS [62]. DC vaccine based on Wilms’ tumor (WT1) class I/II peptide confirmed the immunogenicity and safety of DC vaccine in advanced CRC patients through evaluation of WT1 expression in tissue using the Enzyme-Linked Immunosorbent Spot (ELISPOT) assays [63].
Rodriguez et al. [64] conducted a Randomized Control Trial (RCT) involving 19 patients with surgically amenable liver metastasis of CRC. The patients were divided into two groups, with one group receiving DC vaccinations and the other group undergoing observation after surgery and chemotherapy. The median PFS was significantly longer in the vaccine arm compared to the observation arm [64].
MelCancerVac is a DCs vaccine being investigated in phase II clinical trials [65]. This vaccine is produced by pulsing allogeneic melanoma cell lysate from DDM-1.13, known for its high expression of MAGE-A3, which is a tumor-associated antigen [66] that is also overexpressed in CRC. The overall results of the trial did not demonstrate a significant improvement in OS for the patients. However, five patients experienced prolonged PFS of more than six months, with two patients remaining progression-free for over 27 and 37 months. A phase III trial of MelCancerVac for CRC patients is currently underway, and the results are expected to be available in future.
To enhance the effectiveness of the DC vaccine, further research is required to compare various subsets, including monocyte-derived DCs (moDCs), conventional DCs (cDCs), and plasmacytoid DCs (pDCs) [67]. Additionally, induced pluripotent stem cells (iPSCs) could potentially be a viable option for improving the DC vaccine [68].
Viral Vector Vaccines
Cancer vaccines based on viral vectors are effective carriers for delivering tumor antigens [69]. Vaccinia virus, poxvirus, and adenovirus are naturally immunogenic and can directly infect and activate the APC cells (specifically DCs). Therefore, they are deemed as suitable vectors to transfect tumor antigens based on their ability to stimulate the cellular immune response against CRC antigens. Clinical trials have shown that these vaccines are effective in activating cellular immune response against antigens, such as CEA, EpCAM/KSA, p53, and 5T4, which are associated with CRC [70].
Morse et al. [71] conducted a phase I/II trial using adenovirus subtype 5 (Ad5) [E1- E2b-]-CEA (6D) to enhance CEA-specific T cell-mediated immune response in 32 mCRC patients. The treatment was proven to be safe and effective, with a 48% overall survival rate at 12 months. Another trial using E1/E3-deleted Ad5 with GUCY2C and PADRE sequences has also shown safety in a phase I trial [72] and a phase IIa trial is currently being explored (NCT04111172).
One promising approach involves utilizing a DC vaccine along with pox vectors encoding CEA and MUC1 (PANVAC) in patients with resected mCRC. This combination has shown promising results, with vaccinated patients exhibiting longer survival compared to an unvaccinated group. These findings indicate the potential of the vaccine to stimulate immune responses against cancers that overexpress CEA and MUC1 [71].
Several other vaccines with intrinsic outcomes are currently in phase I/II clinical trials. These include TroVax (modified vaccinia Ankara encoding 5T4 antigen) [73, 74]. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma [75, 76].
Live-Attenuated Bacteria
Bacterial vaccine vectors employed as live vaccine vectors over the last 30 years [77]. They possess many advantages based on their easy and inexpensive manufacture. Their susceptibility to antibiotics and well-defined mutations for reducing virulence make it straightforward to manage any adverse reactions caused by viral vector-based vaccines [77]. Moreover, these vaccines can be administered via multiple routes, including oral mucosal delivery [78]. The use of enteric bacterial vectors presents a distinct advantage in developing mucosal vaccines because they have a propensity to target lymphoid antigen-presenting cells (such as dendritic cells and macrophages) in the intestinal mucosal tract [79]. A phase 1 trial (NCT03189030) is currently underway to assess the safety of a personalized live-attenuated, double-deleted (pLADD) Listeria monocytogenes vaccine in patients with CRC. Another ongoing phase I trial (NCT03265080) is investigating the combination of the L. monocytogenes platform with neoantigens in patients with metastatic solid tumors, known as ADX-NEO [22]. The phase 1 trial of pLADD Listeria monocytogenes vaccine in patients with CRC (NCT03189030) has completed enrollment and is ongoing. The phase 1 trial of ADX-NEO in patients with metastatic solid tumors (NCT03265080) is also ongoing. The primary endpoint of NCT03189030 and NCT03265080 is to assess the safety of the combination of the L. monocytogenes platform with neoantigens. The secondary endpoints of the trials are to assess the immunogenicity of the combination therapy and its efficacy in treating metastatic solid tumors. In addition to these two trials, there are several other trials that are investigating the use of L. monocytogenes in cancer treatment. For example, a phase 2 trial (NCT04225205) is currently underway to assess the efficacy of L. monocytogenes in combination with Pembrolizumab in patients with mCRC. The results of these trials are still pending, but the early results are promising. These trials suggest that L. monocytogenes has the potential to be a promising new candidate for the treatment of CRC.
Yeast-Based Vaccines
Saccharomyces cerevisiae, also known as baker’s yeast, is a non-harmful yeast strain widely used as a vector in therapeutic vaccines [80]. Research has shown that utilizing whole recombinant S. cerevisiae can activate dendritic cells, leading to the stimulation of specific cytotoxic T lymphocyte (CTL) responses. Moreover, it has demonstrated the ability to induce cell-mediated immunity that provides protection against tumor challenges in mice [80, 81]. There is some vaccine using Heat-killed Saccharomyces cerevisiae as a vector, encoded with tumor-associated antigens or carcinoembryonic antigen (CEA) to form GI-6207 [82], (GI-4000) [83], and in multiple phase I trials, (GI-6301), which encodes brachyury [84], was deemed safe with no indication of serious adverse effects or autoimmunity. Yeast-derived β-glucan particles loaded with short DNA sequences that contain unmethylated CpG motifs and MC38 lysates (lysed cells from the MC38 tumor cell line) showed a strong antibody response in murine models [85]. Different trials are investigating the effects of most vaccine trials that either use one or more antigenic elements in the form of whole tumor cell vaccines which contain irradiated tumor cells or tumor cell lysate, dendritic cell-based cancer (DC-linked peptides) vaccines or viral vector-based cancer vaccines [70]. Currently, clinical trials are underway to explore the use of different tumor antigens, such as CEA, PDL1, MUC1, and SART3, which are known to be highly expressed in the digestive system, for vaccination of CRC patients [86]. These trials are also evaluating various types of vaccines, including DC-based vaccines, DNA vaccines, RNA vaccines, and viral vaccine vectors. The clinical trials are primarily in phase I and II, aiming to assess the safety and efficacy of these vaccine approaches [87]. A summary of clinical trials of immunotherapy in CRC is presented in Table 4.
Table 4.
