Abstract
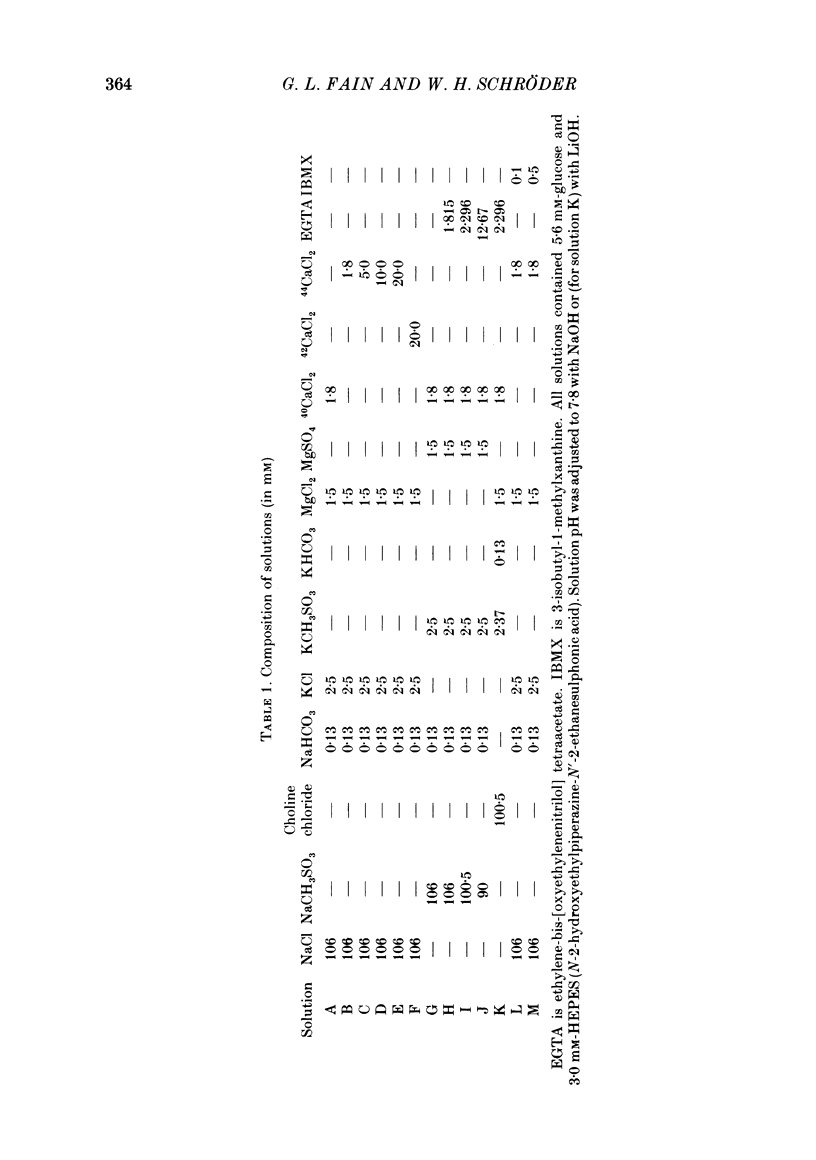
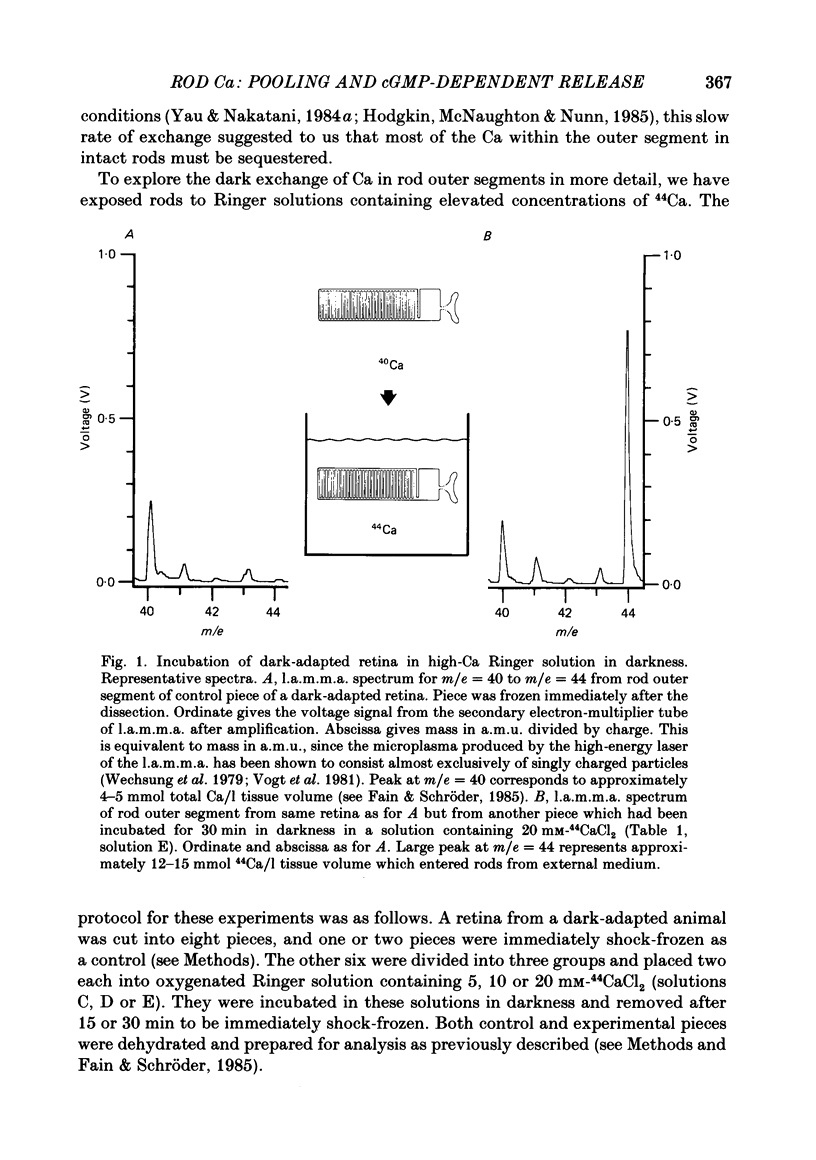
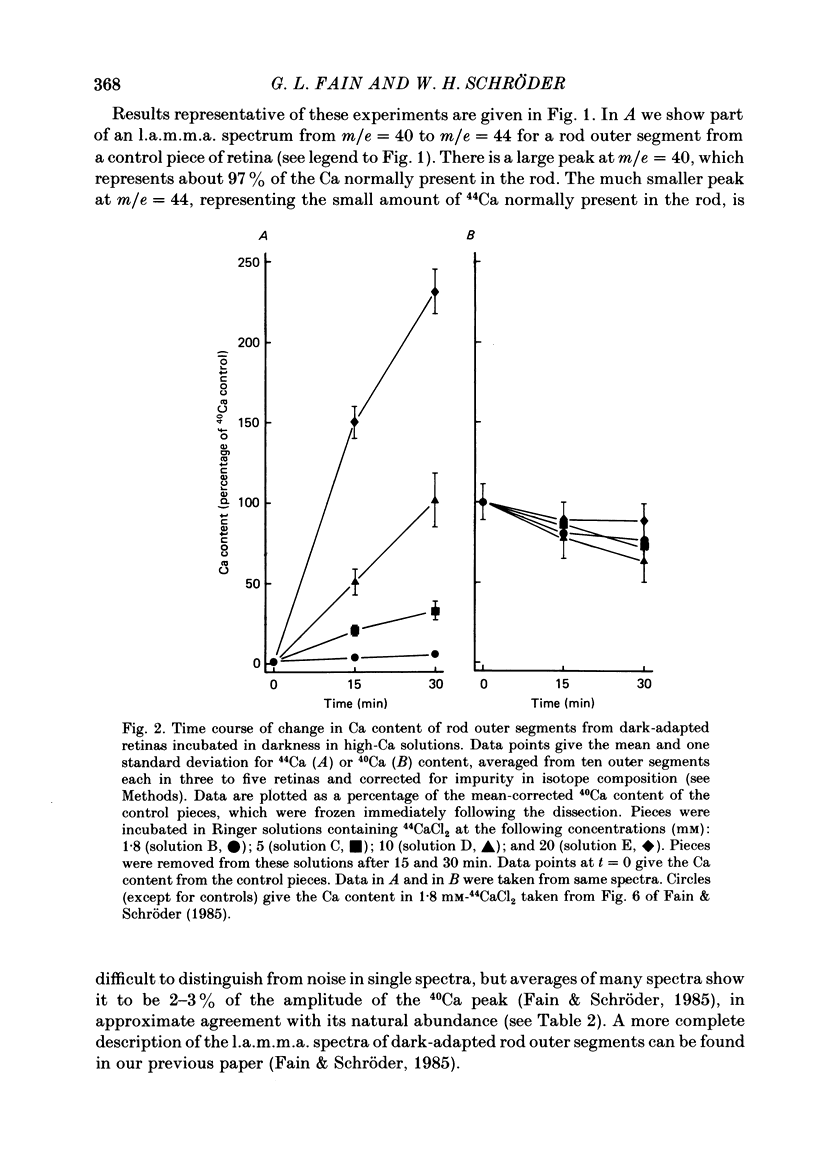
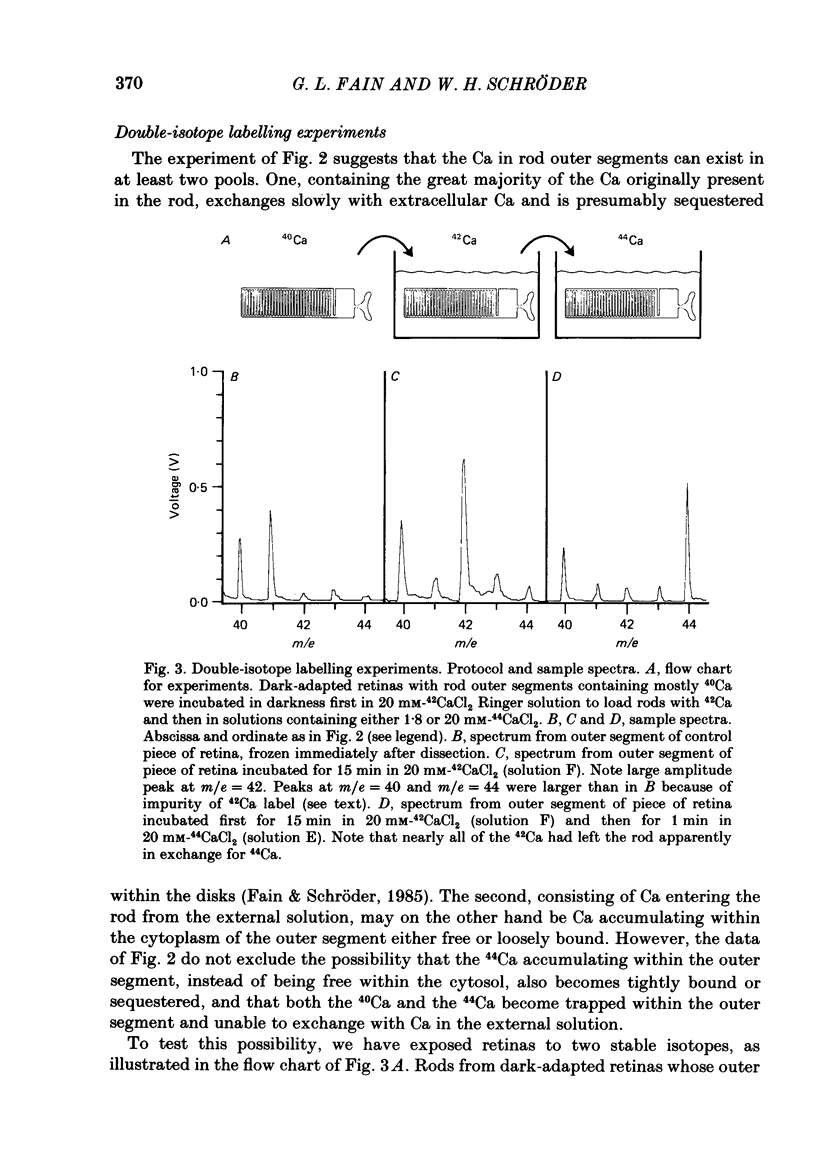
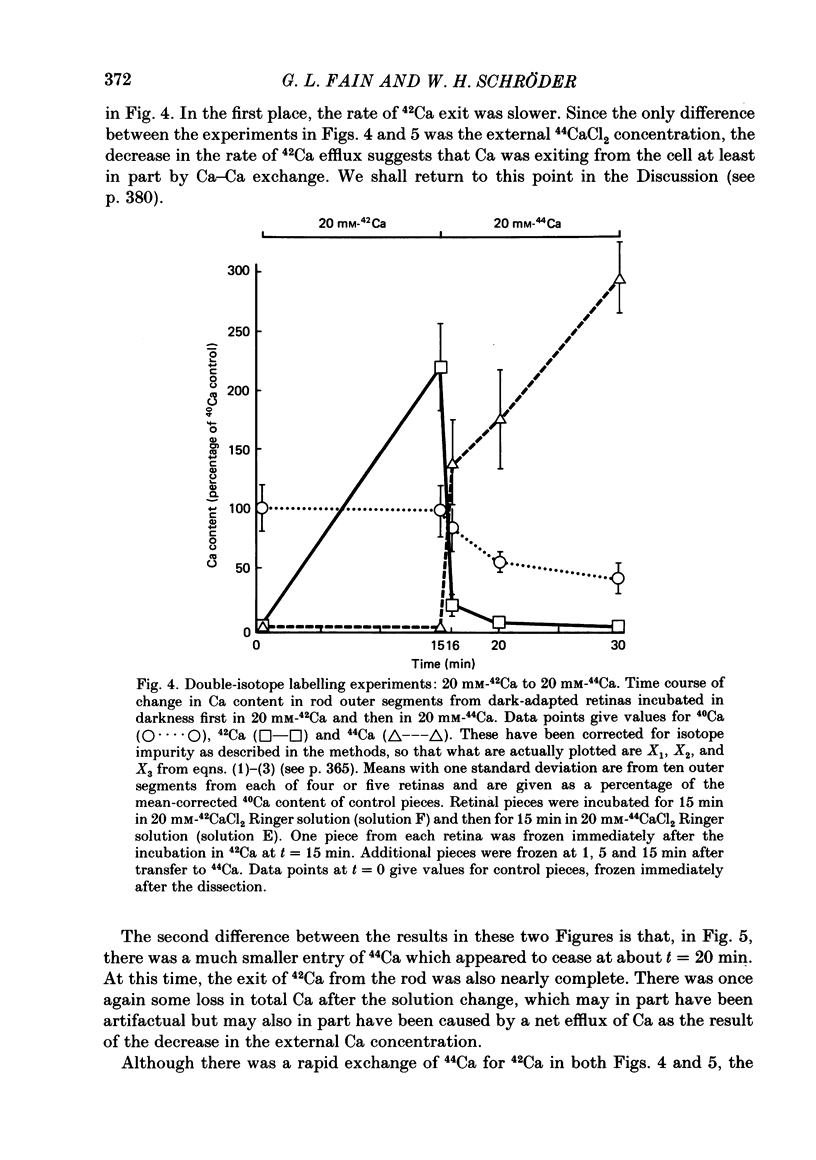
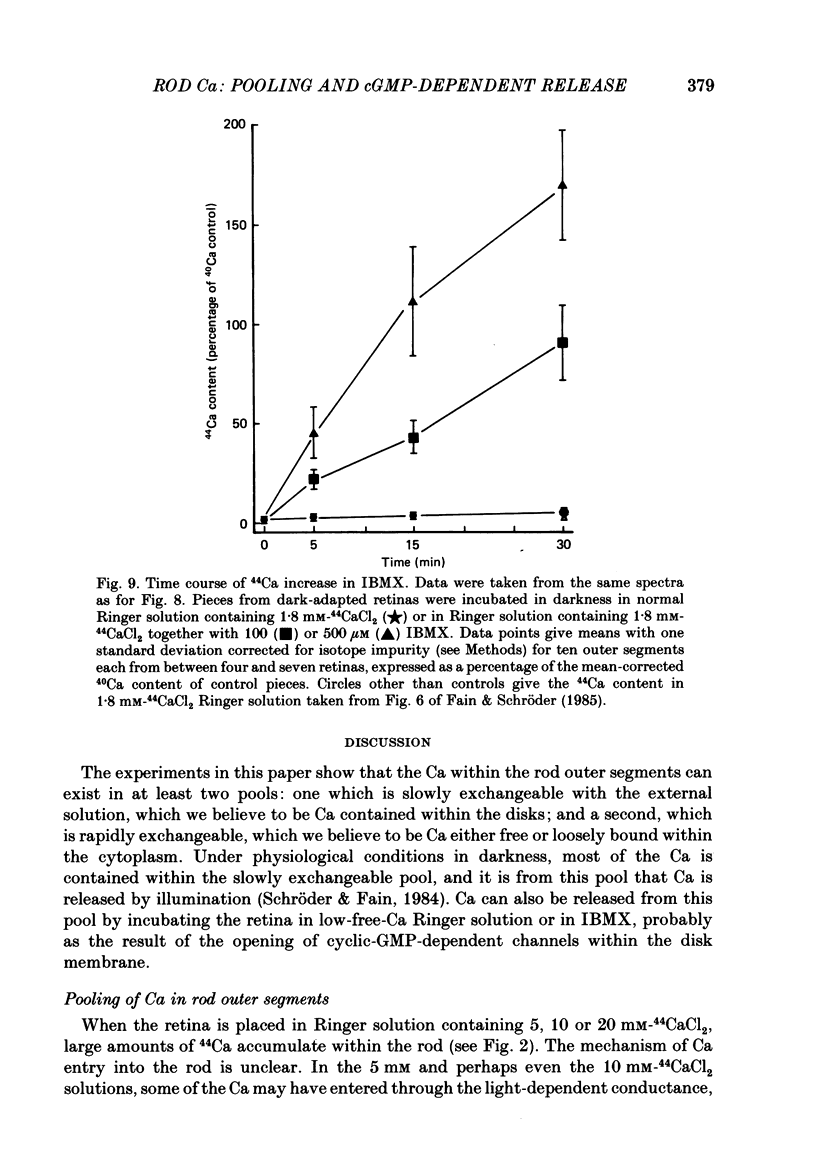
1. We have used laser micromass analysis (l.a.m.m.a.) to investigate Ca uptake and release in intact 'red' rod photoreceptors in the dark-adapted retina of the toad, Bufo marinus. 2. With l.a.m.m.a. it is possible to measure separately the concentrations of each of the Ca isotopes. Rods normally containing almost exclusively 40Ca can be incubated in Ringer solution containing the stable isotopes 42Ca or 44Ca. In this way, the movements of Ca into and out of the rod can be separately determined. 3. When rods are incubated in darkness in high 44Ca (up to 20 mM), large amounts of 44Ca accumulate in the outer segment at a rate which increases with increasing external 44Ca concentration. However, this 44Ca appears not to exchange with the 40Ca