Abstract
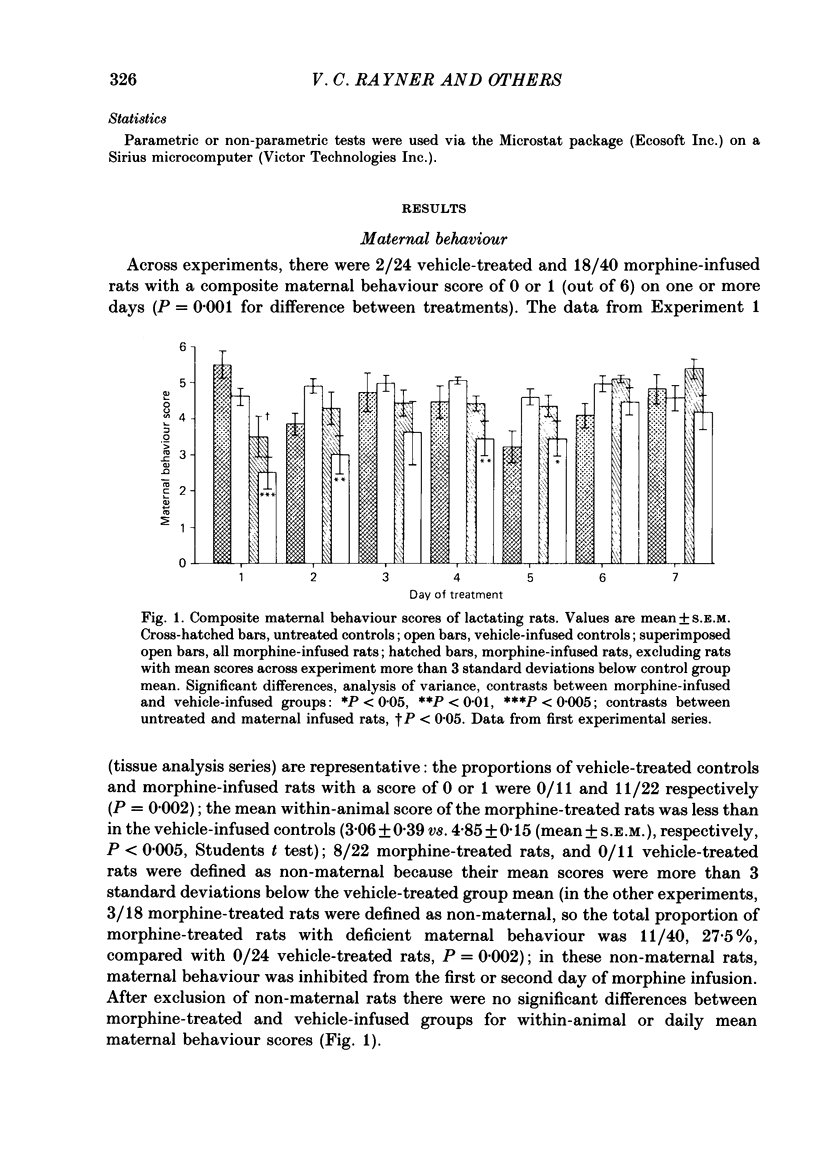
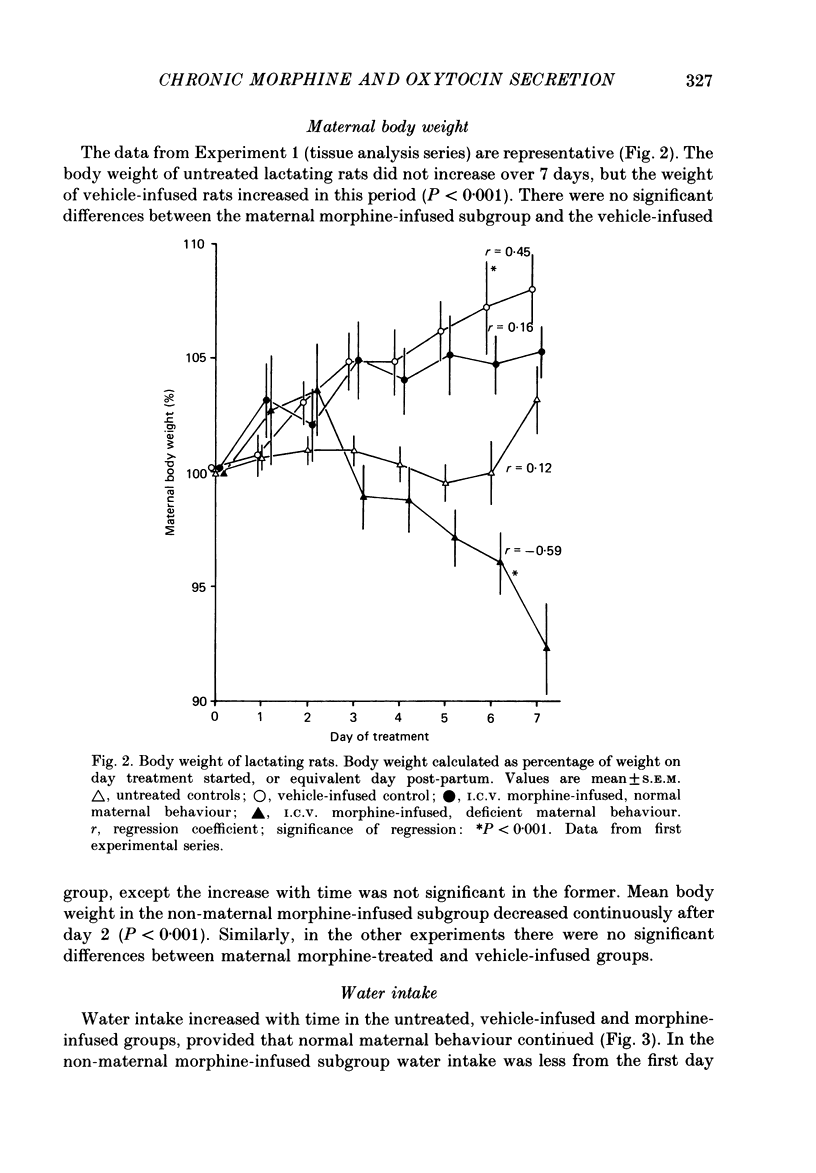
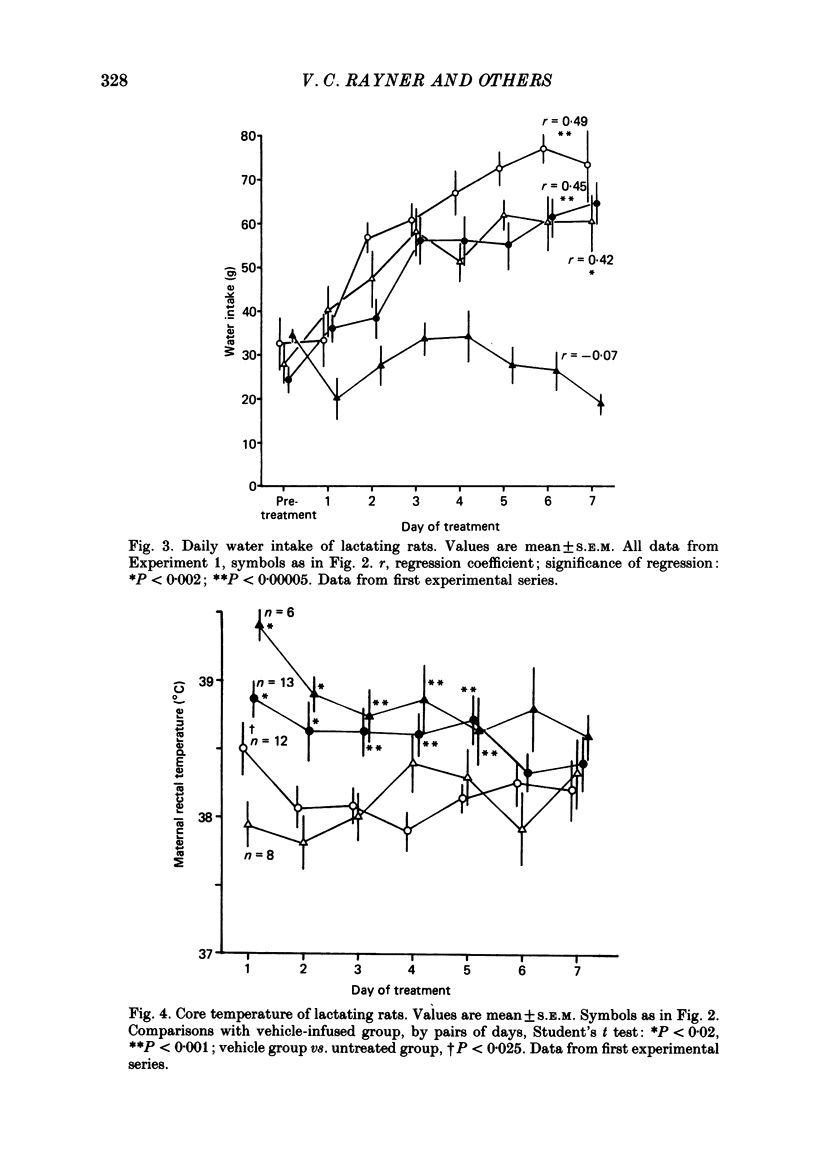
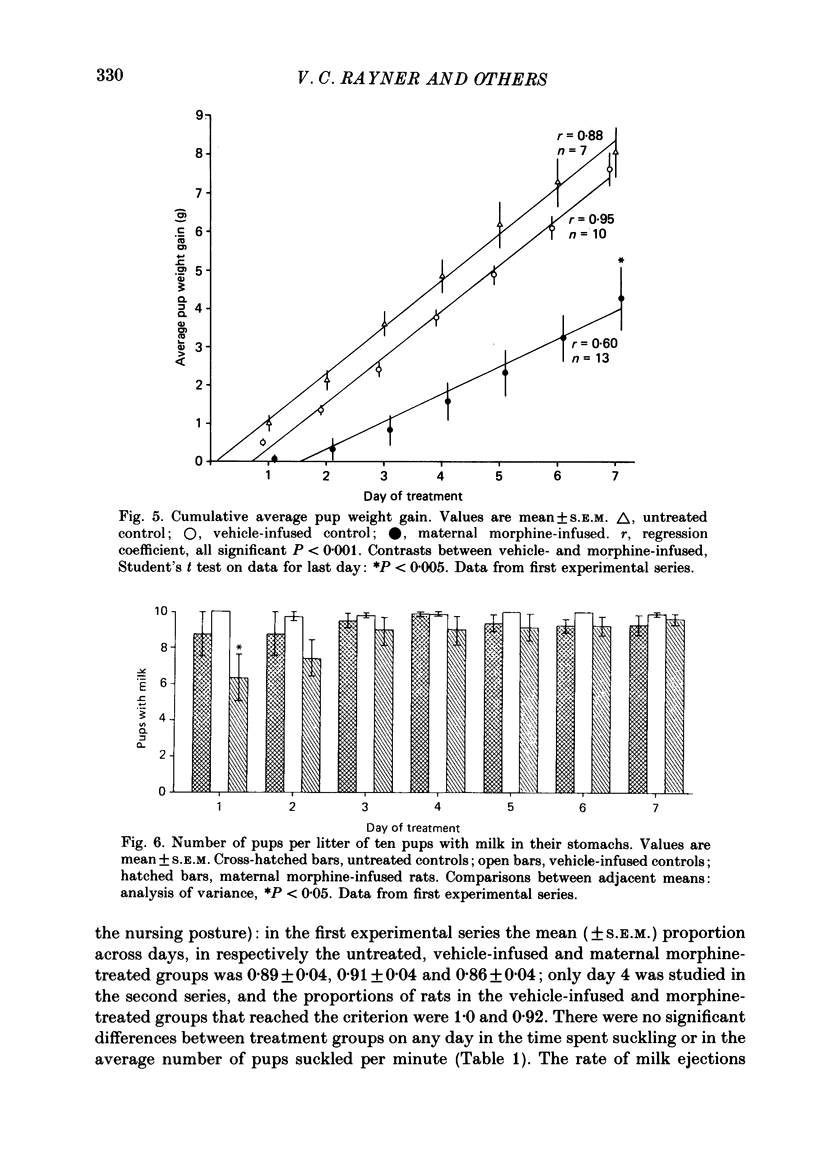
1. Acutely, opioids inhibit oxytocin secretion. To study the responses of oxytocin neurones during chronic opioid exposure, forty-five lactating rats were infused continuously from a subcutaneous osmotically driven mini-pump via a lateral cerebral ventricle with morphine sulphate solution from day 2 post-partum for 5-7 days; the infusion rate was increased 2- or 2.5-fold each 40 h from 10 micrograms/h initially up to 50 micrograms/h; controls were infused with vehicle (1 microliter/h, twenty-eight rats) or were untreated (eight rats). 