A summary of vaccines used in clinical trials of CRC treatment
| Name | Vaccine Type | Outcome | Stage | References |
|---|---|---|---|---|
| Fowlpox and vaccinia viruses encoding the CEA antigen and TRICOM (B7.1, ICAM-1, and LFA-3) | Viral vector | Induction of CEA-specific CTL | Metastatic | [145] |
| Cholera | Viral vector | Decreased the mortality rate of CRC, | I–IV | [106] |
| Nonreplicating canarypox virus (ALVAC-CEA/B7.1) | Viral vector | Increases in CEA-specific T cells were detected in patients treated with chemotherapy and booster vaccination | Metastatic | [75] |
| pVAX1-HER2, coding HER2 antigen | DNA-based vaccines | Increases the susceptibility of cancer cells to lysis by CTL | Metastatic | [146] |
| pcDNA-hNIS, expressing human sodium/iodide symporter (hNIS) | DNA-based vaccines | Promote IFN-γ production and reduced the tumor growth | – | [147] |
| OX40L OX86 | DNA-based vaccines | Murine OX40L promote T-cell proliferation | – | [148] |
| MYB | DNA-based vaccines | Enhanced tumor target lysis and memory of T cells | – | [149] |
| CpVR-MS and CpDV-IL2-MS, encoding a fusion gene of human surviving S8 and human 33 MUC1, plus IL2) | DNA-based vaccines |
Increase in anti-tumor effects and extended the rate of survival about 2.5-fold |
Metastatic | [56] |
| NDV-infected irradiated autologous tumor cells | Whole tumor cell | Did not significantly improve overall survival | [150] | |
| Autologous tumor cells combined with BCG | Whole tumor cell | Benefits in terms of disease-free survival (P = 0.078) and overall survival (P = 0.12) | stage II—III | [151] |
| DC pulsed with CEA | DC-based vaccines | The majority of CRC patients demonstrated induction of CEA-specific T-cell responses | IV | [152] |
| DCs with CEA- altered peptides combined with the Flt3 ligand | DC-based vaccines | Expansion of CD8 + T cells | IV | [153] |
| Autologous tumor antigen-loaded DC | DC-based vaccines | Increased IL-12 production for immunization against neoantigens | Metastatic | [154] |
| 13-mer mutant ras | Peptide | The anti-tumor immune response was significantly associated with prolonged overall survival | – | [155] |
| β-hCG | Peptide | Prolongation of survival and induction of serum antipeptide antibody | Metastatic | [156] |
| SART3 | Peptide | Increased CTL activity and induction of serum antipeptide | Metastatic | [157] |
| Survivin-2B | Peptide | Increase of Survivin-2B-specific CTL frequency | Metastatic | [158] |
| Set of 10 overlapping p53 synthetic long peptides | Peptide | Induction of p53-specific CD4 + and CD8 + T-cell responses and p53-specific CTL reactivity | Metastatic | [159] |
| mRNA-4157 | RNA-based vaccine | Induce strong neoantigen-specific T=cell responses | Metastatic | [42] |
| NCI 4650 (mRNA 4650) | RNA-based vaccine | A portion of results showed that this vaccine was safe and significantly induced neoantigen-specific CD8 and CD4 T cells responses against CRC neoepitopes | Metastatic | [160] |
| GI-6207 | Yeast | Decreased serum CEA and calcitonin, and improve CD8 + T cells and CD4 + T responses, | Metastatic | [82] |
| GI-6301 | Yeast |
Decreased tumor density and level of serum CEA of in CRC-treated patients therapeutic |
Metastatic | [84] |
| GI-4000 | Yeast | Demonstrated a favorable safety profile and immunogenicity in the majority of subjects | Metastatic | [83] |
Hurdles of Cancer Vaccines
Therapeutic cancer vaccines have been demonstrated to improve the clinical outcomes of patients and increased tumor burden, despite the great efforts to discover possible target antigens for CRC vaccines and several clinical trials over the past two decades [88]. These therapeutic vaccines do not have a significant effect as monotherapy in CRC, especially in the advanced stages based on the existence of Co-inhibitory and inhibitory receptors that suppress T-cell function in the TME and make them dysfunctional. Also, most clinical trials showed no considerable survival benefit when using a single-peptide vaccine [89]. A promising new approach could be genetically modified cancer vaccines that harness both innate and adaptive immune responses to elicit long-lasting anti-tumor effects and prevent tumor recurrence. Unlike whole-cell vaccines, autologous vaccines do not employ the entire tumor cell in their formulation [58]. Autologous vaccines are isolated and altered to be injected back into the same patient’s body [59]. Utilizing the individual’s own cells in autologous vaccines reduces the likelihood of rejection, and these cells specifically contain the antigens that are highly efficient in triggering an immune response [58]. Future research on antigens, adjuvants, and methods of delivery are crucial to develop new cancer vaccines with low toxicity and high benefits in many patients.
Cell Therapy Strategies
CAR-T-Cell Therapy
CAR-T-cell immunotherapy is a new method of treatment that genetically modifies T cells to fight against cancer [90].
Several techniques are currently under development to improve CAR-T-cell therapy. These include methods to enable them to counteract the immunosuppressive microenvironment of cancer by targeting and destroying PD1 and CTLA-4, as well as CARs that can target two different cancer antigens, among other approaches, to enhance specificity and safety [91]. Besides, there are ongoing efforts to develop genetic modification techniques that would make CAR-T cells from healthy donors suitable for allogeneic use as a treatment option [92–94].
To address the challenges of reducing complications associated with CAR-T-cell therapy in CRC, new approaches are being proposed. These include techniques such as engineering T cells with immune-activating molecules, administering T cells regionally, using bispecific T-cell engagers, and employing combinatorial target antigen recognition [95]. Patient-derived xenograft (PDX) mouse models of CRC are created by engrafting tumor tissue from patients. These models accurately represent the molecular heterogeneity of the patients and replicate the tumor’s immunosuppressive microenvironment. PDX models have been established as a trustworthy model, and humanized models have been developed for two preclinical studies of anti-CRC CAR-T cells [96]. In xenograft models, CAR-T cells are either injected together with cancer cells or separately a few days after the cancer cell administration, once the tumor has formed. A few examinations utilizing CAR-T cells focusing on CEA or EpCAM showed that co-injection of CAR-T and growth cells repressed or deferred cancer development [97]. In 2017, Ang et al. conducted preclinical tests on CAR-T cells targeting CRC, focusing on the cytotoxic effects of EpCAM-directed CAR-T cells. Their study found that multiple infusions of EpCAM CAR-T cells, created through mRNA electroporation, delayed the progression of cancer in mice with CRC xenografts and showed promising results [98].
One study demonstrated significant anti-tumor effectiveness of third-generation MSLN-CAR-T cells, which remained active for at least 10 days after their administration. Another study showed that HER2-CAR-T cells could lead to tumor relapse or elimination in a PDX mouse model of CRC and protect the treated animals from cancer recurrence [99]. Due to the positive results obtained in preclinical studies, numerous CAR-T-cell therapies developed against CRC are currently undergoing evaluation in clinical trials to determine their effectiveness and safety in combating tumors.
Furthermore, second-generation CAR-T cells that target CEA have shown excellent anti-tumor effects both in laboratory tests and animal studies, and their effectiveness can be increased by combining them with interleukins, such as IL-12 [100]. Tandem CAR-T cells that target both CEA and CD30 have demonstrated increased cytotoxicity, persistence, and the release of Perforin and granzyme B compared to CAR-T cells that target only CEA. Similarly, CD30/TAG72-CAR-T cells have shown increased cytotoxicity compared to TAG72-CAR-T cells [101]. By contrast, CAR-T cells that target both CEA and CD25 showed increased cell persistence but similar cytotoxicity compared to anti-CEA CAR-T cells in CRC models. In in vivo studies using mouse models of CRC, the CAR-T-cell doses typically range from 2_106 to 2_107 [102]. Moreover, when CEA-CAR-T cells were co-administered with mesenchymal stem cells expressing IL-7 and IL-12, tumor inhibition was significantly enhanced, leading to prolonged survival. Typically, in treatments where CAR-T cells are administered after tumor formation, multiple doses are usually given [95]. One of these studies, conducted by Huang et al., demonstrated cancer eradication using EGFRvIII-CAR-T cells in combination with miR-153, which suppresses indoleamine 2,3-dioxygenase 1 (IDO1), a protein that is negatively associated with patient survival [103]. In addition, combining CEA-CAR-T cells with recombinant human IL-12 has shown to have a superior anti-tumor effect compared to using CAR-T cells alone. These findings indicate that the use of cytokines or other therapeutic approaches in conjunction with CAR-T cells can improve their ability to fight tumors, as seen in animal models of CRC. However, preclinical studies have also shown that CAR-T-cell therapy directed toward CRC antigens such as CEA, EpCAM, GUCY2C, NKG2DL, and PLAP can be effective in inhibiting tumor growth [104]. In addition to intravenous administration, the effectiveness and safety of CAR-T cells targeting EpCAM and GUCY2C have also been evaluated through local administration via intraperitoneal implantation [105].
There has been much progress in developing CAR-T cells to treat hematologic tumors in clinical trials, but fewer trials are conducted on solid tumors, such as CRC. This is partly because of the lack of appropriate tumor-specific antigens that can be targeted by CAR-T cells, potentially causing on/off-tumor toxicity due to the accidental killing of surrounding non-malignant cells that express the target antigen [106, 107].
In Table 5, we provide an overview of all of the clinical trials involving CAR-T cells against CRC that are currently registered at clinicaltrials.gov. Various studies were found on the effect of CAR-T cells on the removal of colorectal tumor cells. There are 11 different antigens on CRC that have been targeted by CAR-T-cell studies registered at clinicaltrials.gov, including HER2 [108], MUC1, guanylate cyclase-C [56], NKG2D ligands (7), CD133 [108], EPCAM [108], CEA (7), B7-H3, mesothelin [56], C-met [108], and EGFR [56].
Table 5.
