Abstract
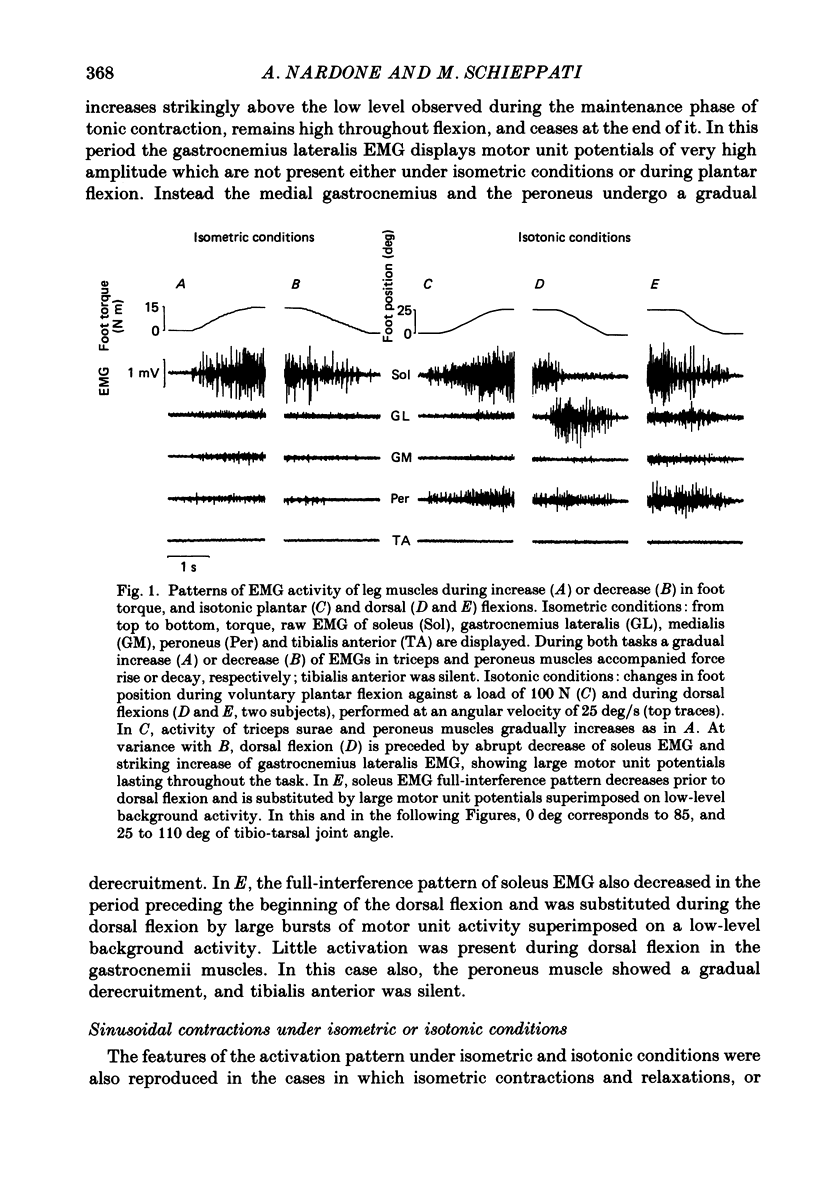
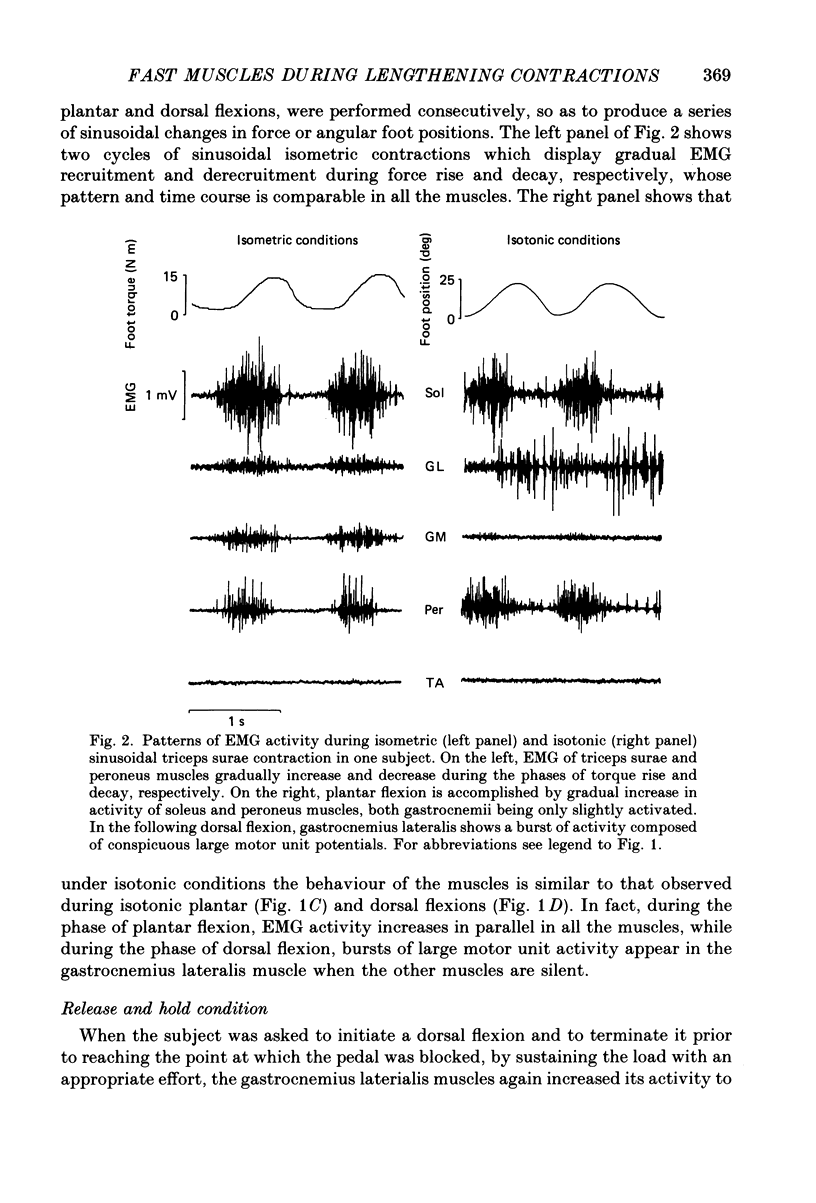
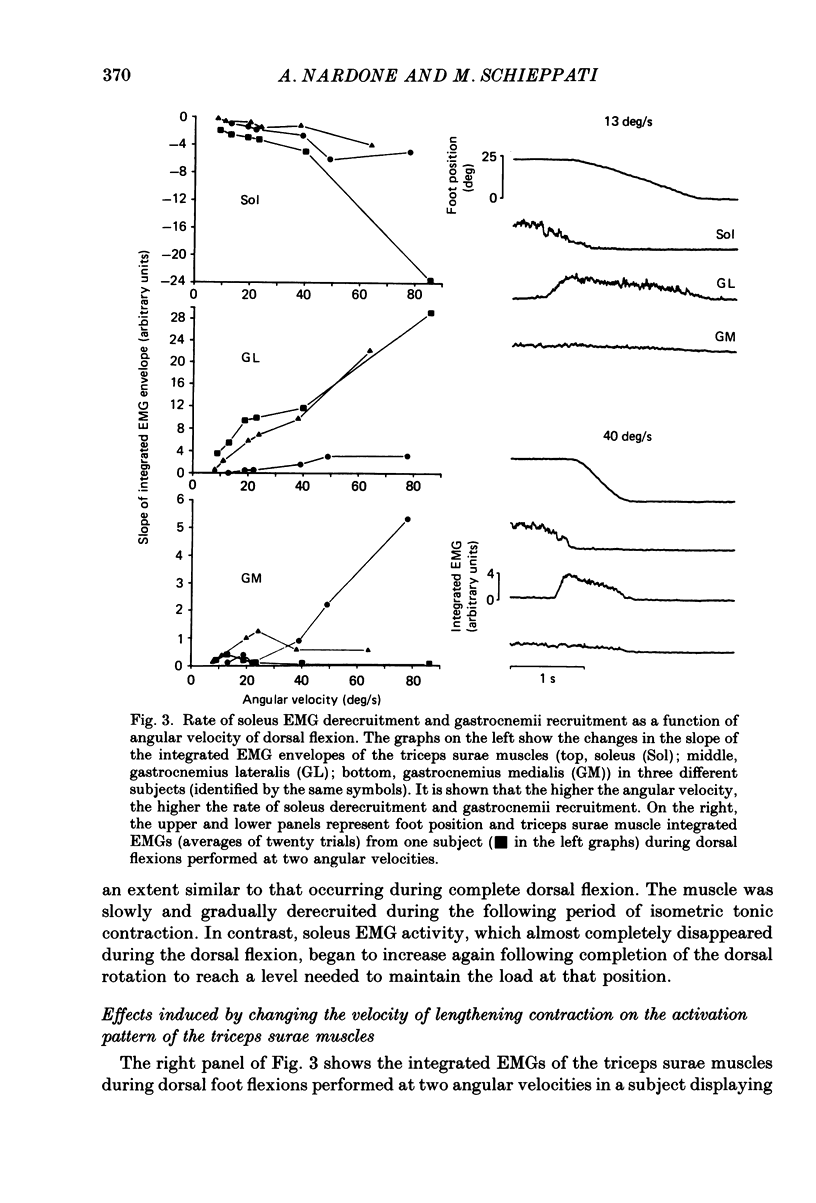
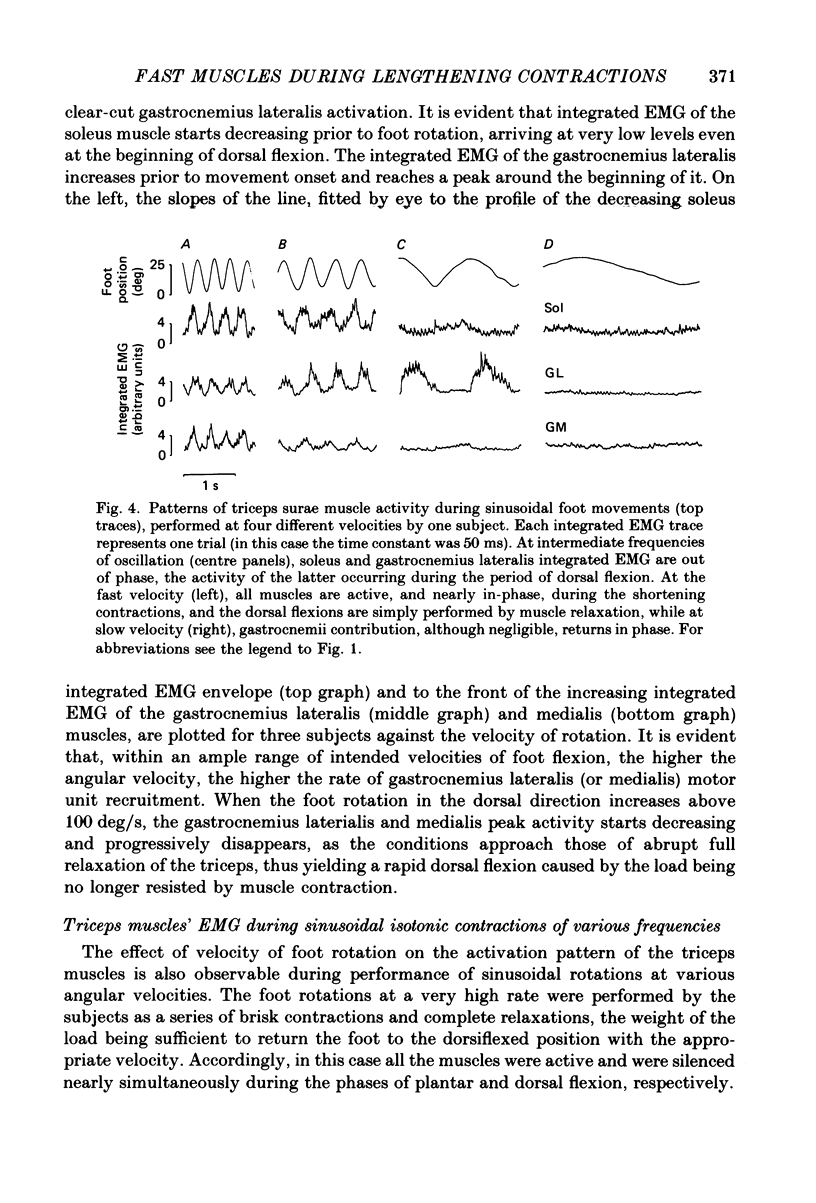
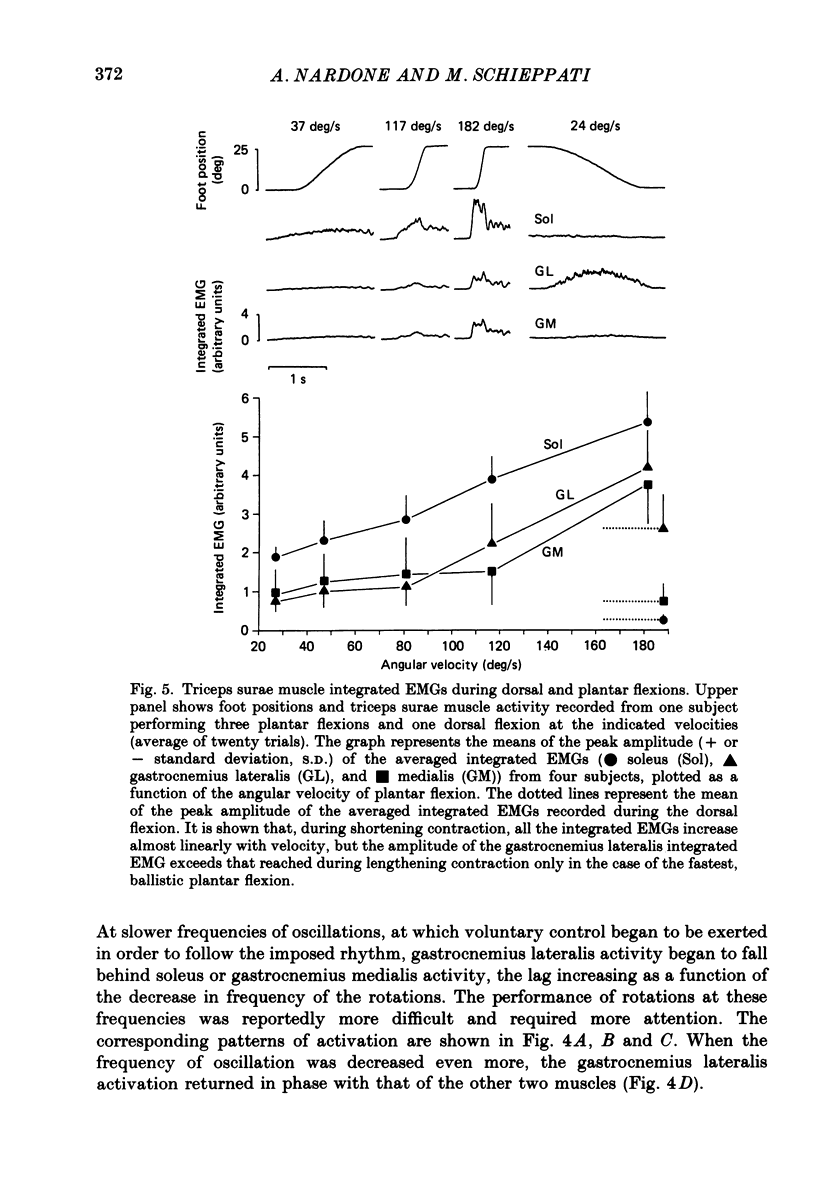
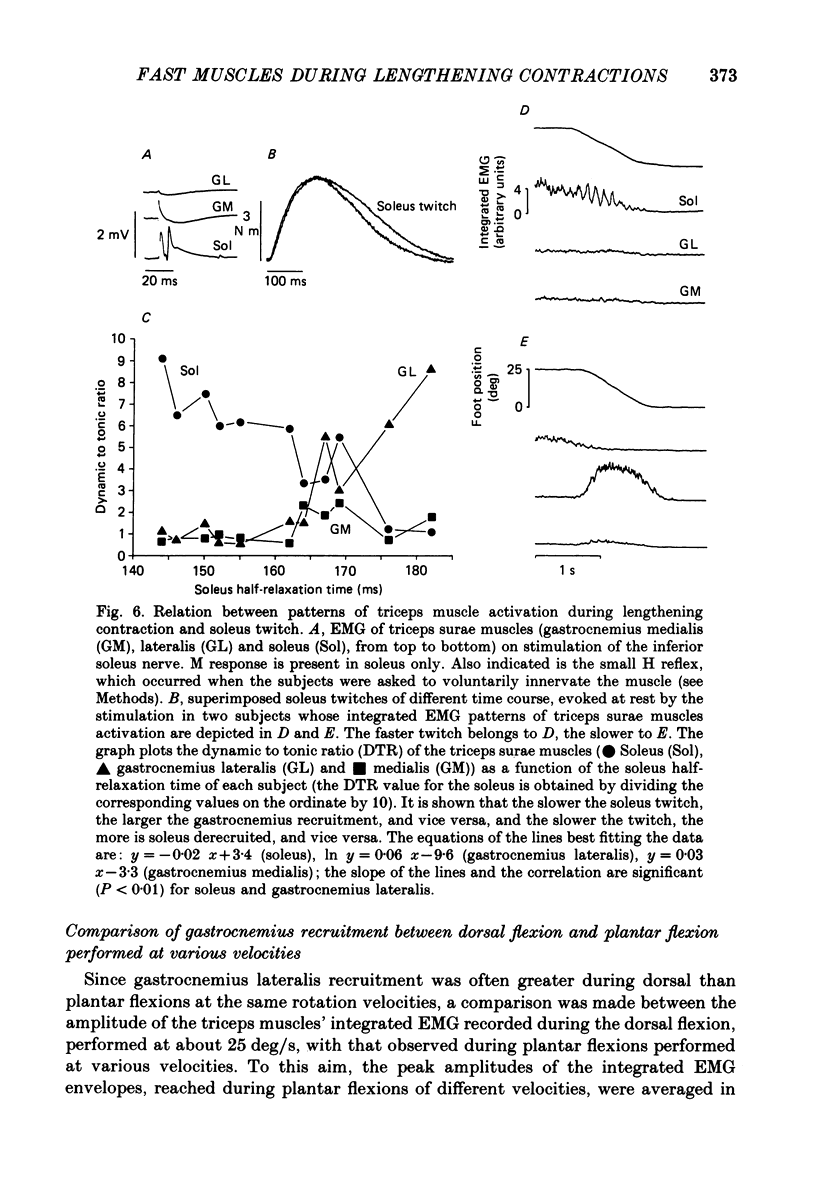
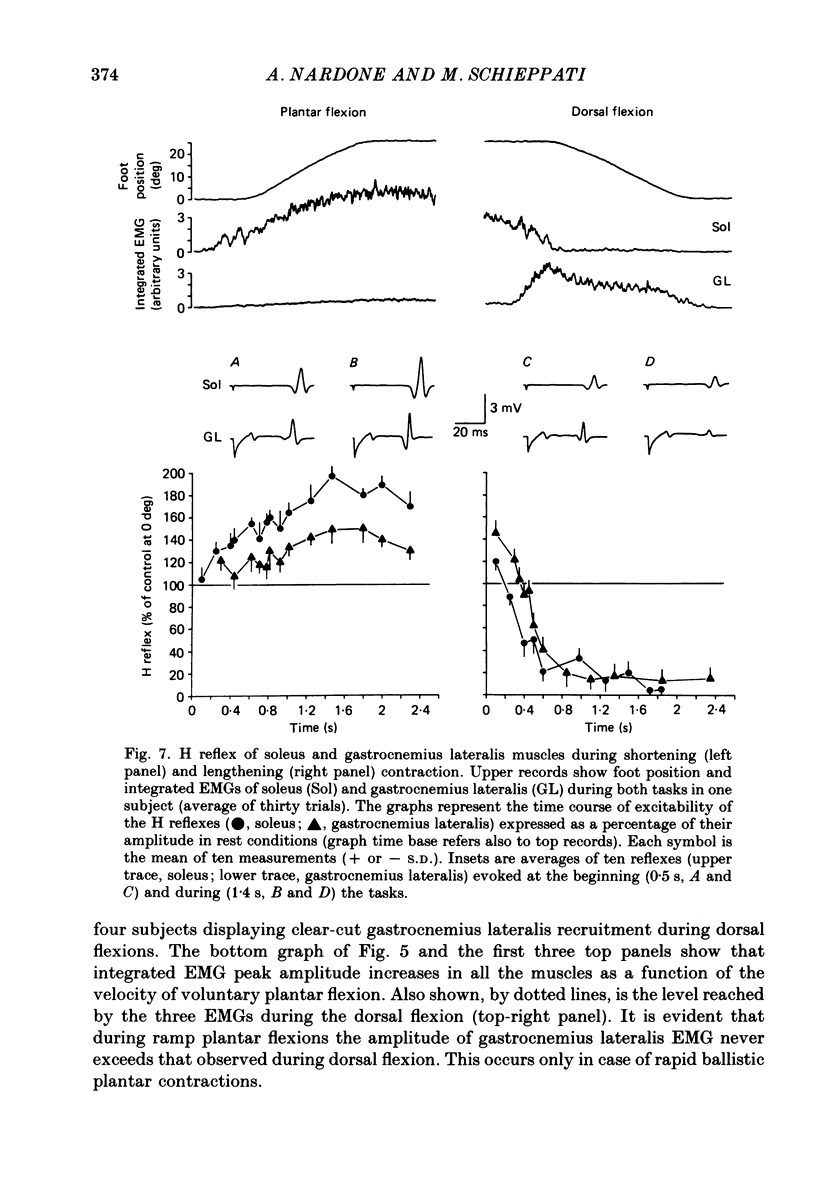
1. Raw or rectified and integrated electromyograms (integrated EMGs) of the leg muscles were recorded during (a) isotonic ramp shortening or lengthening contractions consisting of foot plantar flexions against a constant load, or dorsal flexions accomplished by braking the load and yielding to it, respectively, and (b) isometric increasing or decreasing plantar torques accomplished by graded contractions or relaxations of the triceps muscles. 2. During plantar flexions or increasing torques, the EMG of soleus, gastrocnemius lateralis, medialis, and peroneus increased in parallel. During decreasing torques, motor unit derecruitment took place gradually and simultaneously. The tibialis anterior was silent. During dorsal flexions, one of two characteristic patterns was observed in different subjects: (a) soleus was abruptly derecruited at the beginning of the task, while gastrocnemius lateralis (or medialis) exhibited