Abstract
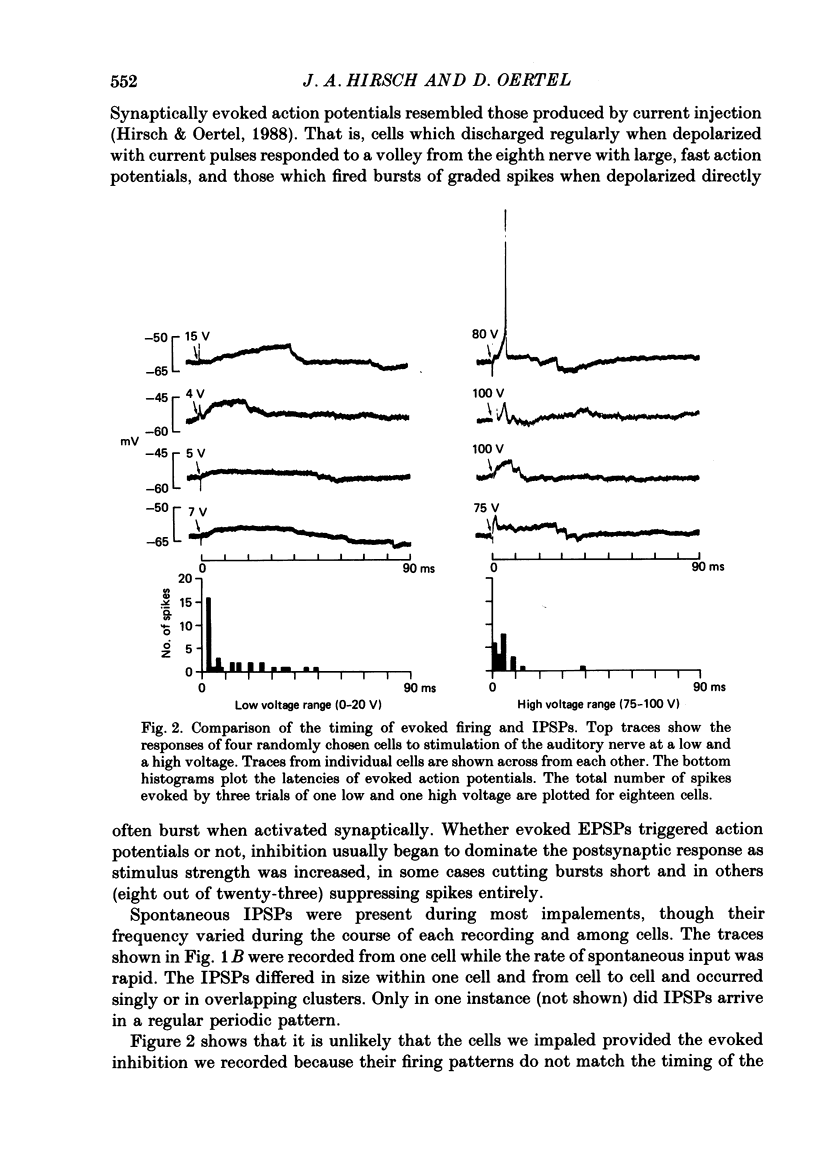
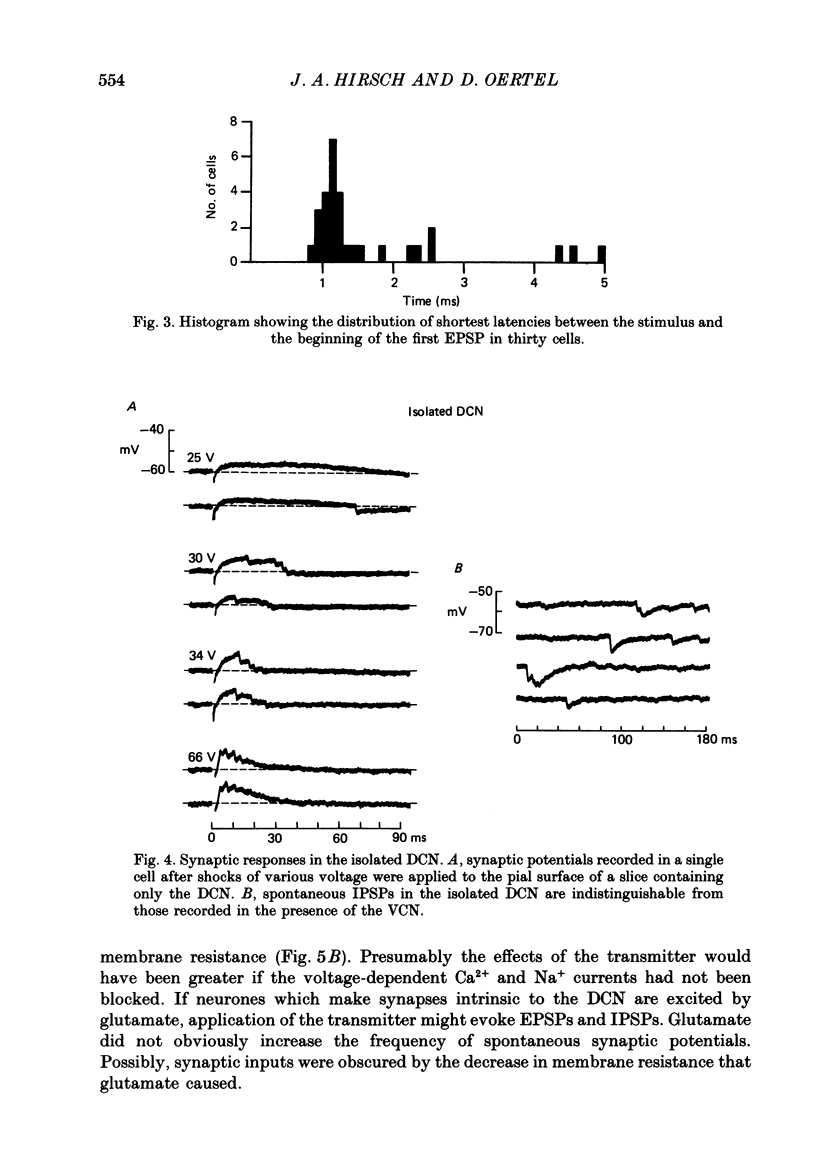
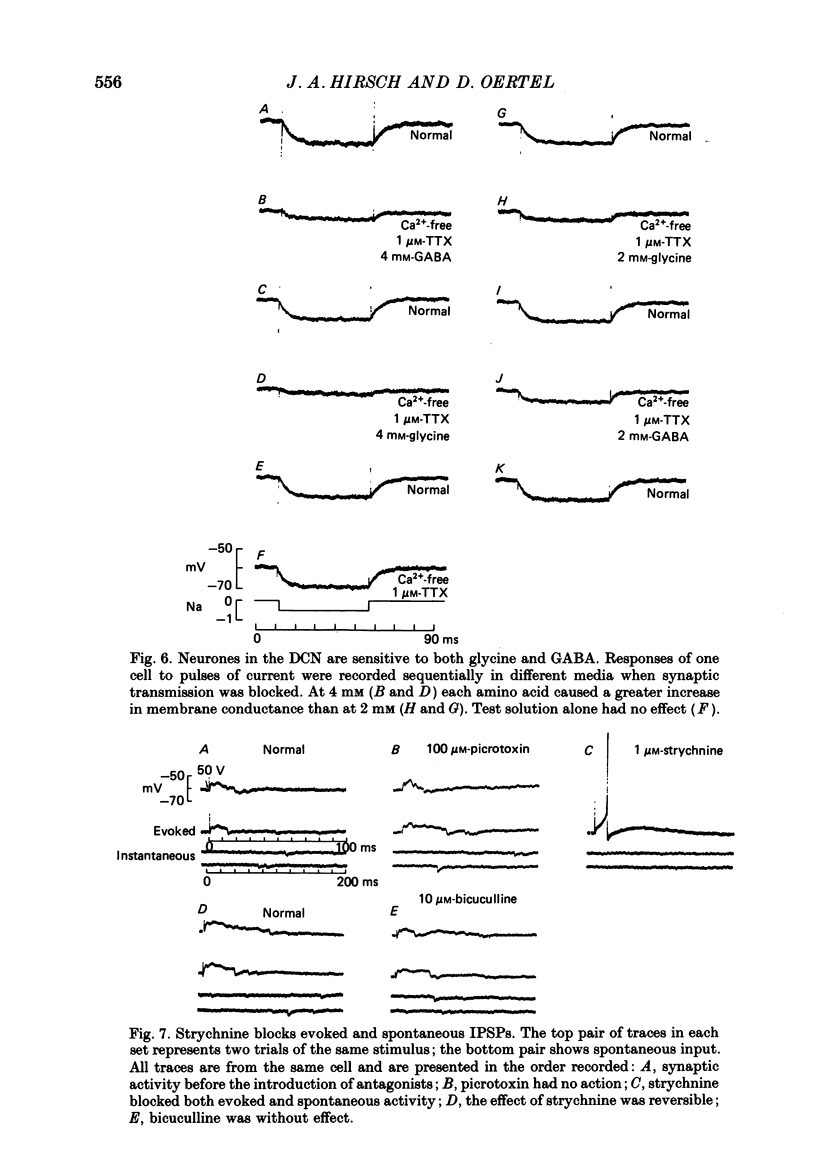
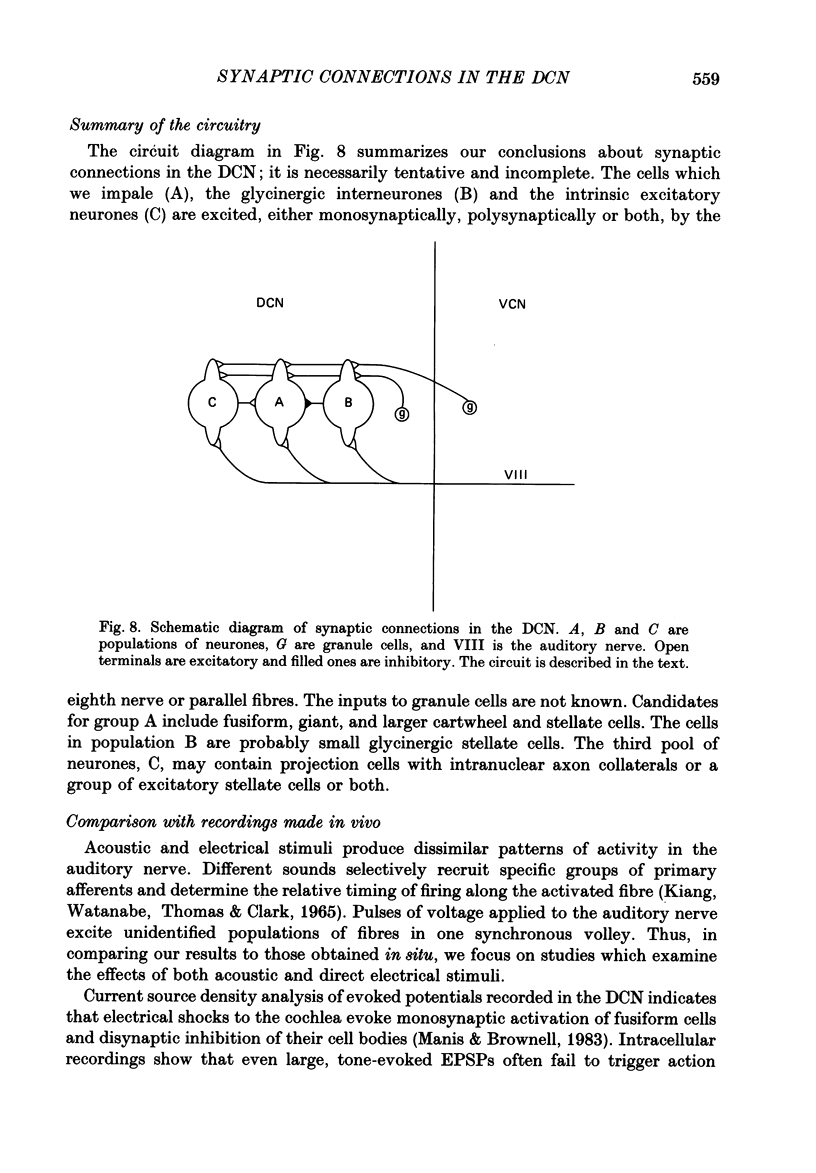
1. Intracellular recordings were made from the dorsal cochlear nucleus (DCN) in slices that contained the root of the auditory nerve and parts of the dorsal and ventral cochlear nuclei. Probably the largest and most common cells were impaled. 2. Weak shocks to the nerve usually evoked an excitatory postsynaptic potential (EPSP) that lasted about 90 ms and whose latency was often less than 1.2 ms, indicating monosynaptic input. 3. Stronger shocks elicited a larger EPSP and a later train of inhibitory postsynaptic potentials (IPSPs). Increasing the stimulus voltage shortened the latency of the train of IPSPs and increased its efficacy so that at large stimulus strengths inhibition dominated the synaptic response. 4. To determine whether any of the neuronal circuitry which generated the synaptic responses involved the ventral cochlear nucleus, recordings were made from slices containing only the dorsal nucleus. Synaptic responses to stimulation of the pial surface of the isolated DCN resembled those driven from the nerve root. That is, weak shocks evoked long-lasting, monosynaptic EPSPs and stronger stimuli elicited a larger EPSP followed by trains of IPSPs. The DCN, therefore, contains intrinsic inhibitory interneurones. 5. The parallel fibres of the DCN course superficially, near the stimulating electrodes, whereas the axons of the auditory nerve terminate in deeper areas. Thus, the monosynaptic EPSPs evoked from the pial surface are probably generated by parallel fibres. Apparently the inhibitory interneurones are also excited by a circuit including parallel fibres. 