CAR-T cells used in clinical trials of CRC patients
| Number | Identifier | phase | Type of cancer | Status | Antigen | CAR Formats | participant | References |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT02349724 | 1 | Colorectal, lung, gastric, breast, and pancreas cancer | Unknown | CEA | scFv-CD28/CD3ζ | 75 | https://clinicaltrials.gov/ct2/show/NCT02349724 |
| 2 | NCT03682744 | 1 | Colorectal, peritoneal, gastric, breast, and pancreas cancer, peritoneal metastases | Withdrawn | CEA | N/A | 0 | https://clinicaltrials.gov/ct2/show/NCT03682744 |
| 3 | NCT03542799 | 1 | Metastatic CRC | Unknown | EGFR/IL-12 | TRUCKs | 20 | https://clinicaltrials.gov/ct2/show/NCT03542799 |
| 4 | NCT03152435 | ½ | EGFR-positive Colorectal Cancer | Unknown | EGFR | 4-1BB:CD28:CD3 | 20 | https://clinicaltrials.gov/ct2/show/NCT03152435 |
| 5 | NCT02617134 | ½ | Malignant Glioma of Brain, Colorectal Carcinoma, Gastric Carcinoma | Unknown | MUC1 | N/A | 20 | https://clinicaltrials.gov/ct2/show/NCT02617134 |
| 6 | NCT03310008 | 1 | Colon Cancer Liver Metastasis | Unknown | NKG2D ligands | NKG2D link to CD3ζ | 36 | https://clinicaltrials.gov/ct2/show/NCT03310008 |
| 7 | NCT03370198 | 1 | Colon Cancer Liver Metastasis | Unknown | NKG2D ligands | N/A | 1 | https://clinicaltrials.gov/ct2/show/NCT03370198 |
| 8 | NCT03692429 | 1 | Unresectable Metastatic Colorectal Cancer | Recruiting | NKG2D ligands | NKG2D link to CD3ζ | 49 | https://clinicaltrials.gov/ct2/show/NCT03692429 |
| 9 | NCT02959151 | ½ | Hepatocellular, Pancreatic Cancer, Metastatic Colorectal Cancer | Unknown | CEA | N/A | 20 | https://clinicaltrials.gov/ct2/show/NCT02959151 |
| 10 | NCT04503980 | 1 | Colorectal Cancer, Ovarian Cancer | Recruiting | mesothelin | N/A | 10 | https://clinicaltrials.gov/ct2/show/NCT04503980 |
| 11 | NCT05089266 | 1 | Colorectal Cancer | Not yet recruiting | mesothelin | N/A | 30 | https://clinicaltrials.gov/ct2/show/NCT05089266 |
| 12 | NCT05240950 | 1 | Colorectal Cancer, Metastatic Liver Cancer | Recruiting | CEA | N/A | 18 | https://clinicaltrials.gov/ct2/show/NCT05240950 |
| 13 | NCT05248048 | 1 | Refractory Metastatic Colorectal Cancer | Recruiting | NKG2D ligands | N/A | 9 | https://clinicaltrials.gov/ct2/show/NCT05248048 |
| 14 | NCT04513431 | 1 | Stage III Colorectal Cancer, Colorectal Cancer Liver Metastasis | Not yet recruiting | CEA | N/A | 18 | https://clinicaltrials.gov/ct2/show/NCT04513431 |
| 15 | NCT05190185 | 1 | Malignant Melanoma, Lung Cancer, or Colorectal Cancer | Recruiting | B7-H3 | N/A | 18 | https://clinicaltrials.gov/ct2/show/NCT05190185 |
| 16 | NCT04550663 | 1 | Solid Tumor, Hepatocellular Carcinoma, Colorectal Cancer, Glioma | Not yet recruiting | NKG2D ligands | 10 | https://clinicaltrials.gov/ct2/show/NCT04550663 | |
| 17 | NCT04348643 | ½ | Solid Tumor, Lung Cancer, Colorectal Cancer, Liver Cancer, Pancreatic Cancer, Gastric Cancer, Breast Cancer | Recruiting | CEA | N/A | 40 | https://clinicaltrials.gov/ct2/show/NCT04348643 |
| 18 | NCT05415475 | 1 | Colorectal Cancer, Esophageal Cancer, Stomach Cancer, Pancreatic Cancer, Metastatic Tumor Recurrent Cancer | Recruiting | CEA | N/A | 36 | https://clinicaltrials.gov/ct2/show/NCT05415475 |
| 19 | NCT05396300 | 1 | Colorectal Cancer, Esophageal Cancer, Stomach Cancer, Pancreatic Cancer, Metastatic Tumor Recurrent Cancer | Recruiting | CEA | N/A | 60 | https://clinicaltrials.gov/ct2/show/NCT05396300 |
| 20 | NCT02713984 | ½ | Breast Cancer, Ovarian Cancer, Lung Cancer, Gastric Cancer, Colorectal Cancer, Glioma, Pancreatic Cancer | Withdrawn | HER2 | N/A | 0 | https://clinicaltrials.gov/ct2/show/NCT02713984 |
| 21 | NCT03638206 | ½ | B-cell Acute Lymphoblastic Leukemia, Lymphoma, Myeloid Leukemia, Multiple Myeloma, Hepatoma, Gastric Cancer, Pancreatic Cancer, Mesothelioma, Colorectal Cancer, Esophagus Cancer, Lung Cancer, Glioma, Melanoma, Synovial Sarcoma, Ovarian Cancer, Renal Carcinoma | Recruiting | C-met | N/A | 73 | https://clinicaltrials.gov/ct2/show/NCT03638206 |
| 22 | NCT05028933 | 1 | Advanced Hepatocellular Carcinoma, Advanced Colorectal Cancer, Advanced Gastric Cancer, Advanced Pancreatic Cancer | Recruiting | EPCAM | N/A | 48 | https://clinicaltrials.gov/ct2/show/NCT05028933 |
| 23 | NCT05239143 | 1 | Breast Cancer, Ovarian Cancer, Non-Small Cell Lung Cancer, Colorectal Cancer, Pancreatic Cancer, Renal Cell Carcinoma, Nasopharyngeal Cancer, Head and Neck Squamous Cell Carcinoma, Gastric Cancer | Recruiting | MUC1 | N/A | 100 | https://clinicaltrials.gov/ct2/show/NCT05239143 |
| 24 | NCT03740256 | 1 | Bladder Cancer, Head and Neck Squamous Cell Carcinoma, Cancer of the Salivary Gland, Lung Cancer, Breast Cancer, Gastric Cancer, Esophageal Cancer, Colorectal Cancer, Pancreatic Adenocarcinoma, Solid Tumor | Recruiting | HER2 | N/A | 45 | https://clinicaltrials.gov/ct2/show/NCT03740256 |
| 25 | NCT04991948 | 1 | Unresectable Metastatic Colorectal Cancer | Recruiting | NKG2D ligands | N/A | 34 | https://clinicaltrials.gov/ct2/show/NCT04991948 |
| 26 | NCT04107142 | 1 | Colorectal Cancer, Triple-Negative Breast Cancer, Sarcoma, Nasopharyngeal Carcinoma, Prostate Cancer, Gastric Cancer | Unknown | NKG2D ligands | N/A | 10 | https://clinicaltrials.gov/ct2/show/NCT04107142 |
| 27 | NCT02541370 | ½ | Liver Cancer, Pancreatic Cancer, Brain Tumor, Breast Cancer, Ovarian Tumor Colorectal Cancer, Acute Myeloid and Lymphoid Leukemias | Completed | CD133 | anti-CD133 scFv-CD137ζ | 20 | https://clinicaltrials.gov/ct2/show/NCT02541370 |
| 28 | NCT05319314 | 1 | Colorectal Cancer | Not yet Recruiting | guanylate cyclase-C | N/A | 30 | https://clinicaltrials.gov/ct2/show/NCT05319314 |
| 29 | NCT05287165 | 1 | Advanced Solid Tumors, Digestive System Neoplasms, Pancreatic Cancer, Resectable Colorectal (Colon or Rectal) Cancer | Recruiting | guanylate cyclase-C | N/A | 19 | https://clinicaltrials.gov/ct2/show/NCT05287165 |
For example, B7-H3 is a co-stimulatory molecule for T cells that make T cells proliferate and differentiate into cytotoxic T cells. Healthy tissues only express a very limited level of B7-H3. Nonetheless, B7-H3 is overexpressed in CRC, causing NK cells to be unable to recognize and kill tumor cells [109].
Some CRC cells have MUC1 overexpression, an immunogenic molecule related to TCR and BCR epitopes, which mediates the disease’s metastasis, chemical resistance, and worse prognosis [110].
Most studies do not specify what method was used to introduce chimeric antigen receptors to T cells. Only two studies have used lentiviral methods for this purpose. CAR Formats are not explained in most studies; only in a few cases that are listed in Table 5.