a large recruitment lasting throughout the lengthening contraction; (b) soleus remained active during the task, showing large motor unit potentials, while the gastrocnemius lateralis recruitment was of a lesser extent than in (a). Peroneus derecruitment was gradual and tibialis anterior activity was absent in both cases. 3. The EMG patterns observed during plantar flexions or in increasing and decreasing torques, and the two patterns observed during shortening or lengthening contractions, were closely reproduced during sinusoidal oscillations of the foot or in isometric contractions and relaxations. 4. When recruitment of the gastrocnemius lateralis was present during dorsal flexion, the slope of its integrated EMG envelope was steeper, the higher the velocity of lengthening contraction. The most rapid and the slowest tasks, however, did not require its activation. Gastrocnemius lateralis integrated EMGs of an amplitude similar to those occurring during lengthening contractions were observed only during ballistic plantar flexions. 5. The two patterns of triceps activation occurring during lengthening contraction could be traced to different mechanical characteristics of the soleus muscles, the gastrocnemius lateralis being activated preferentially in subjects with long soleus half-relaxation times, and the soleus in subjects with short soleus half-relaxation times. 6. The soleus and gastrocnemius lateralis H reflexes were tested during shortening and lengthening contractions.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bawa P., Binder M. D., Ruenzel P., Henneman E. Recruitment order of motoneurons in stretch reflexes is highly correlated with their axonal conduction velocity. J Neurophysiol. 1984 Sep;52(3):410–420. doi: 10.1152/jn.1984.52.3.410. [DOI] [PubMed] [Google Scholar]
- Broman H., De Luca C. J., Mambrito B. Motor unit recruitment and firing rates interaction in the control of human muscles. Brain Res. 1985 Jul 1;337(2):311–319. doi: 10.1016/0006-8993(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Buchthal F., Schmalbruch H. Contraction times of twitches evoked by H-reflexes. Acta Physiol Scand. 1970 Nov;80(3):378–382. doi: 10.1111/j.1748-1716.1970.tb04801.x. [DOI] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L. Muscle spindle activity in man during shortening and lengthening contractions. J Physiol. 