6. The putative neurotransmitter of parallel fibres, glutamate, excited all neurones tested. 7. Cells were sensitive both to glycine and to gamma-aminobutyric acid (GABA). Only strychnine, however, not picrotoxin or bicuculline, blocked IPSPs.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Multipolar cells in the ventral cochlear nucleus project to the dorsal cochlear nucleus and the inferior colliculus. Neurosci Lett. 1983 Jun 30;37(3):205–208. doi: 10.1016/0304-3940(83)90431-7. [DOI] [PubMed] [Google Scholar]
- Altschuler R. A., Betz H., Parakkal M. H., Reeks K. A., Wenthold R. J. Identification of glycinergic synapses in the cochlear nucleus through immunocytochemical localization of the postsynaptic receptor. Brain Res. 1986 Mar 26;369(1-2):316–320. doi: 10.1016/0006-8993(86)90542-1. [DOI] [PubMed] [Google Scholar]
- Blackstad T. W., Osen K. K., Mugnaini E. Pyramidal neurones of the dorsal cochlear nucleus: a Golgi and computer reconstruction study in cat. Neuroscience. 1984 Nov;13(3):827–854. doi: 10.1016/0306-4522(84)90099-x. [DOI] [PubMed] [Google Scholar]
- Brawer J. R., Morest D. K., Kane E. C. The neuronal architecture of the cochlear nucleus of the cat. J Comp Neurol. 1974 Jun 1;155(3):251–300. doi: 10.1002/cne.901550302. [DOI] [PubMed] [Google Scholar]
- Caspary D. M., Havey D. C., Faingold C. L. Effects of microiontophoretically applied glycine and GABA on neuronal response patterns in the cochlear nuclei. Brain Res. 1979 Aug 17;172(1):179–185. doi: 10.1016/0006-8993(79)90909-0. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Farb D. H., Fischbach G. D. GABA-mediated synaptic potentials in chick spinal cord and sensory neurons. J Neurophysiol. 1981 Apr;45(4):632–643. doi: 10.1152/jn.1981.45.4.632. [DOI] [PubMed] [Google Scholar]