NK-Cell Therapy
NK cells are a type of immune cell that play a critical role in the body’s defense against infectious agents and cancer cells. The mechanism of action involves their ability to recognize and eliminate abnormal cells without prior sensitization or the need for the immune system to be activated [111]. NK cells have a range of receptors on their surface that can detect the presence of stress-related molecules or proteins on the surfaces of abnormal cells, such as infected cells or tumor cells [112]. These receptors include killer cell immunoglobulin-like receptors (KIRs), natural cytotoxicity receptors (NCRs), and NKG2D receptors. When NK cells recognize abnormal cells, they release cytotoxic granules containing Perforin and Granzymes. Perforin creates pores in the target cell’s membrane, allowing the entry of Granzymes. Granzymes then trigger a cascade of events within the target cell, leading to cell death through apoptosis or programmed cell death [113]. Additionally, these cells can also induce cell death by directly engaging death receptors on the target cell surface, such as Fas receptor and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptor. NK cells can also produce cytokines, such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), which help in stimulating other immune cells, enhancing the immune response, and aiding in the clearance of infected or abnormal cells [114]. NK-cell therapy involves usage of these specialized immune cells as a treatment for various types of cancer. The therapy focuses on enhancing the activity and efficacy of NK cells to target and destroy cancer cells more effectively. NK-cell therapy can be used for various types of cancer, including CRC, as NK cells can recognize and target a broad range of cancer cells [115]. These cells can selectively attack cancer cells while sparing healthy cells, reducing the likelihood of side effects compared to traditional treatments like chemotherapy or radiation. Unlike other forms of cell-based immunotherapy, such as T-cell therapy, NK cells do not carry the risk of causing GVHD, a potentially severe immune reaction [115]. Despite all of these advantages, NK cells have a relatively short lifespan compared to other immune cells, which may limit the long-term efficacy of the therapy. Obtaining a sufficient number of high-quality NK cells for therapy can be challenging and may require advanced laboratory techniques, making it less accessible than other treatments [116]. Fortunately, in the context of CRC, NK-cell therapy has shown promising results in preclinical and early-phase clinical trials. Studies have demonstrated that NK-cell therapy can enhance the anti-tumor immune response and improve patient outcomes. However, further research is needed to optimize NK-cell therapy approaches and explore combination treatments to maximize efficacy against CRC [117]. Several clinical trials have explored the intratumoral infusion of NK cells in CRC patients. Intratumoral administration allows direct delivery of NK cells to the tumor microenvironment, enhancing their cytotoxicity and potential anti-tumoral effect. A phase I/II clinical trial by Hsu et al. (2015) demonstrated the feasibility and safety of intratumoral NK-cell infusion in mCRC patients and reported objective tumor responses. Adoptive NK-cell transfer involves expanding and activating autologous or allogeneic NK cells ex vivo and subsequently infusing them back into the patient to enhance anti-tumor activity [118]. The results of a phase II trial evaluating the efficacy of adoptive transfer of activated NK cells in patients with advanced CRC. The trial reported significant improvements in progression-free survival and overall survival in the NK-cell therapy cohort compared to the control group [119]. Several ongoing clinical trials are exploring the potential of combining NK cell-based therapies with other treatment modalities. For example, a phase I/II trial by Hu, Hung, and Huang (2020) is investigating the safety and efficacy of combining NK-cell therapy with immune checkpoint inhibitors in advanced CRC patients. Preliminary results showed enhanced anti-tumor responses and improved overall survival of patients receiving the combination therapy. The genetic engineering of NK cells has emerged as a promising strategy to enhance their anti-tumor activity. Clinical trials exploring the use of NK cells in CRC have shown promising results, both as monotherapy and in combination with other treatment approaches. Intratumoral NK-cell infusion, adoptive NK-cell transfer, and combination therapies have demonstrated improved clinical outcomes in CRC patients. Further studies incorporating NK-cell engineering and optimized treatment protocols are necessary to fully harness the potential of NK cell-based therapies for CRC treatment.
CAR-NK-Cell Therapy
Chimeric antigen receptor (CAR) NK cells are a type of immunotherapy that involves engineering NK cells to express a CAR. A CAR is a synthetic receptor that can be designed to recognize specific tumor antigens. When CAR NK cells encounter tumor cells that express their target antigen, they can kill them directly or activate other immune cells to attack the tumor [120]. CAR NK cells have several advantages over other types of immunotherapy, such as CAR-T cells. First, NK cells are less likely to cause cytokine release syndrome (CRS), a severe side effect that can occur with CAR-T-cell therapy. Second, NK cells can be obtained from allogeneic donors, which means that they can be used to treat patients who are not eligible for autologous CAR-T-cell therapy (i.e., therapy that uses the patient’s own NK cells). Third, NK cells can be expanded and engineered more efficiently than T cells [121]. Several clinical trials are currently underway to evaluate the safety and efficacy of CAR NK cells for the treatment of colorectal cancer (CRC). For example, the results of a phase I trial of CAR NK cells targeting the EpCAM. The trial showed that CAR NK-cell therapy was safe and feasible, with no patients experiencing CRS or other serious side effects. In addition, 8 of the 11 patients who received CAR NK-cell therapy had a clinical response to treatment, including 2 patients who achieved complete remission [122]. Another clinical trial is evaluating the safety and efficacy of CAR NK cells targeting the NKG2D ligand MICA/B in patients with advanced CRC. The trial is still ongoing, but preliminary results have shown that CAR NK-cell therapy is safe and well tolerated. In addition, some patients have experienced tumor shrinkage and/or prolonged survival. Overall, the results of early clinical trials suggest that CAR NK-cell therapy is a promising new treatment approach for CRC. However, more research is needed to confirm the long-term safety and efficacy of this therapy.
Hurdles of Cell Therapy Strategies
Cell therapy strategies, specifically CAR-T-cell therapy, have been successful in treating blood cancers but face challenges when it comes to treating solid tumors. These challenges include finding an ideal target antigen, reaching the tumor, and survive in the tumor. Additionally, the toxicity of CAR-T cells to the human body and the potential for antigen escape are other concerns [123]. Solid tumors pose difficulties in targeting antigens because they are expressed at different levels on normal tissues, increasing the risk of on-target off-tumor toxicity. Furthermore, the dense extracellular matrix [124] and lack of certain chemokines [125] in solid tumors hinder the migration and invasion of CAR-T cells. However, there are potential strategies to address these hurdles, such as targeting tumor-specific post-translational modifications and using anti-angiogenic therapy to normalize tumor vasculature [126]. The tumor microenvironment also plays a role in limiting the effectiveness of CAR-T-cell therapy, and efforts are being made to modify the metabolic profiles of CAR-T cells to enhance their function. For example, several chemotherapeutic drugs, including sunitinib, modulate tumor microenvironment components (such as Treg and MDSCs). Researchers reported that sunitinib combined with carbonic anhydrase IX (CAIX) targeting CAR-T cells increased infiltration and proliferation of CAIX-specific CAR-T cells by decreasing the presence of MDSCs and increasing the expression of the target antigen [5]. Toxicities associated with CAR-T-cell therapy include anaphylaxis, tumor lysis syndrome, infectious diseases, cytokine release syndrome (CRS), and neurologic toxicities [127–129]. Although most of these symptoms have not been reported in solid tumors, they should be considered when designing CAR-T cells for solid tumors, such as CRC. Various approaches, such as using complete human sequences in CAR construction to prevent allergic reactions and engineering CAR-T cells to secrete IL-1 receptor antagonists to control CRS, are being explored to address these toxicities [23, 130]. Another challenge is tumor resistance to antigen-targeting CAR-T cells, with loss of antigen expression in malignant cells being reported in most patients [131, 132].
Conclusion and Future Perspectives
In conclusion, the treatment landscape for CRC has witnessed significant evolution with the advent of targeted therapies. Chemotherapy backbones like FOLFOX/CAPOX and FOLFIRI remain central for CRC treatment, while newer combinations with oxaliplatin may pose neurotoxicity concerns. Targeted therapies such as Cetuximab and Panitumumab benefit RAS/RAF wild-type, microsatellite stable CRC, while immunotherapies like Pembrolizumab and Nivolumab offer promise for high microsatellite instability/mismatch repair-deficient cases. Combination therapies with immune checkpoint inhibitors are being explored to tackle resistance. Specific molecular subtypes, like BRAF mutant CRC, HER2-amplified CRC, and NTRK fusions, have seen breakthroughs with targeted therapies, expanding treatment options. Recent advancements in immunotherapy, encompassing monoclonal antibodies, checkpoint inhibitors, and oncolytic viruses, provide hope for novel CRC treatments (Table 6). Although these approaches are in early stages, they hold potential for improving outcomes. Continued research and clinical trials are essential for refining these therapies, overcoming resistance, and advancing CRC treatment in future.
Table 6.
