Abstract
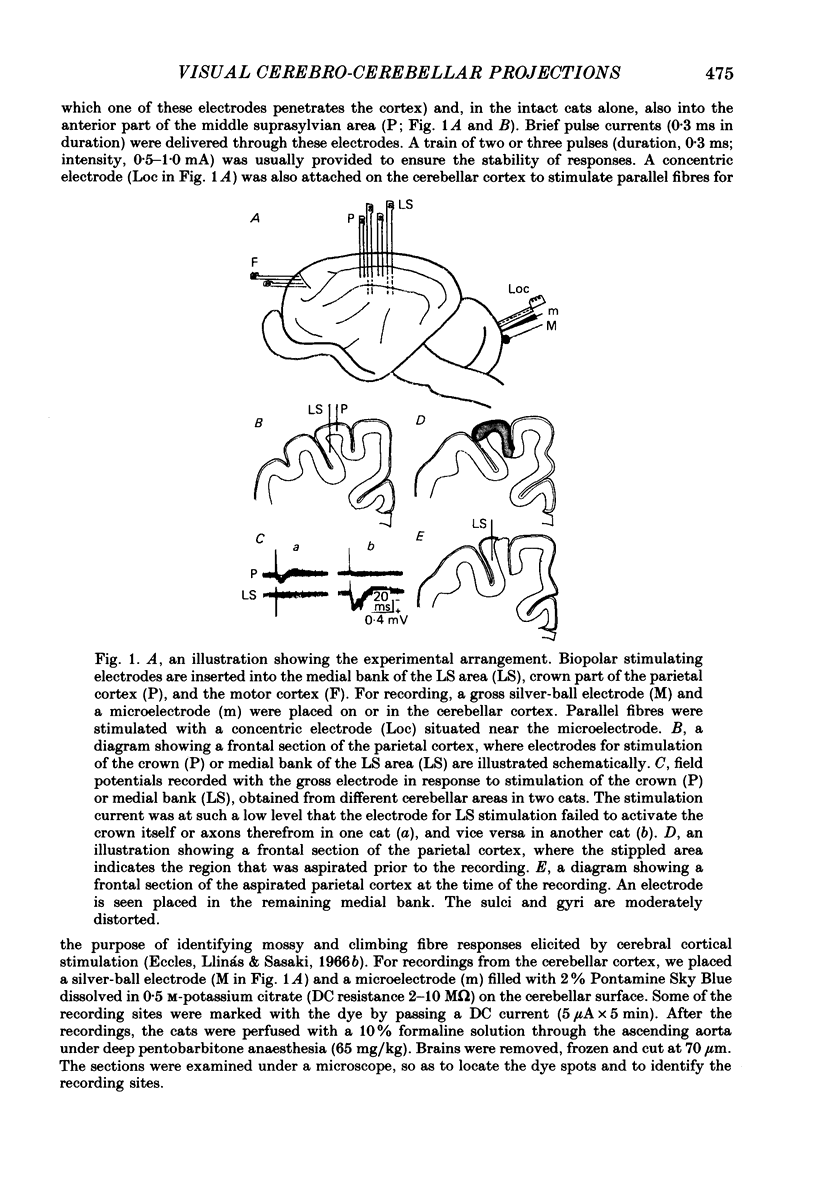
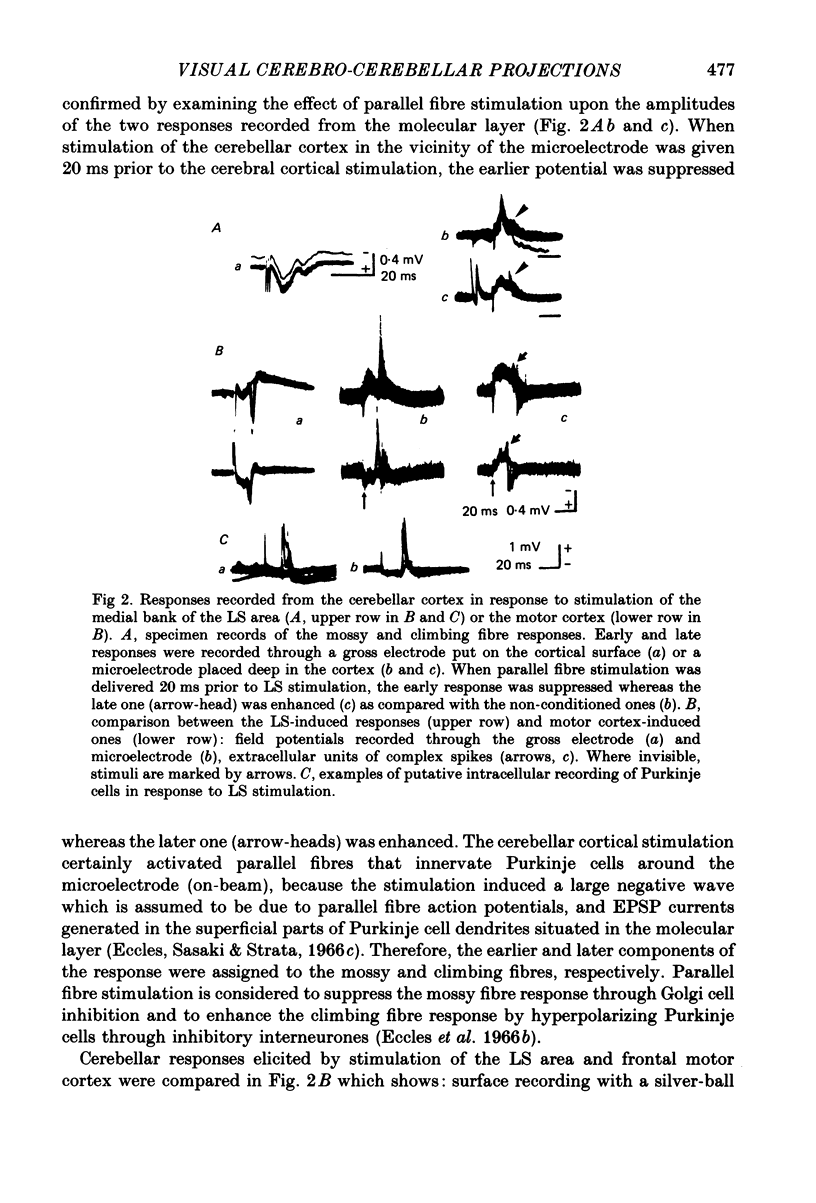
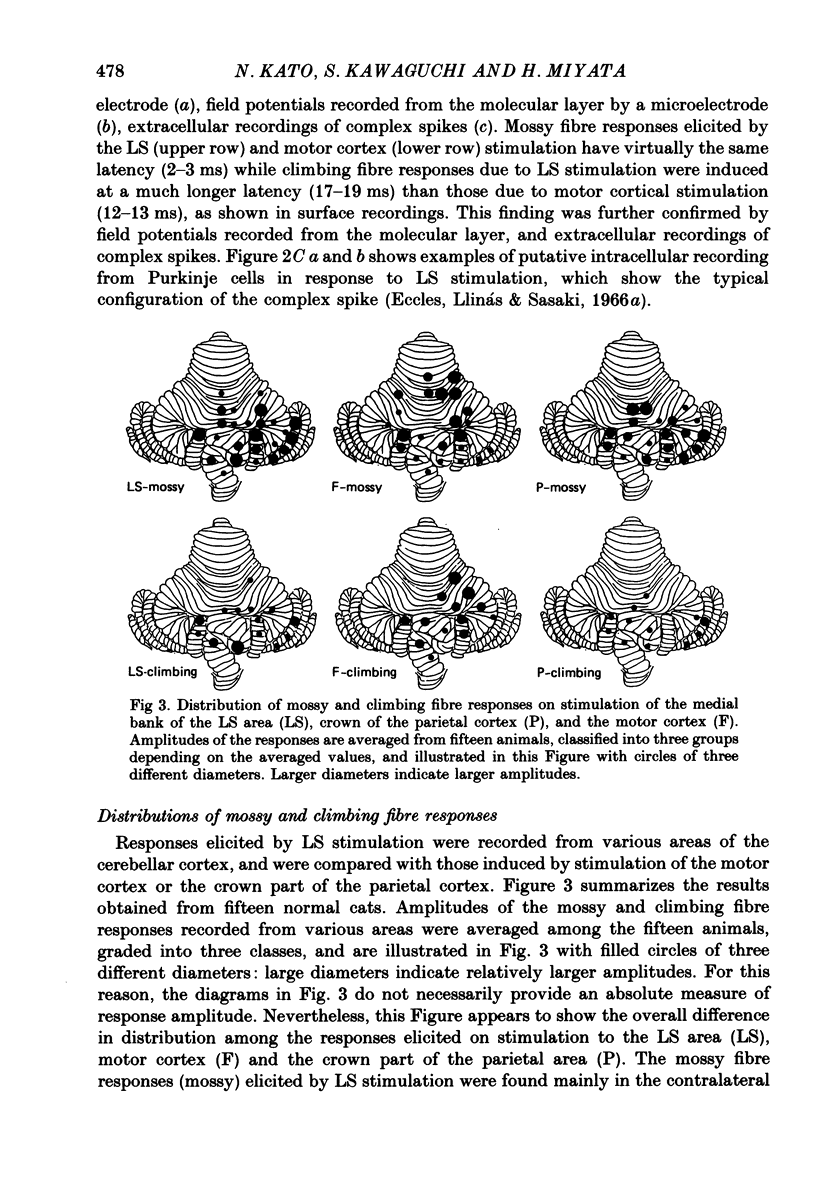
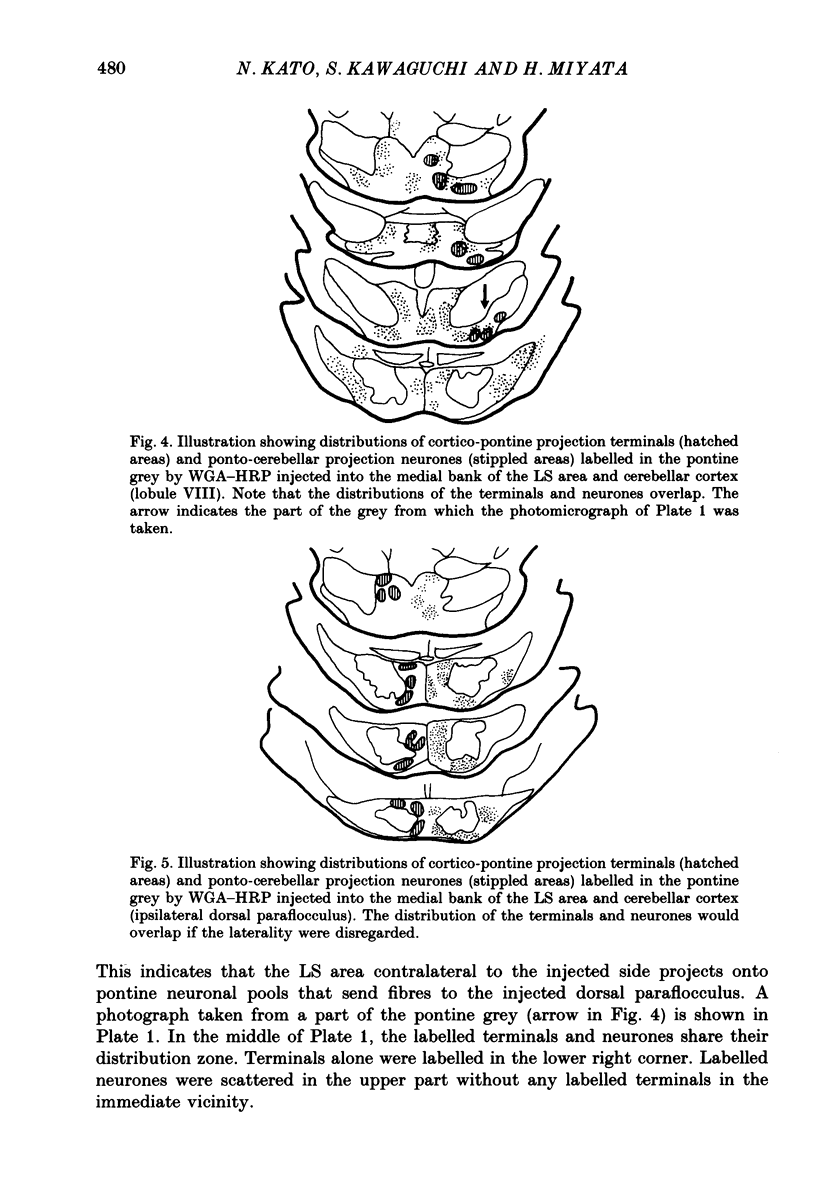
1. A projection from the medial bank of the lateral suprasylvian visual area, one of the targets of the cerebello-cerebral projection, back to the cerebellar cortex was demonstrated electrophysiologically in the cat. The anatomical pathways underlying this projection were investigated using orthograde and retrograde transport of wheatgerm-agglutinin-conjugated horseradish peroxidase (WGA-HRP). 2. Responses were recorded in the cerebellar cortex on stimulation of the medial bank of the lateral suprasylvian area, and were compared with those evoked by stimulation of the motor cortex and the crown part of the parietal association cortex. 3. Responses induced by stimulation of the lateral suprasylvian area were shown to consist of early mossy and late climbing fibre responses. The mossy fibre response was evoked, at a latency of 2-3 ms, predominantly in the lateral part of the contralateral cerebellar cortex (mainly, crus I, crus II, dorsal paraflocculus and paramedian lobule) and the posterior part of the vermis (mainly, lobules VII and VIII). Climbing fibre responses were elicited with the preceding mossy fibre responses and were elicited at a much longer latency than the motor cortex-induced climbing fibre response. 4. The orthograde and retrograde HRP studies suggested that the mossy fibre response is mediated by the pontine grey whereas the climbing fibre response is conveyed indirectly to the inferior olive which sends the climbing fibres to the cerebellar cortex. After WGA-HRP injections into both the medial bank of the lateral