2. Maternal behaviour was disrupted in 27% of the morphine-treated rats; in rats that remained maternal morphine did not affect body weight or water intake but increased rectal temperature by 0.82 +/- 0.14 degrees C (mean +/- S.E.M.) across the first 4 days. 3. Weight gain of the litters of maternal morphine-treated rats was reduced by 32% during 7 days, predominantly in the first day of treatment when milk transfer was also reduced. Observation of pup behaviour during suckling showed decreased frequency of milk ejections on only the second day of morphine treatment. Plasma concentration of prolactin after 6 days was similar in maternal morphine-treated and control rats, but reduced by 90% in non-maternal morphine-treated rats, indicating normal control of prolactin secretion by suckling in morphine-treated rats. 4. Oxytocin and vasopressin contents, measured by radioimmunoassay, in the supraoptic and paraventricular nuclei and in the neurohypophysis were similar between fourteen maternal morphine-treated, twelve vehicle-treated and eight untreated lactating rats; thus exposure to morphine did not involve increased production and storage of oxytocin. 5. Distribution of [3H]morphine infused intracerebroventricularly into six virgin female rats for 6 days was measured by scintillation counting of tissue extracts. Morphine concentration in the hypothalamus and neurohypophysis was 2.7 and 12.8 micrograms/g, respectively, and in blood plasma 0.75 micrograms/g. Tolerance