1978 Apr;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Group Ia synaptic input to fast and slow twitch motor units of cat triceps surae. J Physiol. 1968 Jun;196(3):605–630. doi: 10.1113/jphysiol.1968.sp008526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Jankowska E., ten Bruggencate G. A comparison of peripheral and rubrospinal synaptic input to slow and fast twitch motor units of triceps surae. J Physiol. 1970 May;207(3):709–732. doi: 10.1113/jphysiol.1970.sp009090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büdingen H. J., Freund H. J. The relationship between the rate of rise of isometric tension and motor unit recruitment in a human forearm muscle. Pflugers Arch. 1976 Mar 11;362(1):61–67. doi: 10.1007/BF00588682. [DOI] [PubMed] [Google Scholar]
- Calancie B., Bawa P. Voluntary and reflexive recruitment of flexor carpi radialis motor units in humans. J Neurophysiol. 1985 May;53(5):1194–1200. doi: 10.1152/jn.1985.53.5.1194. [DOI] [PubMed] [Google Scholar]
- Citterio G., Agostoni E. Selective activation of quadriceps muscle fibers according to bicycling rate. J Appl Physiol Respir Environ Exerc Physiol. 1984 Aug;57(2):371–379. doi: 10.1152/jappl.1984.57.2.371. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different functional types of muscle unit. J Physiol. 