suprasylvian area and the cerebellar responsive area, orthogradely labelled terminals of cortico-pontine projection fibres and retrogradely labelled ponto-cerebellar neurones were found in the pontine grey, where distributions of the two kinds of labelling overlapped. On the other hand, retrograde neuronal labelling alone was found in the inferior olive, implying that the climbing fibre responses evoked from the lateral suprasylvian area were relayed via indirect cortico-olivary pathways.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K., Donate-Oliver F., Sanides D., Fries W. The distribution of pontine projection cells in visual and association cortex of the cat: an experimental study with horseradish peroxidase. J Comp Neurol. 1981 Sep 10;201(2):175–189. doi: 10.1002/cne.902010204. [DOI] [PubMed] [Google Scholar]
- Allen G. I., Azzena G. B., Ono T. Contribution of the cerebro-reticulo-cerebellar pathway to the early mossy fibre response in the cerebellar cortex. Brain Res. 1972 Sep 29;44(2):670–675. doi: 10.1016/0006-8993(72)90333-2. [DOI] [PubMed] [Google Scholar]
- Baker J., Gibson A., Glickstein M., Stein J. Visual cells in the pontine nuclei of the cat. J Physiol. 1976 Feb;255(2):415–433. doi: 10.1113/jphysiol.1976.sp011287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando T., Yamamoto N., Tsukahara N. Cortical neurons related to lens accommodation in posterior lateral suprasylvian area in cats. J Neurophysiol. 1984 Nov;52(5):879–891. doi: 10.1152/jn.1984.52.5.879. [DOI] [PubMed] [Google Scholar]
- Bishop G. A., McCrea R. A., Kitai S. T. A horseradish peroxidase study of the cortico-olivary projection in the cat. Brain Res. 1976 Nov 5;116(2):306–311. doi: 10.1016/0006-8993(76)90908-2. [DOI] [PubMed] [Google Scholar]
