Abstract
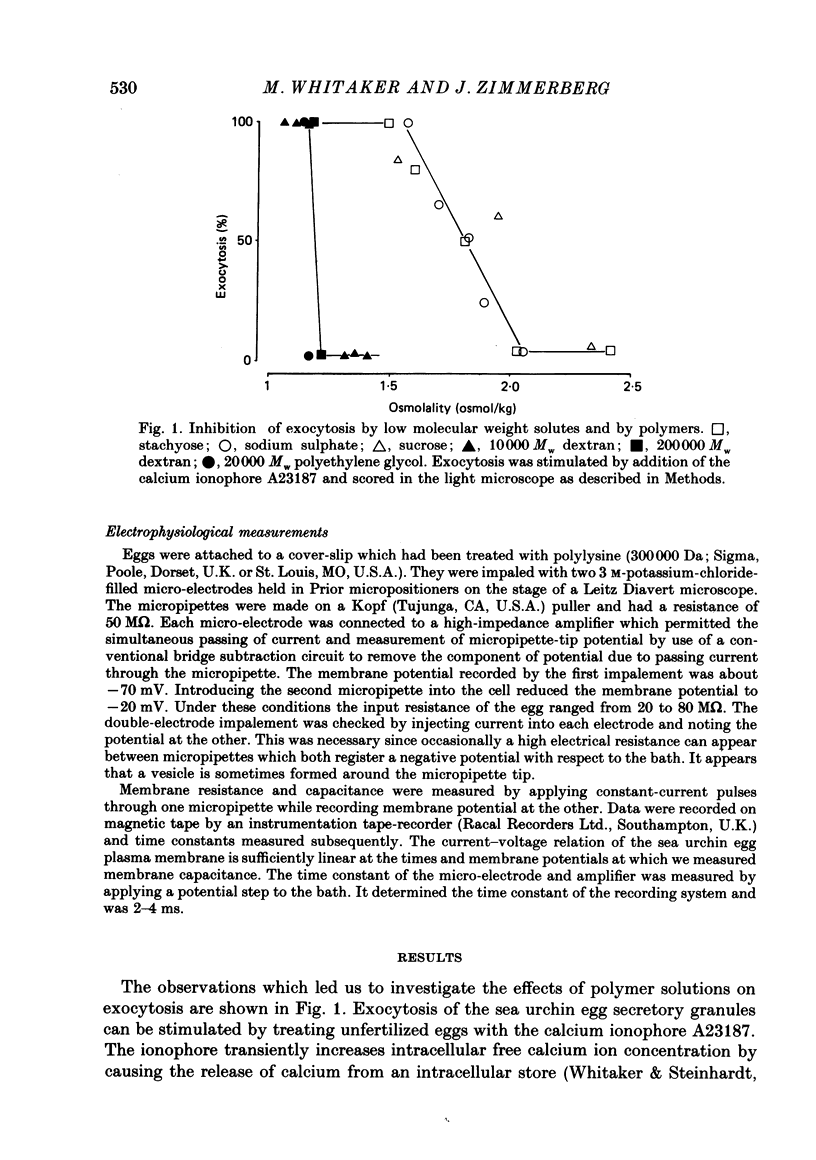
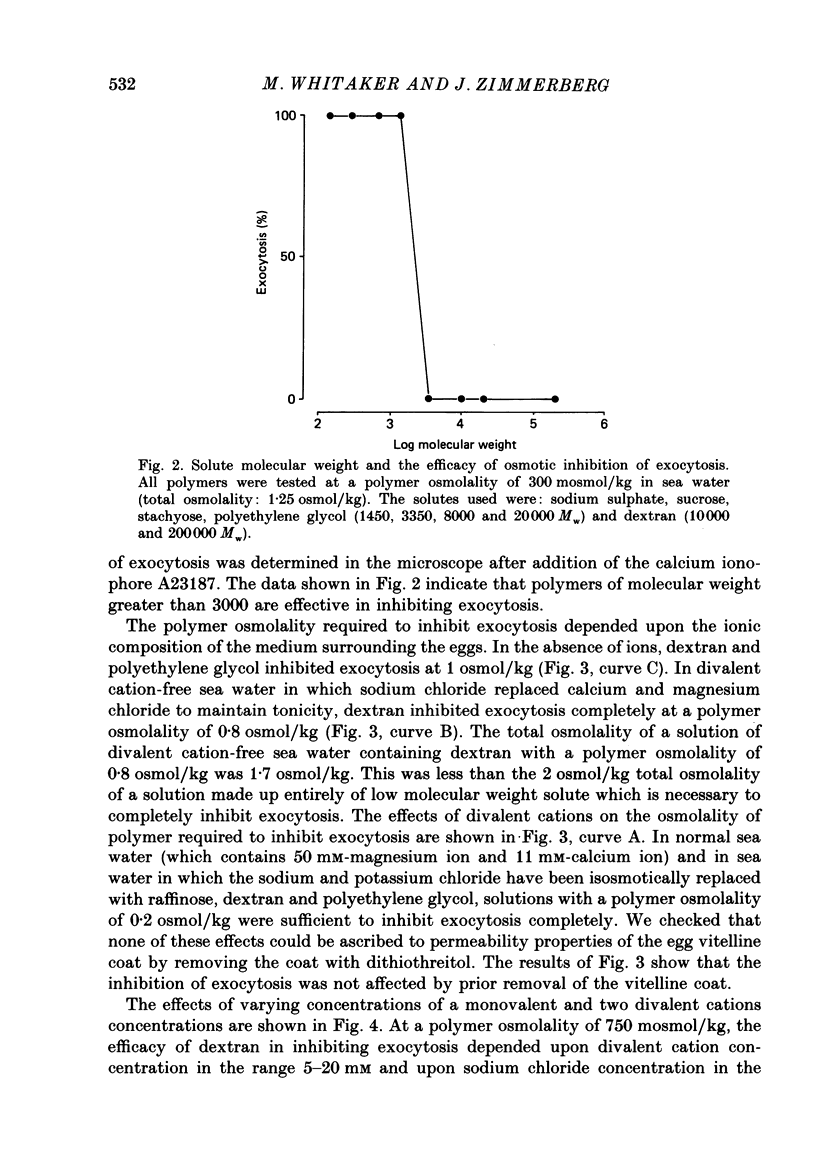
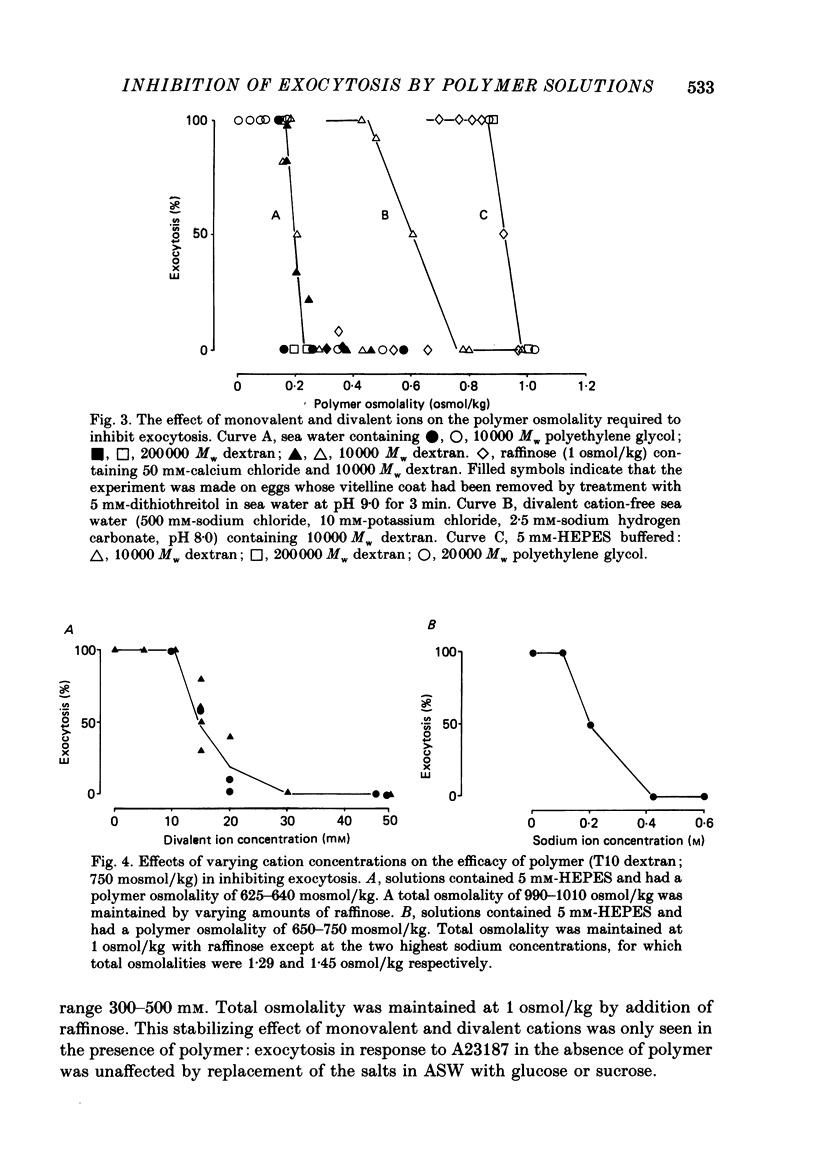
1. We have investigated the effects of the osmotic pressure exerted by polymer solutions on exocytosis in sea urchin eggs. 2. Exocytosis is prevented by including a variety of polymers of different chemical composition and molecular weight in the sea water surrounding the eggs. Inhibition is complete at a polymer osmolality of 250 mosmol/kg. 3. The increase in membrane capacitance which occurs during exocytosis and which corresponds to the fusion of secretory granules with the plasma membrane is not substantially altered by inhibitory concentrations of polymer. 4. In the absence of the ions present in sea water, these polymers inhibit exocytosis at an osmolality of 950 mosmol/kg. 5. Calcium, magnesium and sodium ions reduce the polymer osmolality required to prevent exocytosis. 6. A comparison of the effects of monovalent and divalent ions indicates that the divalent ions bind to and stabilize the secretory granule contents. 7. These results demonstrate that polymers prevent exocytosis by preventing dispersal of the granule contents once fusion of the secretory granule with the plasma-lemma has occurred. The ions present in sea water do not promote dispersal, rather, they hinder it. The contribution of ionic fluxes to granule swelling during exocytosis is discussed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aborg C. H., Novotný J., Uvnäs B. Ionic binding of histamine in mast cell granules. Acta Physiol Scand. 