1978 Aug;281:301–313. doi: 10.1113/jphysiol.1978.sp012423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J. E. Size principle of motoneuron recruitment and the calibration of muscle force and speed in man. Adv Neurol. 1983;39:227–251. [PubMed] [Google Scholar]
- Dum R. P., Kennedy T. T. Synaptic organization of defined motor-unit types in cat tibialis anterior. J Neurophysiol. 1980 Jun;43(6):1631–1644. doi: 10.1152/jn.1980.43.6.1631. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., ITO M. Distribution of recurrent inhibition among motoneurones. J Physiol. 1961 Dec;159:479–499. doi: 10.1113/jphysiol.1961.sp006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edström L., Grimby L. Effect of exercise on the motor unit. Muscle Nerve. 1986 Feb;9(2):104–126. doi: 10.1002/mus.880090203. [DOI] [PubMed] [Google Scholar]
- Friedman W. A., Sypert G. W., Munson J. B., Fleshman J. W. Recurrent inhibition in type-identified motoneurons. J Neurophysiol. 1981 Dec;46(6):1349–1359. doi: 10.1152/jn.1981.46.6.1349. [DOI] [PubMed] [Google Scholar]
- Garnett R., Stephens J. A. Changes in the recruitment threshold of motor units produced by cutaneous stimulation in man. J Physiol. 1981 Feb;311:463–473. doi: 10.1113/jphysiol.1981.sp013598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby L. Firing properties of single human motor units during locomotion. J Physiol. 1984 Jan;346:195–202. doi: 10.1113/jphysiol.1984.sp015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V. THE EFFECT OF LOAD ON THE HEAT OF SHORTENING OF MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:297–318. doi: 10.1098/rspb.1964.0004. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. I. Effects on alpha-motoneurones innervating hindlimb muscles in cats. Exp Brain Res. 1969;7(4):344–364. doi: 10.1007/BF00237320. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Polgar J., Weightman D., Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973 Jan;18(1):111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M., Westbury D. R. The mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J Physiol. 1969 Oct;204(2):461–474. doi: 10.1113/jphysiol.1969.sp008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K., Burke R. E., Walmsley B. Differential control of fast and slow twitch motor units in the decerebrate cat. Exp Brain Res. 1977 Aug 8;29(1):57–74. doi: 10.1007/BF00236875. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson C. B., Carpenter D. O., Henneman E. Orderly recruitment of muscle action potentials. Arch Neurol. 1968 Dec;19(6):591–597. doi: 10.1001/archneur.1968.00480060061008. [DOI] [PubMed] [Google Scholar]
- PRESTON J. B., WHITLOCK D. G. A comparison of motor cortex effects on slow and fast muscle innervations in the monkey. Exp Neurol. 1963 Apr;7:327–341. doi: 10.1016/0014-4886(63)90079-7. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Bergego C., Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42(3-4):337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Romanò C., Schieppati M. Reflex excitability of human soleus motoneurones during voluntary shortening or lengthening contractions. J Physiol. 1987 Sep;390:271–284. doi: 10.1113/jphysiol.1987.sp016699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall R. W., Piercey M. F., Polosa C., Goldfarb J. Excitation of Renshaw cells in relation to orthodromic and antidromic excitation of motoneurons. J Neurophysiol. 1972 Jan;35(1):137–148. doi: 10.1152/jn.1972.35.1.137. [DOI] [PubMed] [Google Scholar]
- Schieppati M., Nardone A., Musazzi M. Modulation of the Hoffmann reflex by rapid muscle contraction or release. Hum Neurobiol. 1986;5(1):59–66. [PubMed] [Google Scholar]