- Bjaalie J. G. Distribution of corticopontine neurons in visual areas of the middle suprasylvian sulcus: quantitative studies in the cat. Neuroscience. 1986 Aug;18(4):1013–1033. doi: 10.1016/0306-4522(86)90114-4. [DOI] [PubMed] [Google Scholar]
- Buchtel H. A., Iosif G., Marchesi G. F., Provini L., Strata P. Analysis of the activity evoked in the cerebellar cortex by stimulation of the visual pathways. Exp Brain Res. 1972;15(3):278–288. doi: 10.1007/BF00235912. [DOI] [PubMed] [Google Scholar]
- Burne R. A., Woodward D. J. Visual cortical projections to the paraflocculus in the rat. An electrophysiologic study. Exp Brain Res. 1983;49(1):55–67. doi: 10.1007/BF00235541. [DOI] [PubMed] [Google Scholar]
- Donaldson I. M., Hawthorne M. E. Coding of visual information by units in the cat cerebellar vermis. Exp Brain Res. 1979 Jan 2;34(1):27–48. doi: 10.1007/BF00238339. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The mossy fibre-granule cell relay of the cerebellum and its inhibitory control by Golgi cells. Exp Brain Res. 1966;1(1):82–101. doi: 10.1007/BF00235211. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sasaki K., Strata P. The profiles of physiological events produced by a parallel fibre volley in the cerebellar cortex. Exp Brain Res. 1966;2(1):18–34. doi: 10.1007/BF00234358. [DOI] [PubMed] [Google Scholar]
- Kato N., Kawaguchi S., Miyata H. Post-natal development of the retinal and cerebellar projections onto the lateral suprasylvian area in the cat. J Physiol. 1987 Feb;383:729–743. doi: 10.1113/jphysiol.1987.sp016438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Kawaguchi S., Miyata H. Postnatal development of afferent projections to the lateral suprasylvian visual area in the cat: an HRP study. J Comp Neurol. 1986 Oct 22;252(4):543–554. doi: 10.1002/cne.902520410. [DOI] [PubMed] [Google Scholar]