Comparison of different immunotherapies
| Treatment Type | CAGR | Advantages | Disadvantages | Commercial products | Number of clinical trials |
|---|---|---|---|---|---|
| NK-Cell Therapy | 45–50% from 2021 to 2028 | Enhanced anticancer response | Limited persistence | Yescarta (axicabtagene ciloleucel) by Kite Pharma/Gilead Sciences | Over 200 registered trials |
| Oncolytic Virus | 30–35% from 2021 to 2028 | Direct tumor cell killing | Potential off-target effects | Imlygic (talimogene laherparepvec) by Amgen | Over 600 registered trials |
| Cytokines | 5–10% from 2021 to 2028 | Stimulate immune response | Systemic toxicity | Proleukin (aldesleukin) by Prometheus Laboratories | Over 100 registered trials |
| Immune Checkpoint Inhibitor Therapy | 20–25% from 2021 to 2028 | Enhanced immune response | Autoimmune side effects | Keytruda (Pembrolizumab) by Merck, Opdivo (Nivolumab) by Bristol Myers Squibb, and others | Over 2,500 registered trials |
| Cell-Based Therapy | 30–40% from 2021 to 2028 | Target-specific cancer cells | Difficult manufacturing | Provenge (sipuleucel-T) by Dendreon Pharmaceuticals, Kymriah (tisagenlecleucel) by Novartis, and Yescarta (axicabtagene ciloleucel) by Kite Pharma/Gilead Sciences | Over 1000 registered trials |
| Monoclonal Antibodies | 10–15% from 2021 to 2028 | High specificity | Risk of immunogenicity | Herceptin (Trastuzumab) by Genentech/Roche, Rituxan (Rituximab) by Roche, and numerous others | Over 1000 registered trials |
| Cancer Cell Vaccines | 5–10% from 2021 to 2028 | Induce immune response | Variable effectiveness | Provenge (sipuleucel-T) by Dendreon Pharmaceuticals | Over 100 registered trials |
| CAR-T-Cell Therapy | 40–45% from 2021 to 2028 | Highly effective in some cases | Costly and complex | Commercial products: Kymriah (tisagenlecleucel) by Novartis, Yescarta (axicabtagene ciloleucel) by Kite Pharma/Gilead Sciences, Tecartus (brexucabtagene autoleucel) by Kite Pharma/Gilead Sciences | Over 100 registered trials |
All of these methods have a variety of advantages and disadvantages
The compound annual growth rate (CAGR) is a useful index for comparison
Author’s contribution
NF, SNM, ST, FS, SS, MA, MA, MMR, and PN wrote the manuscript and prepared the tables and figures; NF and SNM collected the references and carried out the primary literature search; and ENM and MT designed and revised the article. All the authors read and approved the final manuscript.
Funding
The authors have not disclosed any funding.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alluri P, Newman LA. Basal-like and triple-negative breast cancers: searching for positives among many negatives. Surg Oncol Clin N Am 2014;23:567–577. 10.1016/j.soc.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators GBDCC. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol Hepatol 2022;7:627–647. 10.1016/S2468-1253(22)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Palma FDE, D’Argenio V, Pol J, Kroemer G, Maiuri MC, Salvatore F. The molecular hallmarks of the serrated pathway in colorectal cancer. Cancers (Basel). 2019. 10.3390/cancers11071017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefani C, Miricescu D, Stanescu S II et al. Growth factors, PI3K/AKT/mTOR and MAPK signaling pathways in colorectal cancer pathogenesis: where are we now? Int J Mol Sci. 2021. 10.3390/ijms221910260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fennell LJ, Kane A, Liu C et al. APC mutation marks an aggressive subtype of BRAF mutant colorectal cancers. Cancers (Basel). 2020. 10.3390/cancers12051171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiwari A, Saraf S, Verma A, Panda PK, Jain SK. Novel targeting approaches and signaling pathways of colorectal cancer: an insight. World J Gastroenterol 2018;24:4428–4435. 10.3748/wjg.v24.i39.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from mypathway, an open-label, phase iia multiple basket study. J Clin Oncol 2018;36:536–542. 10.1200/JCO.2017.75.3780. [DOI] [PubMed] [Google Scholar]
- 8.Jin JY, Kim DW, Lee JW, Han CW, Min WS, Park CW et al. Immune suppression therapy in aplastic anemia: influencing factors on response and survival. Korean J Intern Med 1995;10:25–31. 10.3904/kjim.1995.10.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar M, Thangavel C, Becker RC, Sadayappan S. Monoclonal antibody-based immunotherapy and its role in the development of cardiac toxicity. Cancers (Basel). 2020. 10.3390/cancers13010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan L, Feng HF, Liu HQ et al. Immune checkpoint inhibitors-related thyroid dysfunction: epidemiology, clinical presentation, possible pathogenesis, and management. Front Endocrinol (Lausanne) 2021;12:649863. 10.3389/fendo.2021.649863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem 2006;13:1845–1857. 10.2174/092986706777585059. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Ji Q, Li Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: underlying mechanisms and reversal strategies. J Exp Clin Cancer Res 2021;40:328. 10.1186/s13046-021-02130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 14.Rouyer M, Francois E, Sa Cunha A et al. Effectiveness of first-line cetuximab in wild-type RAS metastatic colorectal cancer according to tumour BRAF mutation status from the EREBUS cohort. Br J Clin Pharmacol 2021;87:1120–1128. 10.1111/bcp.14472. [DOI] [PubMed] [Google Scholar]
- 15.Wei L, Lin Z, Xie S et al. Complete response with cetuximab-based treatment of metastatic colorectal cancers: two case reports and literature review. Front Oncol 2022;12:798515. 10.3389/fonc.2022.798515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopetz S, Guthrie KA, Morris VK, Lenz HJ, Magliocco AM, Maru D et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J Clin Oncol 2021;39:285–294. 10.1200/JCO.20.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe J, Muro K, Shitara K, Yamazaki K, Shiozawa M, Ohori H et al. Panitumumab vs bevacizumab added to standard first-line chemotherapy and overall survival among patients with RAS wild-type, left-sided metastatic colorectal cancer: a randomized clinical trial. JAMA 2023;329:1271–1282. 10.1001/jama.2023.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol 2022;29:3044–3060. 10.3390/curroncol29050247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He R, Zhao X, Liu J, Zhou Y, Zhang X, Cheng F. PD-1 and CTLA-4 inhibitors in combination vs alone for the treatment of advanced melanoma: a systematic review and meta-analysis. Medicine (Baltimore) 2022;101:e30561. 10.1097/md.0000000000030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borelli B, Antoniotti C, Carullo M, Germani MM, Conca V, Masi G. Immune-checkpoint inhibitors (ICIs) in metastatic colorectal cancer (mCRC) patients beyond microsatellite instability. Cancers (Basel). 2022. 10.3390/cancers14204974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol 2013;4:114. 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia W, Zhang T, Huang H et al. Colorectal cancer vaccines: the current scenario and future prospects. Front Immunol 2022;13:942235. 10.3389/fimmu.2022.942235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer 2021;20:41. 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalabi M, Fanchi LF, Dijkstra KK et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566–576. 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 25.Lekshmy M, Dhanya CR, Smrithi JS et al. Peptide vaccines as therapeutic and prophylactic agents for female-specific cancers: the current landscape. Pharmaceuticals 2023;16:1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cercek A, Lumish M, Sinopoli J et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med 2022;386:2363–2376. 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szklarczyk D, Gable AL, Lyon D et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019;47:D607-d613. 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zolaly MA, Mahallawi W, Khawaji ZY, Alahmadi MA. The clinical advances of oncolytic viruses in cancer immunotherapy. Cureus 2023;15:e40742. 10.7759/cureus.40742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiffry J, Thavornwatanayong T, Rao D et al. Oncolytic reovirus (pelareorep) induces autophagy in kras-mutated colorectal cancer. Clin Cancer Res 2021;27:865–876. 10.1158/1078-0432.CCR-20-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parakrama R, Fogel E, Chandy C et al. Immune characterization of metastatic colorectal cancer patients post reovirus administration. BMC Cancer 2020;20:569. 10.1186/s12885-020-07038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C, Chu D, Kalantar-Zadeh K, George J, Young HA, Liu G. Cytokines: from clinical significance to quantification. Advanced Science 2021;8:2004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harbor perspectives in biology 2018;10:a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers (Basel) 2011;3:3856–3893. 10.3390/cancers3043856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhat AA, Nisar S, Singh M et al. Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: emerging avenue for targeted therapy. Cancer Commun (Lond) 2022;42:689–715. 10.1002/cac2.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubensky TW Jr, Reed SG. Adjuvants for cancer vaccines. Semin Immunol 2010;22:155–161. 10.1016/j.smim.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Ni G, Wang T, Walton S et al. Manipulating IL-10 signalling blockade for better immunotherapy. Cell Immunol 2015;293:126–129. 10.1016/j.cellimm.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Perrie Y, Kirby D, Bramwell VW, Mohammed AR. Recent developments in particulate-based vaccines. Recent Pat Drug Deliv Formul 2007;1:117–129. 10.2174/187221107780831897. [DOI] [PubMed] [Google Scholar]