- Cohen E. S., Brawer J. R., Morest D. K. Projections of the cochlea to the dorsal cochlear nucleus in the cat. Exp Neurol. 1972 Jun;35(3):470–479. doi: 10.1016/0014-4886(72)90117-3. [DOI] [PubMed] [Google Scholar]
- Evans E. F., Nelson P. G. On the functional relationship between the dorsal and ventral divisions of the cochlear nucleus of the cat. Exp Brain Res. 1973 Jun 29;17(4):428–442. doi: 10.1007/BF00234104. [DOI] [PubMed] [Google Scholar]
- Evans E. F., Nelson P. G. The responses of single neurones in the cochlear nucleus of the cat as a function of their location and the anaesthetic state. Exp Brain Res. 1973 Jun 29;17(4):402–427. doi: 10.1007/BF00234103. [DOI] [PubMed] [Google Scholar]
- Fekete D. M., Rouiller E. M., Liberman M. C., Ryugo D. K. The central projections of intracellularly labeled auditory nerve fibers in cats. J Comp Neurol. 1984 Nov 1;229(3):432–450. doi: 10.1002/cne.902290311. [DOI] [PubMed] [Google Scholar]
- Frostholm A., Rotter A. Autoradiographic localization of receptors in the cochlear nucleus of the mouse. Brain Res Bull. 1986 Feb;16(2):189–203. doi: 10.1016/0361-9230(86)90033-x. [DOI] [PubMed] [Google Scholar]
- Gerstein G. L., Butler R. A., Erulkar S. D. Excitation and inhibition in cochlear nucleus. I. Tone-burst stimulation. J Neurophysiol. 1968 Jul;31(4):526–536. doi: 10.1152/jn.1968.31.4.526. [DOI] [PubMed] [Google Scholar]
- Godfrey D. A., Carter J. A., Lowry O. H., Matschinsky F. M. Distribution of gamma-aminobutyric acid, glycine, glutamate and aspartate in the cochlear nucleus of the rat. J Histochem Cytochem. 1978 Feb;26(2):118–126. doi: 10.1177/26.2.624832. [DOI] [PubMed] [Google Scholar]