originally present within the rod. This result suggests that the 40Ca may be sequestered within a pool which normally exchanges slowly with external Ca. 4. We explored Ca exchange in high-Ca solutions in more detail with double-isotope labelling. In these experiments, rods were first pre-loaded with Ca of one isotope (42Ca) and then incubated in Ringer solution containing another (44Ca). We could then measure separately the rate of exchange of the pre-loaded 42Ca with the 44Ca in the Ringer solution and with the 40Ca originally present within the rod in the sequestered pool. 5. These experiments show that the pre-loaded-Ca exchanges rapidly with Ca in the Ringer solution, at least in part by Ca-Ca exchange, but much more slowly with the Ca originally present within the rod. Thus Ca in the outer segments can exist in (at least) two pools: one which exchanges rapidly across the plasma membrane and is probably Ca free or loosely bound within the cytosol, and another which exchanges slowly and is probably Ca within the disks. 6. Although Ca sequestered within the outer segment normally exchanges quite slowly, it can be rapidly released if the extracellular free Ca is buffered to low levels with EGTA. The rate-limiting step for Ca release under these conditions appears not to be Na-Ca exchange, since the rate of Ca efflux is unchanged if the Na in the Ringer solution is substituted with choline. 7. Ca can also be released from the sequestered pool if rods are incubated in Ringer solution containing 100 or 500 microM-IBMX (3-isobutyl-1-methylxanthine).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
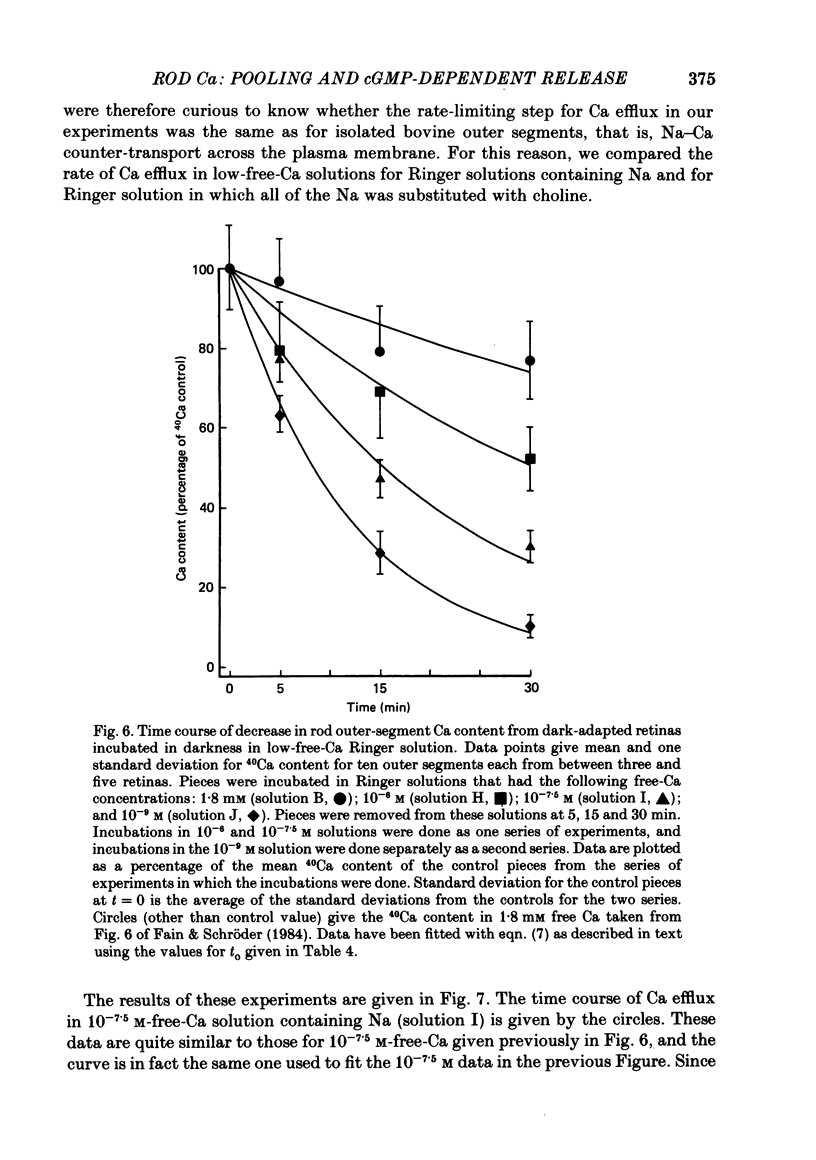
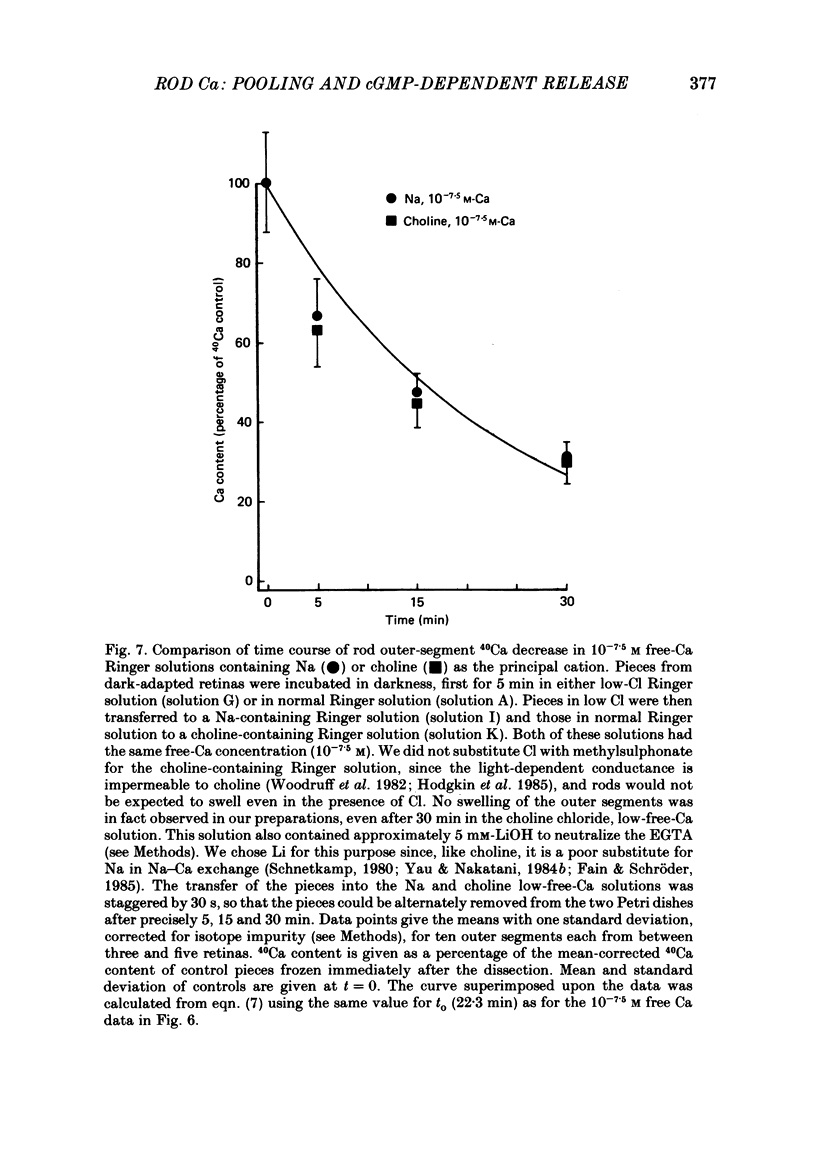
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
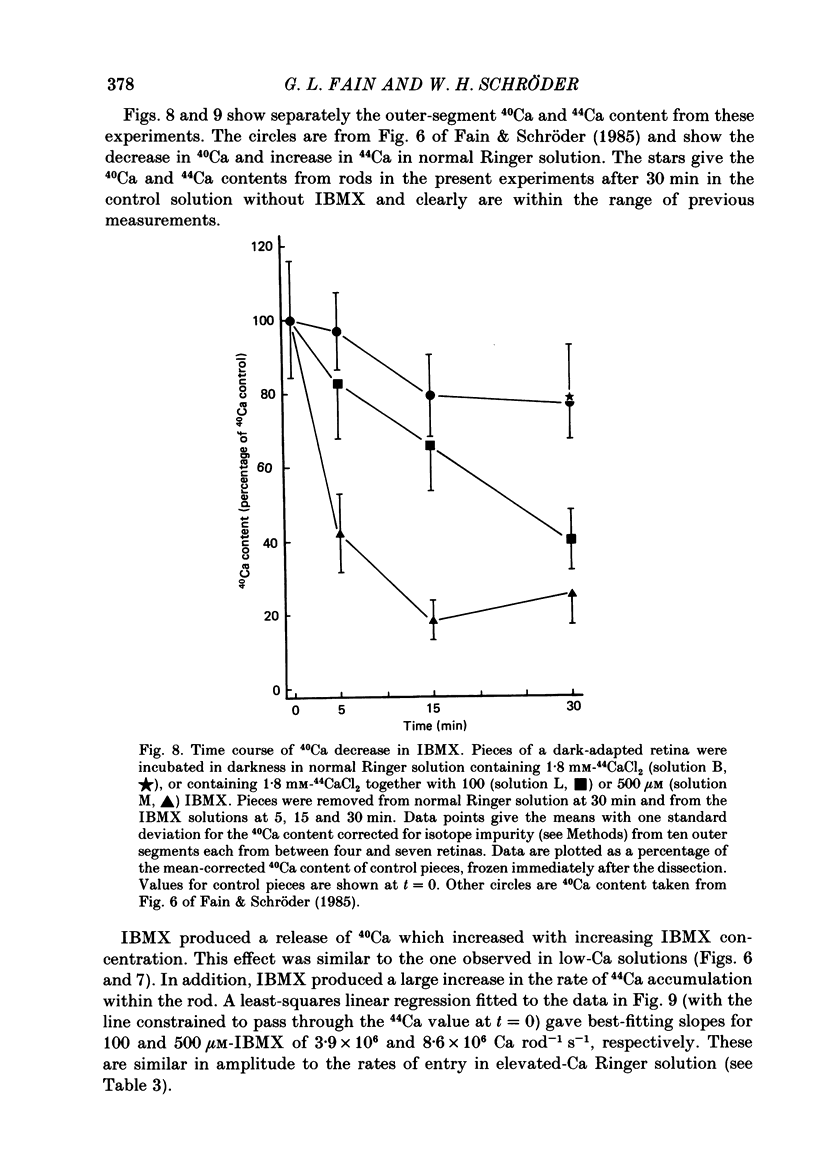
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. Light adaptation in toad rods: requirement for an internal messenger which is not calcium. J Physiol. 