was not due to failure of morphine infusion. In addition, naloxone (5 mg/kg s.c.) provoked typical withdrawal reactions ('wet dog' shakes, defaecation, burrowing) in lactating rats infused with morphine for 5 days. 6. Pups were suckled onto seven maternal morphine-infused and five vehicle-infused rats anaesthetized with urethane for recording of intramammary and arterial blood pressures after treatment for 5 days. The incidence and pattern of milk ejections, and mammary gland sensitivity to oxytocin were similar in the two groups.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
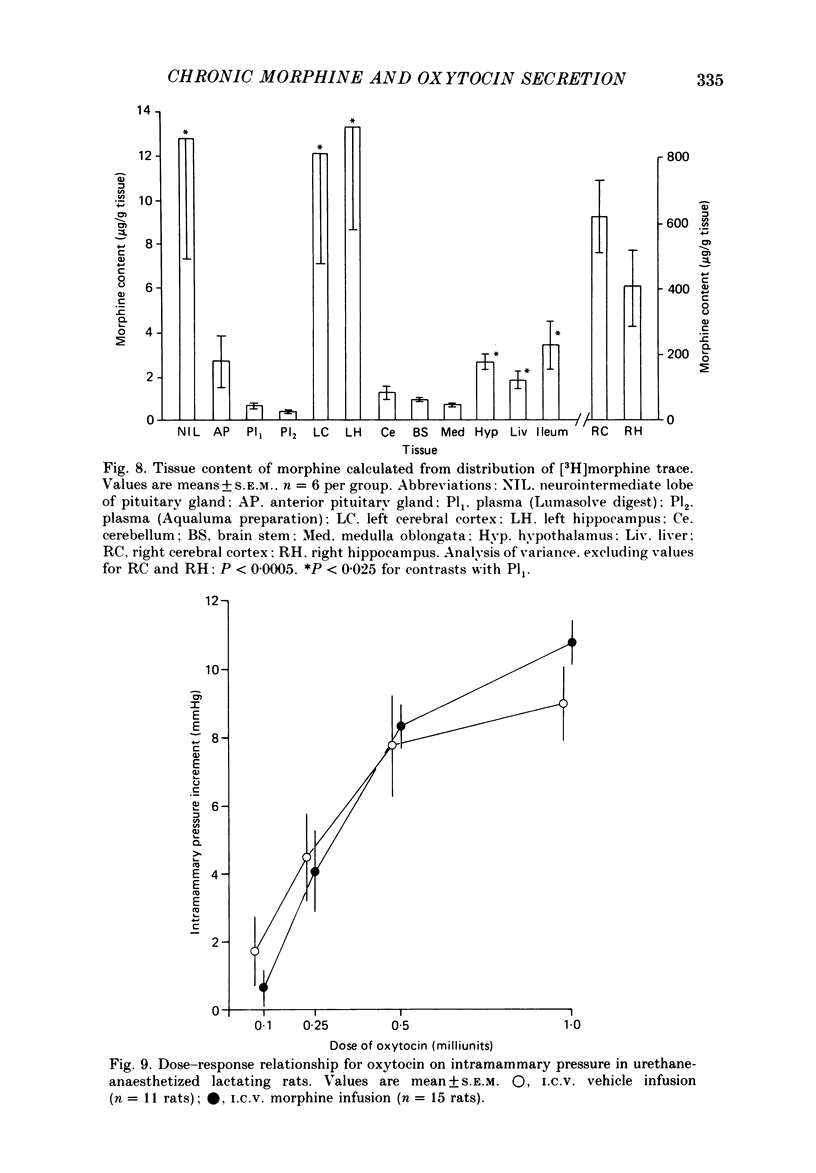
These references are in PubMed. This may not be the complete list of references from this article.