- Godfrey D. A., Kiang N. Y., Norris B. E. Single unit activity in the dorsal cochlear nucleus of the cat. J Comp Neurol. 1975 Jul 15;162(2):269–284. doi: 10.1002/cne.901620207. [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Young A. B., Penney J. B. Quantitative autoradiographic distribution of L-[3H]glutamate-binding sites in rat central nervous system. J Neurosci. 1984 Aug;4(8):2133–2144. doi: 10.1523/JNEUROSCI.04-08-02133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. A., Oertel D. Intrinsic properties of neurones in the dorsal cochlear nucleus of mice, in vitro. J Physiol. 1988 Feb;396:535–548. doi: 10.1113/jphysiol.1988.sp016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. R., Casseday J. H. Projections to laminae in dorsal cochlear nucleus in the tree shrew, Tupaia glis. Brain Res. 1979 Jan 5;160(1):131–133. doi: 10.1016/0006-8993(79)90606-1. [DOI] [PubMed] [Google Scholar]
- Kane E. C. Synaptic organization in the dorsal cochlear nucleus of the cat: a light and electron microscopic study. J Comp Neurol. 1974 Jun 1;155(3):301–329. doi: 10.1002/cne.901550303. [DOI] [PubMed] [Google Scholar]
- Kane E. S., Puglisi S. G., Gordon B. S. Neuronal types in the deep dorsal cochlear nucleus of the cat: I. Giant neurons. J Comp Neurol. 1981 May 20;198(3):483–513. doi: 10.1002/cne.901980308. [DOI] [PubMed] [Google Scholar]
- Manis P. B., Brownell W. E. Synaptic organization of eighth nerve afferents to cat dorsal cochlear nucleus. J Neurophysiol. 1983 Nov;50(5):1156–1181. doi: 10.1152/jn.1983.50.5.1156. [DOI] [PubMed] [Google Scholar]
- Mast T. E. Study of single units of the cochlear nucleus of the chinchilla. J Acoust Soc Am. 1970 Aug;48(2):505–512. doi: 10.1121/1.1912165. [DOI] [PubMed] [Google Scholar]
- Mugnaini E., Osen K. K., Dahl A. L., Friedrich V. L., Jr, Korte G. Fine structure of granule cells and related interneurons (termed Golgi cells) in the cochlear nuclear complex of cat, rat and mouse. J Neurocytol. 1980 Aug;9(4):537–570. doi: 10.1007/BF01204841. [DOI] [PubMed] [Google Scholar]