- 38.Goodwin TJ, Huang L. Investigation of phosphorylated adjuvants co-encapsulated with a model cancer peptide antigen for the treatment of colorectal cancer and liver metastasis. Vaccine 2017;35:2550–2557. 10.1016/j.vaccine.2017.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skwarczynski M, Toth I. Peptide-based synthetic vaccines. Chem Sci 2016;7:842–854. 10.1039/c5sc03892h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Neill CL, Shrimali PC, Clapacs ZE, Files MA, Rudra JS. Peptide-based supramolecular vaccine systems. Acta Biomaterialia 2021;133:153–167. 10.1016/j.actbio.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev 2011;239:27–44. 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hazama S, Nakamura Y, Tanaka H et al. A phase IotaI study of five peptides combination with oxaliplatin-based chemotherapy as a first-line therapy for advanced colorectal cancer (FXV study). J Transl Med 2014;12:108. 10.1186/1479-5876-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murahashi M, Hijikata Y, Yamada K et al. Phase I clinical trial of a five-peptide cancer vaccine combined with cyclophosphamide in advanced solid tumors. Clin Immunol 2016;166–167:48–58. 10.1016/j.clim.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Manickan E, Karem KL, Rouse BT. DNA vaccines—a modern gimmick or a boon to vaccinology? Crit Rev Immunol 2017;37:483–498. 10.1615/CritRevImmunol.v37.i2-6.140. [DOI] [PubMed] [Google Scholar]
- 45.Azadi A, Golchini A, Delazar S et al. Recent advances on immune targeted therapy of colorectal cancer using bi-specific antibodies and therapeutic vaccines. Biol Proced Online 2021;23:13. 10.1186/s12575-021-00147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staff C, Mozaffari F, Haller BK, Wahren B, Liljefors M. A phase I safety study of plasmid DNA immunization targeting carcinoembryonic antigen in colorectal cancer patients. Vaccine 2011;29:6817–6822. 10.1016/j.vaccine.2010.12.063. [DOI] [PubMed] [Google Scholar]
- 47.Huang S, Yu X. Antitumor immunity of DNA vaccine based on CTLA-4 fused with HER2 against colon carcinoma. European Journal of Inflammation 2018;16:2058739218768144. 10.1177/2058739218768144. [Google Scholar]
- 48.Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol 2019;10:594. 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu T, Liang Y, Huang L. Development and delivery systems of mRNA vaccines. Front Bioeng Biotechnol 2021;9:718753. 10.3389/fbioe.2021.718753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faghfuri E, Pourfarzi F, Faghfouri AH, Abdoli Shadbad M, Hajiasgharzadeh K, Baradaran B. Recent developments of RNA-based vaccines in cancer immunotherapy. Expert Opin Biol Ther 2021;21:201–218. 10.1080/14712598.2020.1815704. [DOI] [PubMed] [Google Scholar]
- 51.Li C, Liang S, Zhang C et al. Allogenic dendritic cell and tumor cell fused vaccine for targeted imaging and enhanced immunotherapeutic efficacy of gastric cancer. Biomaterials 2015;54:177–187. 10.1016/j.biomaterials.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 52.Mosolits S, Nilsson B, Mellstedt H. Towards therapeutic vaccines for colorectal carcinoma: a review of clinical trials. Expert Rev Vaccines 2005;4:329–350. 10.1586/14760584.4.3.329. [DOI] [PubMed] [Google Scholar]
- 53.Takamura-Ishii M, Nakaya T, Hagiwara K. Regulation of constitutive interferon-stimulated genes (Isgs) in tumor cells contributes to enhanced antitumor response of newcastle disease virus-infected tumor vaccines. Cancers (Basel). 2018. 10.3390/cancers10060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berry J, Vreeland T, Trappey A et al. Cancer vaccines in colon and rectal cancer over the last decade: lessons learned and future directions. Expert Rev Clin Immunol 2017;13:235–245. 10.1080/1744666X.2016.1226132. [DOI] [PubMed] [Google Scholar]
- 55.Baars A, Claessen AM, Wagstaff J et al. A phase II study of active specific immunotherapy and 5-FU/Leucovorin as adjuvant therapy for stage III colon carcinoma. Br J Cancer 2002;86:1230–1234. 10.1038/sj.bjc.6600254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bever KM, Thomas DL 2nd, Zhang J et al. A feasibility study of combined epigenetic and vaccine therapy in advanced colorectal cancer with pharmacodynamic endpoint. Clin Epigenetics 2021;13:25. 10.1186/s13148-021-01014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim VM, Pan X, Soares KC et al. Neoantigen-based EpiGVAX vaccine initiates antitumor immunity in colorectal cancer. JCI Insight. 2020. 10.1172/jci.insight.136368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Theiler J, Korber B. Graph-based optimization of epitope coverage for vaccine antigen design. Stat Med 2018;37:181–194. 10.1002/sim.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung Y, Qin H, Kang CY, Kim S, Kwak LW, Dong C. An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent antilymphoma immunity. Blood 2007;110:2013–2019. 10.1182/blood-2006-12-061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turnis ME, Rooney CM. Enhancement of dendritic cells as vaccines for cancer. Immunotherapy 2010;2:847–862. 10.2217/imt.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sugiyama H. Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. Expert Rev Vaccines 2005;4:503–512. 10.1586/14760584.4.4.503. [DOI] [PubMed] [Google Scholar]
- 62.Maurel J, Caballero-Baños M, Mila J et al. Phase II randomized trial of autologous tumor lysate dendritic cell vaccine (ADC) plus best supportive care (BSC) compared with BSC, in pre-treated advanced colorectal cancer patients. Journal of Clinical Oncology. 2015. 10.1200/jco.2015.33.15_suppl.3048. [Google Scholar]
- 63.Higuchi Y, Koya T, Yuzawa M et al. Enzyme-linked immunosorbent spot assay for the detection of wilms’ tumor 1-specific t cells induced by dendritic cell vaccination. Biomedicines 2015;3:304–315. 10.3390/biomedicines3040304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodriguez J, Castanon E, Perez-Gracia JL et al. A randomized phase II clinical trial of dendritic cell vaccination following complete resection of colon cancer liver metastasis. J Immunother Cancer 2018;6:96. 10.1186/s40425-018-0405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toh HC, Wang WW, Chia WK et al. Clinical benefit of allogeneic melanoma cell lysate-pulsed autologous dendritic cell vaccine in MAGE-positive colorectal cancer patients. Clin Cancer Res 2009;15:7726–7736. 10.1158/1078-0432.CCR-09-1537. [DOI] [PubMed] [Google Scholar]
- 66.van den Bent MJ, Tesileanu CMS, Wick W et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053–22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol 2021;22:813–823. 10.1016/s1470-2045(21)00090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wooster AL, Girgis LH, Brazeale H, Anderson TS, Wood LM, Lowe DB. Dendritic cell vaccine therapy for colorectal cancer. Pharmacol Res 2021;164:105374. 10.1016/j.phrs.2020.105374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maruoka S, Ojima T, Iwamoto H, Kitadani J, Tabata H, Tominaga S et al. Tumor RNA transfected DCs derived from iPS cells elicit cytotoxicity against cancer cells induced from colorectal cancer patients in vitro. Sci Rep 2022;12:3295. 10.1038/s41598-022-07305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panagioti E, Klenerman P, Lee LN, van der Burg SH, Arens R. Features of effective T cell-inducing vaccines against chronic viral infections. Front Immunol 2018;9:276. 10.3389/fimmu.2018.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang S, Good D, Wei MQ. Vaccinations for colorectal cancer: progress, strategies, and novel adjuvants. Int J Mol Sci. 2019. 10.3390/ijms20143403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morse MA, Chaudhry A, Gabitzsch ES, Hobeika AC, Osada T, Clay TM et al. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer Immunol Immunother 2013;62:1293–1301. 10.1007/s00262-013-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snook AE, Baybutt TR, Xiang B et al. Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced T-cell responses in colon cancer patients. J Immunother Cancer 2019;7:104. 10.1186/s40425-019-0576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harrop R, Connolly N, Redchenko I et al. Vaccination of colorectal cancer patients with modified vaccinia ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res 2006;12:3416–3424. 10.1158/1078-0432.CCR-05-2732. [DOI] [PubMed] [Google Scholar]