- Schieppati M., Poloni M., Nardone A. Voluntary muscle release is not accompanied by H-reflex inhibition in patients with upper moto neuron lesions. Neurosci Lett. 1985 Oct 24;61(1-2):177–181. doi: 10.1016/0304-3940(85)90421-5. [DOI] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28(4):345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Betts B., Edgerton V. R., Zernicke R. F. Rapid ankle extension during paw shakes: selective recruitment of fast ankle extensors. J Neurophysiol. 1980 Mar;43(3):612–620. doi: 10.1152/jn.1980.43.3.612. [DOI] [PubMed] [Google Scholar]
- Spector S. A., Gardiner P. F., Zernicke R. F., Roy R. R., Edgerton V. R. Muscle architecture and force-velocity characteristics of cat soleus and medial gastrocnemius: implications for motor control. J Neurophysiol. 1980 Nov;44(5):951–960. doi: 10.1152/jn.1980.44.5.951. [DOI] [PubMed] [Google Scholar]
- Zengel J. E., Reid S. A., Sypert G. W., Munson J. B. Presynaptic inhibition, EPSP amplitude, and motor-unit type in triceps surae motoneurons in the cat. J Neurophysiol. 1983 Apr;49(4):922–931. doi: 10.1152/jn.1983.49.4.922. [DOI] [PubMed] [Google Scholar]