1967 Apr;69(4):276–283. doi: 10.1111/j.1748-1716.1967.tb03523.x. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Whitaker M. J. Influence of ATP and calcium on the cortical reaction in sea urchin eggs. Nature. 1978 Nov 30;276(5687):513–515. doi: 10.1038/276513a0. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Pazoles C. J., Creutz C. E., Aurbach G. D., Pollard H. B. Role of anions in parathyroid hormone release from dispersed bovine parathyroid cells. Proc Natl Acad Sci U S A. 1978 Feb;75(2):876–880. doi: 10.1073/pnas.75.2.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. E., Heuser J. Membrane fusion during secretion: cortical granule exocytosis in sex urchin eggs as studied by quick-freezing and freeze-fracture. J Cell Biol. 1979 Oct;83(1):91–108. doi: 10.1083/jcb.83.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris R. M., Viveros O. H., Kirshner N. Effects of various agents on the Mg2+-ATP stimulated incorporation and release of catecholamines by isolated bovine adrenomedullary storage vesicles and on secretion from the adrenal medulla. Biochem Pharmacol. 1970 Feb;19(2):505–514. doi: 10.1016/0006-2952(70)90207-8. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Zimmerberg J., Cohen F. S. Osmotic swelling of vesicles: its role in the fusion of vesicles with planar phospholipid bilayer membranes and its possible role in exocytosis. Annu Rev Physiol. 1986;48:163–174. doi: 10.1146/annurev.ph.48.030186.001115. [DOI] [PubMed] [Google Scholar]