- 74.Elkord E, Dangoor A, Drury NL et al. An MVA-based vaccine targeting the oncofetal antigen 5T4 in patients undergoing surgical resection of colorectal cancer liver metastases. J Immunother 2008;31:820–829. 10.1097/CJI.0b013e3181876ab3. [DOI] [PubMed] [Google Scholar]
- 75.Kaufman HL, Lenz HJ, Marshall J et al. Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res 2008;14:4843–4849. 10.1158/1078-0432.ccr-08-0276. [DOI] [PubMed] [Google Scholar]
- 76.Erika JC, Amy CH, Donna N et al. Long-term survival of patients with stage III colon cancer treated with VRP-CEA(6D), an alphavirus vector that increases the CD8+ effector memory T cell to Treg ratio. Journal for ImmunoTherapy of Cancer 2020;8:e001662. 10.1136/jitc-2020-001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shata MT, Stevceva L, Agwale S, Lewis GK, Hone DM. Recent advances with recombinant bacterial vaccine vectors. Mol Med Today 2000;6:66–71. 10.1016/s1357-4310(99)01633-0. [DOI] [PubMed] [Google Scholar]
- 78.da Silva AJ, Zangirolami TC, Novo-Mansur MT, Giordano Rde C, Martins EA. Live bacterial vaccine vectors: an overview. Braz J Microbiol 2014;45:1117–1129. 10.1590/s1517-83822014000400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leclerc C. New technologies for vaccine development. Med Sci (Paris) 2007;23:386–390. 10.1051/medsci/2007234386. [DOI] [PubMed] [Google Scholar]
- 80.Stubbs AC, Martin KS, Coeshott C et al. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med 2001;7:625–629. 10.1038/87974. [DOI] [PubMed] [Google Scholar]
- 81.Lu Y, Bellgrau D, Dwyer-Nield LD et al. Mutation-selective tumor remission with ras-targeted, whole yeast-based immunotherapy. Cancer Res 2004;64:5084–5088. 10.1158/0008-5472.CAN-04-1487. [DOI] [PubMed] [Google Scholar]
- 82.Bilusic M, Heery CR, Arlen PM et al. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol Immunother 2014;63:225–234. 10.1007/s00262-013-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cohn A, Morse MA, O’Neil B et al. Whole recombinant saccharomyces cerevisiae yeast expressing ras mutations as treatment for patients with solid tumors bearing ras mutations: results from a phase 1 trial. J Immunother 2018;41:141–150. 10.1097/CJI.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heery CR, Singh BH, Rauckhorst M et al. Phase I trial of a yeast-based therapeutic cancer vaccine (GI-6301) targeting the transcription factor brachyury. Cancer Immunol Res 2015;3:1248–1256. 10.1158/2326-6066.cir-15-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hou Y, Liu R, Hong X et al. Engineering a sustained release vaccine with a pathogen-mimicking manner for robust and durable immune responses. J Control Release 2021;333:162–175. 10.1016/j.jconrel.2021.03.037. [DOI] [PubMed] [Google Scholar]
- 86.Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med 1965;121:439–462. 10.1084/jem.121.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lopes A, Vandermeulen G, Preat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J Exp Clin Cancer Res 2019;38:146. 10.1186/s13046-019-1154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomas S, Prendergast GC. Cancer vaccines: a brief overview. Methods Mol Biol 2016;1403:755–761. 10.1007/978-1-4939-3387-7_43. [DOI] [PubMed] [Google Scholar]
- 89.Gordon B, Gadi VK. The role of the tumor microenvironment in developing successful therapeutic and secondary prophylactic breast cancer vaccines. Vaccines (Basel). 2020. 10.3390/vaccines8030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Z, Xiao X, Saw PE et al. Chimeric antigen receptor T cells in solid tumors: a war against the tumor microenvironment. Science China Life Sciences 2020;63:180–205. [DOI] [PubMed] [Google Scholar]
- 91.Rodriguez-Garcia A, Palazon A, Noguera-Ortega E, Powell DJ Jr, Guedan S. CAR-T cells hit the tumor microenvironment: strategies to overcome tumor escape. Front Immunol 2020;11:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayakawa T, Harris I, Joung J et al. Report of the international regulatory forum on human cell therapy and gene therapy products. Biologicals 2016;44:467–479. [DOI] [PubMed] [Google Scholar]
- 93.Graham C, Jozwik A, Pepper A, Benjamin R. Allogeneic CAR-T cells: more than ease of access? Cells. 2018. 10.3390/cells7100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinez Bedoya D, Dutoit V, Migliorini D. Allogeneic CAR T cells: an alternative to overcome challenges of CAR T cell therapy in glioblastoma. Front Immunol 2021;12:640082. 10.3389/fimmu.2021.640082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li H, Yang C, Cheng H, Huang S, Zheng Y. CAR-T cells for colorectal cancer: target-selection and strategies for improved activity and safety. Journal of Cancer 2021;12:1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rizzo G, Bertotti A, Leto SM, Vetrano S. Patient-derived tumor models: a more suitable tool for pre-clinical studies in colorectal cancer. J Exp Clin Cancer Res 2021;40:178. 10.1186/s13046-021-01970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martinez M, Moon EK. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol 2019;10:128. 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ang WX, Li Z, Chi Z, Du S-H, Chen C, Tay JC et al. Intraperitoneal immunotherapy with T cells stably and transiently expressing anti-EpCAM CAR in xenograft models of peritoneal carcinomatosis. Oncotarget 2017;8:13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li H, Yang C, Cheng H, Huang S, Zheng Y. CAR-T cells for colorectal cancer: target-selection and strategies for improved activity and safety. J Cancer 2021;12:1804–1814. 10.7150/jca.50509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jin K-T, Chen B, Liu Y-Y, Lan H, Yan J-P. Monoclonal antibodies and chimeric antigen receptor (CAR) T cells in the treatment of colorectal cancer. Cancer Cell International 2021;21:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alnefaie A, Albogami S, Asiri Y et al. Chimeric antigen receptor T-cells: an overview of concepts, applications, limitations, and proposed solutions. Front Bioeng Biotechnol 2022;10:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cha SE, YazakiKujawski MJP, Brown C, Shively JE. Tumor regression and immunity in combination therapy with anti-CEA chimeric antigen receptor T cells and anti-CEA-IL2 immunocytokine. Oncoimmunology 2021;10:1899469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akbari B, Ghahri-Saremi N, Soltantoyeh T, Hadjati J, Ghassemi S, Mirzaei HR. Epigenetic strategies to boost CAR T cell therapy. Molecular Therapy 2021;29:2640–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen N, Xu Y, Mou J et al. Targeting of IL-10R on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood cancer journal 2021;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu J, Meng Q, Sun H et al. HER2-specific chimeric antigen receptor-T cells for targeted therapy of metastatic colorectal cancer. Cell Death & Disease 2021;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18:843–851. 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lamers CH, Sleijfer S, van Steenbergen S et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 2013;21:904–912. 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sur D, Havasi A, Cainap C et al. Chimeric antigen receptor T-cell therapy for colorectal cancer. J Clin Med. 2020. 10.3390/jcm9010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ingebrigtsen VA, Boye K, Tekle C, Nesland JM, Flatmark K, Fodstad O. B7–H3 expression in colorectal cancer: nuclear localization strongly predicts poor outcome in colon cancer. Int J Cancer 2012;131:2528–2536. 10.1002/ijc.27566. [DOI] [PubMed] [Google Scholar]
- 110.Guo M, You C, Dou J. Role of transmembrane glycoprotein mucin 1 (MUC1) in various types of colorectal cancer and therapies: current research status and updates. Biomed Pharmacother 2018;107:1318–1325. 10.1016/j.biopha.2018.08.109. [DOI] [PubMed] [Google Scholar]
- 111.Guillerey C. NK cells in the tumor microenvironment. Adv Exp Med Biol 2020;1273:69–90. 10.1007/978-3-030-49270-0_4. [DOI] [PubMed] [Google Scholar]
- 112.Becker PSA, Suck G, Nowakowska P et al. Selection and expansion of natural killer cells for NK cell-based immunotherapy Cancer Immunology. Immunotherapy 2016;65:477–484. 10.1007/s00262-016-1792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu M, Liang S, Zhang C. NK cells in autoimmune diseases: protective or pathogenic? Front Immunol 2021;12:624687. 10.3389/fimmu.2021.624687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol 2018;9:1869. 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Minetto P, Guolo F, Pesce S et al. Harnessing NK cells for cancer treatment. Front Immunol 2019;10:2836. 10.3389/fimmu.2019.02836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Streltsova MA, Erokhina SA, Kanevskiy LM et al. Recurrent stimulation of natural killer cell clones with K562 expressing membrane-bound interleukin-21 affects their phenotype, interferon-γ production, and lifespan. Int J Mol Sci 2019;20:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Q, Zhang H, Ding J et al. Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models. Journal of Immunology Research 2018;2018:4263520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee S-C, Shimasaki N, Lim JS et al. Phase I trial of expanded, activated autologous NK-cell infusions with trastuzumab in patients with HER2-positive cancers. Clinical Cancer Research 2020;26:4494–4502. [DOI] [PubMed] [Google Scholar]