- Arita J., Porter J. C. Relationship between dopamine release into hypophysial portal blood and prolactin release after morphine treatment in rats. Neuroendocrinology. 1984 Jan;38(1):62–67. doi: 10.1159/000123867. [DOI] [PubMed] [Google Scholar]
- Bicknell R. J., Chapman C., Leng G. Effects of opioid agonists and antagonists on oxytocin and vasopressin release in vitro. Neuroendocrinology. 1985 Aug;41(2):142–148. doi: 10.1159/000124168. [DOI] [PubMed] [Google Scholar]
- Bicknell R. J. Endogenous opioid peptides and hypothalamic neuroendocrine neurones. J Endocrinol. 1985 Dec;107(3):437–446. doi: 10.1677/joe.0.1070437. [DOI] [PubMed] [Google Scholar]
- Bicknell R. J., Leng G., Lincoln D. W., Russell J. A. Naloxone excites oxytocin neurones in the supraoptic nucleus of lactating rats after chronic morphine treatment. J Physiol. 1988 Feb;396:297–317. doi: 10.1113/jphysiol.1988.sp016963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R. S., DiBiase R., Loundes D. D., Doherty P. C. Prolactin stimulation of maternal behavior in female rats. Science. 1985 Feb 15;227(4688):782–784. doi: 10.1126/science.3969568. [DOI] [PubMed] [Google Scholar]
- Bunn S. J., Hanley M. R., Wilkin G. P. Evidence for a kappa-opioid receptor on pituitary astrocytes: an autoradiographic study. Neurosci Lett. 1985 Apr 19;55(3):317–323. doi: 10.1016/0304-3940(85)90455-0. [DOI] [PubMed] [Google Scholar]
- Clark W. G., Bernardini G. L. Morphine-induced hyperthermia: lack of cross-tolerance with enkephalin analogs. Brain Res. 1982 Jan 7;231(1):231–234. doi: 10.1016/0006-8993(82)90025-7. [DOI] [PubMed] [Google Scholar]
- Clarke G., Fall C. H., Lincoln D. W., Merrick L. P. Effects of cholinoceptor antagonists on the suckling-induced and experimentally evoked release of oxytocin. Br J Pharmacol. 1978 Jul;63(3):519–527. doi: 10.1111/j.1476-5381.1978.tb07807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G., Wood P., Merrick L., Lincoln D. W. Opiate inhibition of peptide release from the neurohumoral terminals of hypothalamic neurones. Nature. 1979 Dec 13;282(5740):746–748. doi: 10.1038/282746a0. [DOI] [PubMed] [Google Scholar]
- Clarke G., Wright D. M. A comparison of analgesia and suppression of oxytocin release by opiates. Br J Pharmacol. 1984 Nov;83(3):799–806. doi: 10.1111/j.1476-5381.1984.tb16235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier H. O. Cellular site of opiate dependence. Nature. 1980 Feb 14;283(5748):625–629. doi: 10.1038/283625a0. [DOI] [PubMed] [Google Scholar]
- Deyo S. N., Swift R. M., Miller R. J., Fang V. S. Development of tolerance to the prolactin-releasing action of morphine and its modulation by hypothalamic dopamine. Endocrinology. 1980 May;106(5):1469–1474. doi: 10.1210/endo-106-5-1469. [DOI] [PubMed] [Google Scholar]
- Fahrbach S. E., Morrell J. I., Pfaff D. W. Oxytocin induction of short-latency maternal behavior in nulliparous, estrogen-primed female rats. Horm Behav. 1984 Sep;18(3):267–286. doi: 10.1016/0018-506x(84)90016-3. [DOI] [PubMed] [Google Scholar]
- Fleming A. S. Control of food intake in the lactating rat: role of suckling and hormones. Physiol Behav. 1976 Nov;17(5):841–848. doi: 10.1016/0031-9384(76)90051-2. [DOI] [PubMed] [Google Scholar]
- Haldar J., Hoffman D. L., Zimmerman E. A. Morphine, beta-endorphin and [D-Ala2] Met-enkephalin inhibit oxytocin release by acetylcholine and suckling. Peptides. 1982 Jul-Aug;3(4):663–668. doi: 10.1016/0196-9781(82)90168-1. [DOI] [PubMed] [Google Scholar]