- Mugnaini E., Warr W. B., Osen K. K. Distribution and light microscopic features of granule cells in the cochlear nuclei of cat, rat, and mouse. J Comp Neurol. 1980 Jun 15;191(4):581–606. doi: 10.1002/cne.901910406. [DOI] [PubMed] [Google Scholar]
- Oertel D. Cells in the anteroventral cochlear nucleus are insensitive to L-glutamate and L-aspartate; excitatory synaptic responses are not blocked by D-alpha-aminoadipate. Brain Res. 1984 Jun 8;302(2):213–220. doi: 10.1016/0006-8993(84)90233-6. [DOI] [PubMed] [Google Scholar]
- Oertel D. Synaptic responses and electrical properties of cells in brain slices of the mouse anteroventral cochlear nucleus. J Neurosci. 1983 Oct;3(10):2043–2053. doi: 10.1523/JNEUROSCI.03-10-02043.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D. Use of brain slices in the study of the auditory system: spatial and temporal summation of synaptic inputs in cells in the anteroventral cochlear nucleus of the mouse. J Acoust Soc Am. 1985 Jul;78(1 Pt 2):328–333. doi: 10.1121/1.392494. [DOI] [PubMed] [Google Scholar]
- Osen K. K. Cytoarchitecture of the cochlear nuclei in the cat. J Comp Neurol. 1969 Aug;136(4):453–484. doi: 10.1002/cne.901360407. [DOI] [PubMed] [Google Scholar]
- ROSE J. E., GALAMBOS R., HUGHES J. R. Microelectrode studies of the cochlear nuclei of the cat. Bull Johns Hopkins Hosp. 1959 May;104(5):211–251. [PubMed] [Google Scholar]
- Rhode W. S., Oertel D., Smith P. H. Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat ventral cochlear nucleus. J Comp Neurol. 1983 Feb 1;213(4):448–463. doi: 10.1002/cne.902130408. [DOI] [PubMed] [Google Scholar]
- Rhode W. S., Smith P. H., Oertel D. Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat dorsal cochlear nucleus. J Comp Neurol. 1983 Feb 1;213(4):426–447. doi: 10.1002/cne.902130407. [DOI] [PubMed] [Google Scholar]