- Kawamura K., Brodal A. The tectopontine projection in the cat: an experimental anatomical study with comments on pathweays for teleceptive impulses to the cerebellum. J Comp Neurol. 1973 Jun 1;149(3):371–390. doi: 10.1002/cne.901490306. [DOI] [PubMed] [Google Scholar]
- Kitao Y., Nakamura Y., Okoyama S. An electron microscope study of the cortico-pretecto-olivary projection in the cat by a combined degeneration and horseradish peroxidase tracing technique. Brain Res. 1983 Nov 28;280(1):139–142. doi: 10.1016/0006-8993(83)91181-2. [DOI] [PubMed] [Google Scholar]
- Maekawa K., Takeda T., Kimura M. Neural activity of nucleus reticularis tegmenti pontis--the origin of visual mossy fiber afferents to the cerebellar flocculus of rabbits. Brain Res. 1981 Apr 6;210(1-2):17–30. doi: 10.1016/0006-8993(81)90881-7. [DOI] [PubMed] [Google Scholar]
- Mizuno N., Mochizuki K., Akimoto C., Matsushima R., Sasaki K. Projections from the parietal cortex to the brain stem nuclei in the cat, with special reference to the parietal cerebro-cerebellar system. J Comp Neurol. 1973 Feb 15;147(4):511–522. doi: 10.1002/cne.901470406. [DOI] [PubMed] [Google Scholar]
- Mower G., Gibson A., Glickstein M. Tectopontine pathway in the cat: laminar distribution of cells of origin and visual properties of target cells in dorsolateral pontine nucleus. J Neurophysiol. 1979 Jan;42(1 Pt 1):1–15. doi: 10.1152/jn.1979.42.1.1. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Kitao Y., Okoyama S. Cortico-Darkschewitsch-olivary projection in the cat: an electron microscope study with the aid of horseradish peroxidase tracing technique. Brain Res. 1983 Sep 5;274(1):140–143. doi: 10.1016/0006-8993(83)90529-2. [DOI] [PubMed] [Google Scholar]