- 119.Ishikawa T, Okayama T, Sakamoto N et al. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with IgG1 antibody in patients with gastric or colorectal cancer. Int J Cancer 2018;142:2599–2609. 10.1002/ijc.31285. [DOI] [PubMed] [Google Scholar]
- 120.Della Chiesa M, Setti C, Giordano C et al. NK cell-based immunotherapy in colorectal cancer. Vaccines (Basel). 2022. 10.3390/vaccines10071033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang L, Meng Y, Feng X, Han Z. CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomarker Research 2022;10:12. 10.1186/s40364-022-00364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H et al. Adoptive transfer of NKG2D CAR mRNA-engineered natural killer cells in colorectal cancer patients. Molecular Therapy 2019;27:1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 2016;3:16011. 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Henke E, Nandigama R, Ergun S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci 2019;6:160. 10.3389/fmolb.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Poznansky MC, Olszak IT, Evans RH et al. Thymocyte emigration is mediated by active movement away from stroma-derived factors. J Clin Invest 2002;109:1101–1110. 10.1172/JCI13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Daenen LG, Shaked Y, Man S et al. Low-dose metronomic cyclophosphamide combined with vascular disrupting therapy induces potent antitumor activity in preclinical human tumor xenograft models. Mol Cancer Ther 2009;8:2872–2881. 10.1158/1535-7163.MCT-09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kochenderfer JN, Dudley ME, Kassim SH et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–549. 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Porter DL, Hwang WT, Frey NV et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015. 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Turtle CJ, Hanafi LA, Berger C et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126:2123–2138. 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018;24:731–738. 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015;125:4017–4023. 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer Journal 2021;11:69. 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Diaz LA Jr, Shiu KK, Kim TW et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol 2022;23:659–670. 10.1016/s1470-2045(22)00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jonker DJ, O’Callaghan CJ, Karapetis CS et al. Cetuximab for the treatment of colorectal cancer. New England Journal of Medicine 2007;357:2040–2048. 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 135.Rosen LS, Jacobs IA, Burkes RL. Bevacizumab in colorectal cancer: current role in treatment and the potential of biosimilars. Target Oncol 2017;12:599–610. 10.1007/s11523-017-0518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu Y, Zhang H, Cui H et al. Combined and targeted drugs delivery system for colorectal cancer treatment: conatumumab decorated, reactive oxygen species sensitive irinotecan prodrug and quercetin co-loaded nanostructured lipid carriers. Drug Deliv 2022;29:342–350. 10.1080/10717544.2022.2027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sartore-Bianchi A, Trusolino L, Martino C et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:738–746. 10.1016/s1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 138.Li G, Suzuki H, Ohishi T, Asano T, Tanaka T, Yanaka M et al. Antitumor activities of a defucosylated anti-EpCAM monoclonal antibody in colorectal carcinoma xenograft models. Int J Mol Med. 2023. 10.3892/ijmm.2023.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim RD, Azad NS, Morse MA et al. Phase II study of ensituximab, a novel chimeric monoclonal antibody, in adults with unresectable. Metastatic Colorectal Cancer. Clin Cancer Res 2020;26:3557–3564. 10.1158/1078-0432.ccr-20-0426. [DOI] [PubMed] [Google Scholar]
- 140.Jiffry J, Thavornwatanayong T, Rao D et al. Oncolytic reovirus (pelareorep) induces autophagy in KRAS-mutated colorectal cancerautophagy augments pelareorep activity in KRAS-mutated CRC. Clinical Cancer Research 2021;27:865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang H, Peng T, Li J et al. Treatment of colon cancer with oncolytic herpes simplex virus in preclinical models. Gene Ther 2016;23:450–459. 10.1038/gt.2016.15. [DOI] [PubMed] [Google Scholar]
- 142.Yuan S, Wu Y, Wang Y, Chen J, Chu L. An oncolytic adenovirus expressing SNORD44 and GAS5 exhibits antitumor effect in colorectal cancer cells. Hum Gene Ther 2017;28:690–700. 10.1089/hum.2017.041. [DOI] [PubMed] [Google Scholar]
- 143.Wang J, Liu T, Chen J. Oncolytic measles virus encoding interleukin-12 mediated antitumor activity and immunologic control of colon cancer in vivo and ex vivo. Cancer Biother Radiopharm 2021;36:774–782. 10.1089/cbr.2019.3084. [DOI] [PubMed] [Google Scholar]
- 144.Deng L, Yang X, Fan J et al. IL-24-armed oncolytic vaccinia virus exerts potent antitumor effects via multiple pathways in colorectal cancer. Oncol Res 2021;28:579–590. 10.3727/096504020x15942028641011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hörig H, Lee DS, Conkright W et al. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol Immunother. 2000. 10.1007/s002620000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Danishmalik SN, Sin JI. Therapeutic tumor control of HER2 DNA vaccines is achieved by an alteration of tumor cells and tumor microenvironment by gemcitabine and anti-Gr-1 Ab treatment in a HER2-expressing tumor model. DNA Cell Biol 2017;36:801–811. 10.1089/dna.2017.3810. [DOI] [PubMed] [Google Scholar]
- 147.Son HY, Apostolopoulos V, Chung JK, Kim CW, Park JU. Protective efficacy of a plasmid DNA vaccine against transgene-specific tumors by Th1 cellular immune responses after intradermal injection. Cell Immunol 2018;329:17–26. 10.1016/j.cellimm.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 148.Ali SA, Ahmad M, Lynam J et al. Anti-tumour therapeutic efficacy of OX40L in murine tumour model. Vaccine 2004;22:3585–3594. 10.1016/j.vaccine.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 149.Cross RS, Malaterre J, Davenport AJ et al. Therapeutic DNA vaccination against colorectal cancer by targeting the MYB oncoprotein. Clin Transl Immunology 2015;4:e30. 10.1038/cti.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ockert D, Schirrmacher V, Beck N et al. Newcastle disease virus-infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin Cancer Res 1996;2:21–28. [PubMed] [Google Scholar]
- 151.Harris JE, Ryan L, Hoover HC Jr et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: eastern cooperative oncology group study E5283. J Clin Oncol 2000;18:148–157. 10.1200/JCO.2000.18.1.148. [DOI] [PubMed] [Google Scholar]
- 152.Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol 1998;16:364–369. 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 153.Fong L, Hou Y, Rivas A et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A 2001;98:8809–8814. 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dillman RO, Nistor GI, Keirstead HS. Autologous dendritic cells loaded with antigens from self-renewing autologous tumor cells as patient-specific therapeutic cancer vaccines. Hum Vaccin Immunother 2023;19:2198467. 10.1080/21645515.2023.2198467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Toubaji A, Achtar M, Provenzano M et al. Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers. Cancer Immunol Immunother 2008;57:1413–1420. 10.1007/s00262-008-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moulton HM, Yoshihara PH, Mason DH, Iversen PL, Triozzi PL. Active specific immunotherapy with a beta-human chorionic gonadotropin peptide vaccine in patients with metastatic colorectal cancer: antibody response is associated with improved survival. Clin Cancer Res 2002;8:2044–2051. [PubMed] [Google Scholar]
- 157.Miyagi Y, Imai N, Sasatomi T et al. Induction of cellular immune responses to tumor cells and peptides in colorectal cancer patients by vaccination with SART3 peptides. Clin Cancer Res 2001;7:3950–3962. [PubMed] [Google Scholar]
- 158.Tsuruma T, Hata F, Torigoe T et al. Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med 2004;2:19. 10.1186/1479-5876-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Speetjens FM, Kuppen PJ, Welters MJ et al. Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin Cancer Res 2009;15:1086–1095. 10.1158/1078-0432.CCR-08-2227. [DOI] [PubMed] [Google Scholar]
- 160.Chehelgerdi M, Chehelgerdi M. The use of RNA-based treatments in the field of cancer immunotherapy. Molecular Cancer 2023;22:106. 10.1186/s12943-023-01807-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.