- Higuchi T., Honda K., Fukuoka T., Negoro H., Wakabayashi K. Release of oxytocin during suckling and parturition in the rat. J Endocrinol. 1985 Jun;105(3):339–346. doi: 10.1677/joe.0.1050339. [DOI] [PubMed] [Google Scholar]
- Karras P. J., North R. A. Acute and chronic effects of opiates on single neurons of the myenteric plexus. J Pharmacol Exp Ther. 1981 Apr;217(1):70–80. [PubMed] [Google Scholar]
- Keil L. C., Rosella-Dampman L. M., Emmert S., Chee O., Summy-Long J. Y. Enkephalin inhibition of angiotensin-stimulated release of oxytocin and vasopressin. Brain Res. 1984 Apr 16;297(2):329–336. doi: 10.1016/0006-8993(84)90574-2. [DOI] [PubMed] [Google Scholar]
- Laschka E., Teschemacher H., Mehraein P., Herz A. Sites of action of morphine involved in the development of physical dependence in rats. II. Morphine withdrawal precipitated by application of morphine antagonists into restricted parts of the ventricular system and by microinjection into various brain areas. Psychopharmacologia. 1976 Mar 16;46(2):141–147. doi: 10.1007/BF00421383. [DOI] [PubMed] [Google Scholar]
- Leng G., Mansfield S., Bicknell R. J., Dean A. D., Ingram C. D., Marsh M. I., Yates J. O., Dyer R. G. Central opioids: a possible role in parturition? J Endocrinol. 1985 Aug;106(2):219–224. doi: 10.1677/joe.0.1060219. [DOI] [PubMed] [Google Scholar]
- Lin M. T., Uang W. N., Chan H. K. Hypothalamic neuronal responses to iontophoretic application of morphine in rats. Neuropharmacology. 1984 May;23(5):591–594. doi: 10.1016/0028-3908(84)90035-2. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Hill A., Wakerley J. B. The milk-ejection reflex of the rat: an intermittent function not abolished by surgical levels of anaesthesia. J Endocrinol. 1973 Jun;57(3):459–476. doi: 10.1677/joe.0.0570459. [DOI] [PubMed] [Google Scholar]
- Marchand C., Denis G. Diuretic effect of chronic morphine treatment in rats. J Pharmacol Exp Ther. 1968 Aug;162(2):331–337. [PubMed] [Google Scholar]
- Maysinger D., Vermes I., Tilders F., Seizinger B. R., Gramsch C., Höllt V., Herz A. Differential effects of various opioid peptides on vasopressin and oxytocin release from the rat pituitary in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1984 Dec;328(2):191–195. doi: 10.1007/BF00512071. [DOI] [PubMed] [Google Scholar]
- McNeilly A. S., de Kretser D. M., Sharpe R. M. Modulation of prolactin, luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretion by LHRH and bromocriptine (CB154) in the hypophysectomized pituitary-grafted male rat and its effect on testicular LH receptors and testosterone output. Biol Reprod. 1979 Aug;21(1):141–147. doi: 10.1095/biolreprod21.1.141. [DOI] [PubMed] [Google Scholar]
- North W. G., LaRochelle F. T., Jr, Haldar J., Sawyer W. H., Valtin H. Characterization of an antiserum used in a radioimmunoassay for arginine-vasopressin: implications for reference standards. Endocrinology. 1978 Dec;103(6):1976–1984. doi: 10.1210/endo-103-6-1976. [DOI] [PubMed] [Google Scholar]
- Redmond D. E., Jr, Krystal J. H. Multiple mechanisms of withdrawal from opioid drugs. Annu Rev Neurosci. 1984;7:443–478. doi: 10.1146/annurev.ne.07.030184.002303. [DOI] [PubMed] [Google Scholar]
- Robinson I. C. The development and evaluation of a sensitive and specific radioimmunoassay for oxytocin in unextracted plasma. J Immunoassay. 1980;1(3):323–347. doi: 10.1080/01971528008058475. [DOI] [PubMed] [Google Scholar]
- Robinson J. H., Dunlap C. E., 3rd, Deadwyler S. A. Differences in opiate-induced synaptic excitability of hippocampal slices prepared from tolerant and nontolerant rats. Exp Neurol. 1982 Sep;77(3):590–598. doi: 10.1016/0014-4886(82)90230-8. [DOI] [PubMed] [Google Scholar]