- Schwartz I. R. The differential distribution of label following uptake of 3H-labeled amino acids in the dorsal cochlear nucleus of the cat. An autoradiographic study. Exp Neurol. 1981 Sep;73(3):601–617. doi: 10.1016/0014-4886(81)90199-0. [DOI] [PubMed] [Google Scholar]
- Schweitzer L., Cant N. B. Development of the cochlear innervation of the dorsal cochlear nucleus of the hamster. J Comp Neurol. 1984 May 10;225(2):228–243. doi: 10.1002/cne.902250208. [DOI] [PubMed] [Google Scholar]
- Shofner W. P., Young E. D. Excitatory/inhibitory response types in the cochlear nucleus: relationships to discharge patterns and responses to electrical stimulation of the auditory nerve. J Neurophysiol. 1985 Oct;54(4):917–939. doi: 10.1152/jn.1985.54.4.917. [DOI] [PubMed] [Google Scholar]
- Smith P. H., Rhode W. S. Electron microscopic features of physiologically characterized, HRP-labeled fusiform cells in the cat dorsal cochlear nucleus. J Comp Neurol. 1985 Jul 1;237(1):127–143. doi: 10.1002/cne.902370110. [DOI] [PubMed] [Google Scholar]
- Starr A., Britt R. Intracellular recordings from cat cochlear nucleus during tone stimulation. J Neurophysiol. 1970 Jan;33(1):137–147. doi: 10.1152/jn.1970.33.1.137. [DOI] [PubMed] [Google Scholar]
- Voigt H. F., Young E. D. Evidence of inhibitory interactions between neurons in dorsal cochlear nucleus. J Neurophysiol. 1980 Jul;44(1):76–96. doi: 10.1152/jn.1980.44.1.76. [DOI] [PubMed] [Google Scholar]
- Webster D. B., Trune D. R. Cochlear nuclear complex of mice. Am J Anat. 1982 Feb;163(2):103–130. doi: 10.1002/aja.1001630202. [DOI] [PubMed] [Google Scholar]
- Wenthold R. J. Release of endogenous glutamic acid, aspartic acid and GABA from cochlear nucleus slices. Brain Res. 1979 Feb 23;162(2):338–343. doi: 10.1016/0006-8993(79)90294-4. [DOI] [PubMed] [Google Scholar]