- Oka H., Jinnai K., Yamamoto T. The parieto-rubro-olivary pathway in the cat. Exp Brain Res. 1979 Sep;37(1):115–125. doi: 10.1007/BF01474258. [DOI] [PubMed] [Google Scholar]
- Robinson F. R., Cohen J. L., May J., Sestokas A. K., Glickstein M. Cerebellar targets of visual pontine cells in the cat. J Comp Neurol. 1984 Mar 10;223(4):471–482. doi: 10.1002/cne.902230402. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr J. A. The projection from the motor cortex to the inferior olive in the cat. An experimental study using axonal transport techniques. Neuroscience. 1983 Nov;10(3):667–684. doi: 10.1016/0306-4522(83)90209-9. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Oka H., Matsuda Y., Shimono T., Mizuno N. Electrophysiological studies of the projections from the parietal association area to the cerebellar cortex. Exp Brain Res. 1975 Jul 11;23(1):91–102. doi: 10.1007/BF00238732. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., Mizuno N., Itoh K. An autoradiographic study on the terminal distribution of cerebellothalamic fibers in the cat. Brain Res. 1981 Jun 29;215(1-2):29–47. doi: 10.1016/0006-8993(81)90489-3. [DOI] [PubMed] [Google Scholar]
- Takeda T., Maekawa K. The origin of the pretecto-olivary tract. A study using the horseradish peroxidase method. Brain Res. 1976 Nov 26;117(2):319–325. doi: 10.1016/0006-8993(76)90740-x. [DOI] [PubMed] [Google Scholar]
- Toyama K., Kozasa T. Responses of Clare-Bishop neurones to three dimensional movement of a light stimulus. Vision Res. 1982;22(5):571–574. doi: 10.1016/0042-6989(82)90115-8. [DOI] [PubMed] [Google Scholar]