- Rubin B. S., Bridges R. S. Disruption of ongoing maternal responsiveness in rats by central administration of morphine sulfate. Brain Res. 1984 Jul 30;307(1-2):91–97. doi: 10.1016/0006-8993(84)90464-5. [DOI] [PubMed] [Google Scholar]
- Russell J. A. Changes in nucleolar dry mass of neurones of the paraventricular and supraoptic nuclei in the rat during pregnancy and lactation. Cell Tissue Res. 1980;208(2):313–325. doi: 10.1007/BF00234879. [DOI] [PubMed] [Google Scholar]
- Russell J. A. Combined morphometric and immunocytochemical evidence that in the paraventricular nucleus of the rat oxytocin- but not vasopressin-neurones respond to the suckling stimulus. Prog Brain Res. 1983;60:31–38. doi: 10.1016/S0079-6123(08)64372-1. [DOI] [PubMed] [Google Scholar]
- Russell J. A., Harrison D. J., McNeilly A. S. Bromocriptine and alpha-ergocryptine do not inhibit oxytocin secretion in the lactating rat. J Endocrinol. 1981 Apr;89(1):91–98. doi: 10.1677/joe.0.0890091. [DOI] [PubMed] [Google Scholar]
- Russell J. A. Milk yield, suckling behaviour and milk ejection in the lactating rat nursing litters of different sizes. J Physiol. 1980 Jun;303:403–415. doi: 10.1113/jphysiol.1980.sp013295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson W. K., McDonald J. K., Lumpkin M. D. Naloxone-induced dissociation of oxytocin and prolactin releases. Neuroendocrinology. 1985 Jan;40(1):68–71. doi: 10.1159/000124053. [DOI] [PubMed] [Google Scholar]
- Sanger D. J., McCarthy P. S. Increased food and water intake produced in rats by opiate receptor agonists. Psychopharmacology (Berl) 1981;74(3):217–220. doi: 10.1007/BF00427097. [DOI] [PubMed] [Google Scholar]
- Summy-Long J. Y., Miller D. S., Rosella-Dampman L. M., Hartman R. D., Emmert S. E. A functional role for opioid peptides in the differential secretion of vasopressin and oxytocin. Brain Res. 1984 Sep 10;309(2):362–366. [PubMed] [Google Scholar]
- Tepperman F. S., Hirst M., Gowdey C. W. Hypothalamic injection of morphine: feeding and temperature responses. Life Sci. 1981 Jun 1;28(22):2459–2467. doi: 10.1016/0024-3205(81)90587-7. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. Intermittent release of oxytocin during suckling in the rat. Nat New Biol. 1971 Oct 6;233(40):180–181. doi: 10.1038/newbio233180a0. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Noble R., Clarke G. Effects of morphine and D-Ala, D-Leu enkephalin on the electrical activity of supraoptic neurosecretory cells in vitro. Neuroscience. 1983 Sep;10(1):73–81. doi: 10.1016/0306-4522(83)90081-7. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Brown R. D., Hinson J. L., Dailey J. W. Pharmacokinetic pitfalls in the estimation of the breast milk/plasma ratio for drugs. Annu Rev Pharmacol Toxicol. 1985;25:667–689. doi: 10.1146/annurev.pa.25.040185.003315. [DOI] [PubMed] [Google Scholar]
- Woodside B., Pelchat R., Leon M. Acute elevation of the heat load of mother rats curtails maternal nest bouts. J Comp Physiol Psychol. 1980 Feb;94(1):61–68. doi: 10.1037/h0077653. [DOI] [PubMed] [Google Scholar]
- Yim G. K., Lowy M. T. Opioids, feeding, and anorexias. Fed Proc. 1984 Nov;43(14):2893–2897. [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. Effects of chronic morphine administration on pregnant rats and their offspring. Pharmacology. 1977;15(4):302–310. doi: 10.1159/000136703. [DOI] [PubMed] [Google Scholar]