- Holz R. W. The role of osmotic forces in exocytosis from adrenal chromaffin cells. Annu Rev Physiol. 1986;48:175–189. doi: 10.1146/annurev.ph.48.030186.001135. [DOI] [PubMed] [Google Scholar]
- Jaffe L. A., Hagiwara S., Kado R. T. The time course of cortical vesicle fusion in sea urchin eggs observed as membrane capacitance changes. Dev Biol. 1978 Nov;67(1):243–248. doi: 10.1016/0012-1606(78)90314-7. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D. Activation of sea-urchin eggs by a calcium ionophore. Proc Natl Acad Sci U S A. 1974 May;71(5):1915–1919. doi: 10.1073/pnas.71.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. Ionic regulation of egg activation. Q Rev Biophys. 1982 Nov;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Sardet C., Epel D. Exocytosis of sea urchin egg cortical vesicles in vitro is retarded by hyperosmotic sucrose: kinetics of fusion monitored by quantitative light-scattering microscopy. J Cell Biol. 1985 Dec;101(6):2398–2410. doi: 10.1083/jcb.101.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Whitaker M. Irreversible swelling of secretory granules during exocytosis caused by calcium. Nature. 1985 Jun 13;315(6020):581–584. doi: 10.1038/315581a0. [DOI] [PubMed] [Google Scholar]