1979 Dec;297(0):493–520. doi: 10.1113/jphysiol.1979.sp013053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. The effects of low calcium and background light on the sensitivity of toad rods. J Physiol. 1982 Sep;330:307–329. doi: 10.1113/jphysiol.1982.sp014343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A., Cavaggioni A. Fast ionic flux activated by cyclic GMP in the membrane of cattle rod outer segments. Eur J Biochem. 1983 Apr 15;132(1):1–8. doi: 10.1111/j.1432-1033.1983.tb07317.x. [DOI] [PubMed] [Google Scholar]
- Cervetto L., McNaughton P. A. The effects of phosphodiesterase inhibitors and lanthanum ions on the light-sensitive current of toad retinal rods. J Physiol. 1986 Jan;370:91–109. doi: 10.1113/jphysiol.1986.sp015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M. Trigger and amplification mechanisms in visual phototransduction. Annu Rev Biophys Biophys Chem. 1985;14:331–360. doi: 10.1146/annurev.bb.14.060185.001555. [DOI] [PubMed] [Google Scholar]
- Cohen A. I., Hall I. A., Ferrendelli J. A. Calcium and cyclic nucleotide regulation in incubated mouse retinas. J Gen Physiol. 1978 May;71(5):595–612. doi: 10.1085/jgp.71.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Gerschenfeld H. M., Quandt F. N. Calcium spikes in toad rods. J Physiol. 1980 Jun;303:495–513. doi: 10.1113/jphysiol.1980.sp013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Schröder W. H. Calcium content and calcium exchange in dark-adapted toad rods. J Physiol. 1985 Nov;368:641–665. doi: 10.1113/jphysiol.1985.sp015881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Ames A. A., 3rd, Gander J. E., Walseth T. F. Magnitude of increase in retinal cGMP metabolic flux determined by 18O incorporation into nucleotide alpha-phosphoryls corresponds with intensity of photic stimulation. J Biol Chem. 1983 Aug 10;258(15):9213–9219. [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Ionic mechanisms in excitation of photoreceptors. Ann N Y Acad Sci. 1975 Dec 30;264:314–325. doi: 10.1111/j.1749-6632.1975.tb31492.x. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. W., Kaupp U. B. Cyclic GMP directly regulates a cation conductance in membranes of bovine rods by a cooperative mechanism. J Biol Chem. 1985 Jun 10;260(11):6788–6800. [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Ornberg R. L., Reese T. S. A freeze-substitution method for localizing divalent cations: examples from secretory systems. Fed Proc. 1980 Aug;39(10):2802–2808. [PubMed] [Google Scholar]
- Puckett K. L., Aronson E. T., Goldin S. M. ATP-dependent calcium uptake activity associated with a disk membrane fraction isolated from bovine retinal rod outer segments. Biochemistry. 1985 Jan 15;24(2):390–400. doi: 10.1021/bi00323a023. [DOI] [PubMed] [Google Scholar]
- Puckett K. L., Goldin S. M. Guanosine 3',5'-cyclic monophosphate stimulates release of actively accumulated calcium in purified disks from rod outer segments of bovine retina. Biochemistry. 1986 Apr 8;25(7):1739–1746. doi: 10.1021/bi00355a044. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Calcium translocation and storage of isolated intact cattle rod outer segments in darkness. Biochim Biophys Acta. 1979 Jul 5;554(2):441–459. doi: 10.1016/0005-2736(79)90383-3. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Ion selectivity of the cation transport system of isolated intact cattle rod outer segments: evidence for a direct communication between the rod plasma membrane and the rod disk membranes. Biochim Biophys Acta. 1980 May 8;598(1):66–90. doi: 10.1016/0005-2736(80)90266-7. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Sodium-calcium exchange in the outer segments of bovine rod photoreceptors. J Physiol. 1986 Apr;373:25–45. doi: 10.1113/jphysiol.1986.sp016033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder W. H., Fain G. L. Light-dependent calcium release from photoreceptors measured by laser micro-mass analysis. Nature. 1984 May 17;309(5965):268–270. doi: 10.1038/309268a0. [DOI] [PubMed] [Google Scholar]
- Slaughter R. S., Sutko J. L., Reeves J. P. Equilibrium calcium-calcium exchange in cardiac sarcolemmal vesicles. J Biol Chem. 1983 Mar 10;258(5):3183–3190. [PubMed] [Google Scholar]
- Somlyo A. P., Walz B. Elemental distribution in Rana pipiens retinal rods: quantitative electron probe analysis. J Physiol. 1985 Jan;358:183–195. doi: 10.1113/jphysiol.1985.sp015547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler G. L., Bitensky M. W. A light-activated GTPase in vertebrate photoreceptors: regulation of light-activated cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4238–4242. doi: 10.1073/pnas.74.10.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M. L., Fain G. L., Bastian B. L. Light-dependent ion influx into toad photoreceptors. J Gen Physiol. 1982 Oct;80(4):517–536. doi: 10.1085/jgp.80.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984 May 24;309(5966):352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985 Sep 19;317(6034):252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]