- Wouterlood F. G., Mugnaini E. Cartwheel neurons of the dorsal cochlear nucleus: a Golgi-electron microscopic study in rat. J Comp Neurol. 1984 Jul 20;227(1):136–157. doi: 10.1002/cne.902270114. [DOI] [PubMed] [Google Scholar]
- Wouterlood F. G., Mugnaini E., Osen K. K., Dahl A. L. Stellate neurons in rat dorsal cochlear nucleus studies with combined Golgi impregnation and electron microscopy: synaptic connections and mutual coupling by gap junctions. J Neurocytol. 1984 Aug;13(4):639–664. doi: 10.1007/BF01148083. [DOI] [PubMed] [Google Scholar]
- Wu S. H., Oertel D. Inhibitory circuitry in the ventral cochlear nucleus is probably mediated by glycine. J Neurosci. 1986 Sep;6(9):2691–2706. doi: 10.1523/JNEUROSCI.06-09-02691.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. H., Oertel D. Intracellular injection with horseradish peroxidase of physiologically characterized stellate and bushy cells in slices of mouse anteroventral cochlear nucleus. J Neurosci. 1984 Jun;4(6):1577–1588. doi: 10.1523/JNEUROSCI.04-06-01577.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. D., Brownell W. E. Responses to tones and noise of single cells in dorsal cochlear nucleus of unanesthetized cats. J Neurophysiol. 1976 Mar;39(2):282–300. doi: 10.1152/jn.1976.39.2.282. [DOI] [PubMed] [Google Scholar]
- Young E. D. Identification of response properties of ascending axons from dorsal cochlear nucleus. Brain Res. 1980 Oct 27;200(1):23–37. doi: 10.1016/0006-8993(80)91091-4. [DOI] [PubMed] [Google Scholar]
- Young E. D., Voigt H. F. Response properties of type II and type III units in dorsal cochlear nucleus. Hear Res. 1982 Feb;6(2):153–169. doi: 10.1016/0378-5955(82)90051-x. [DOI] [PubMed] [Google Scholar]


