Abstract
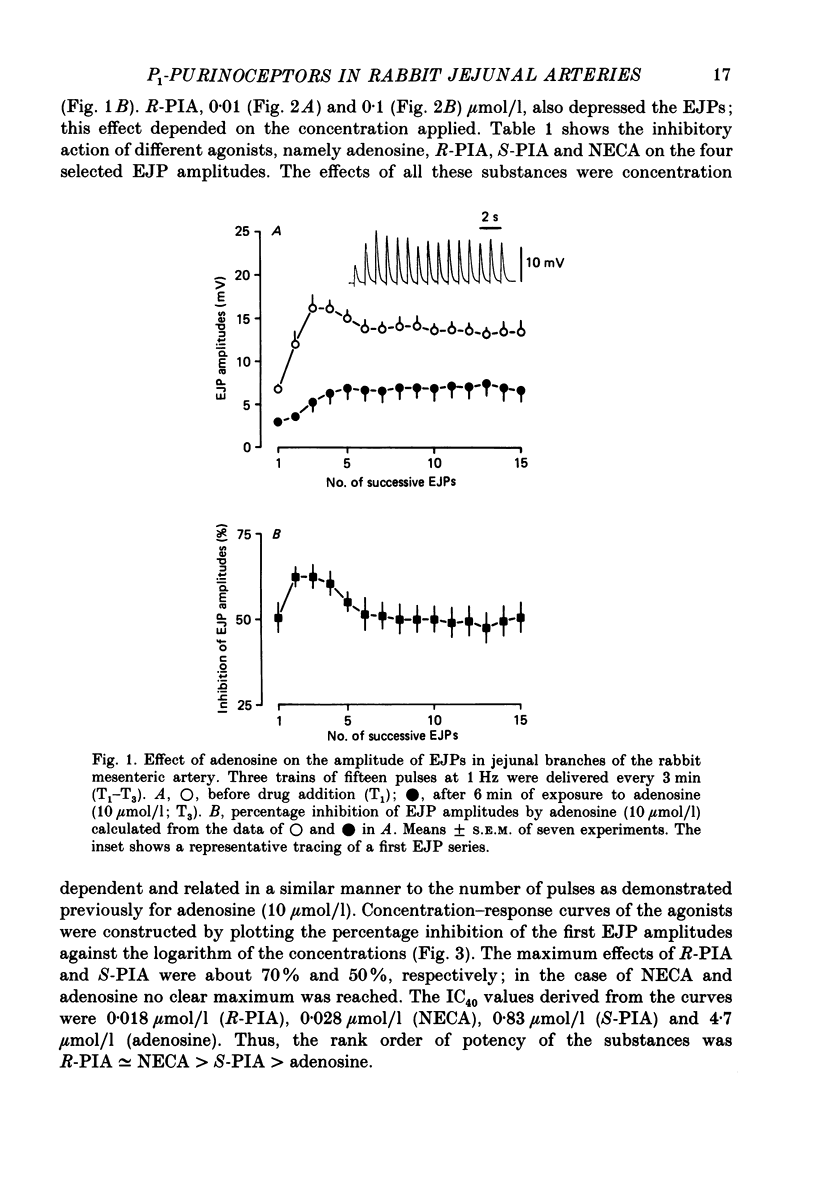
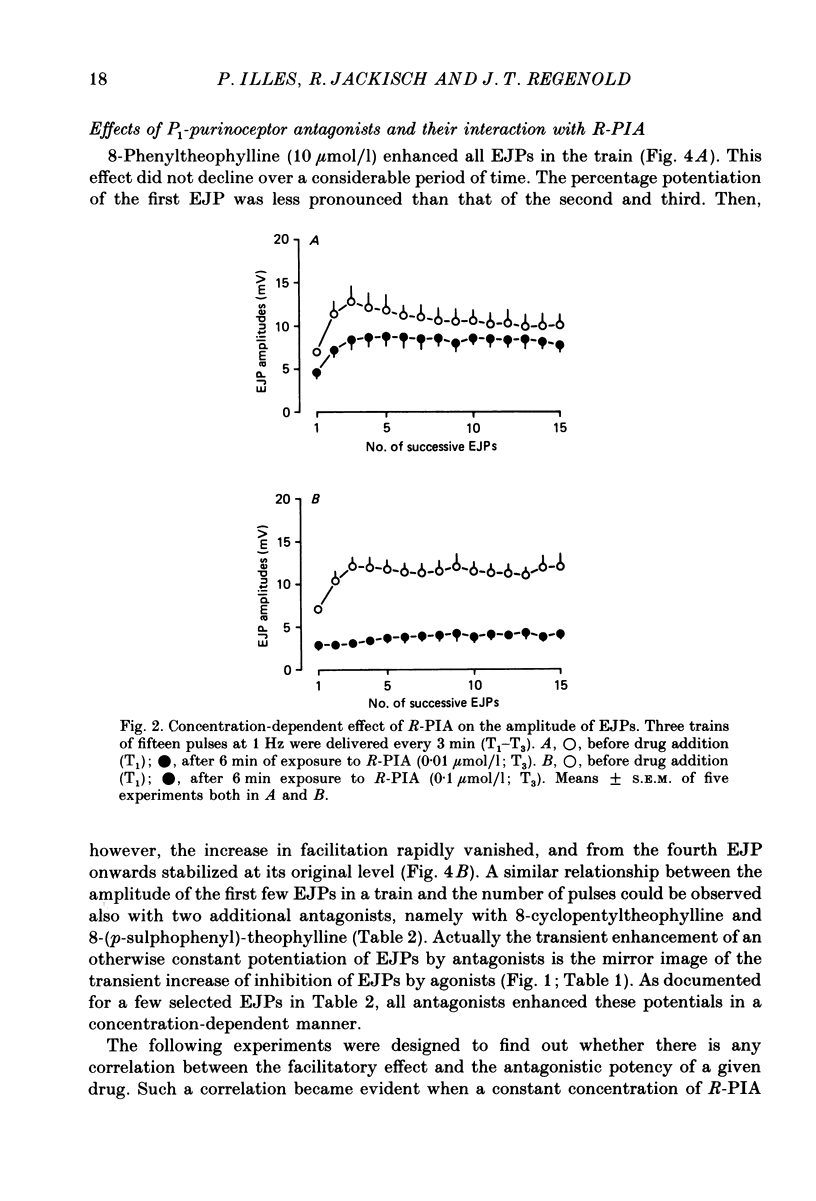
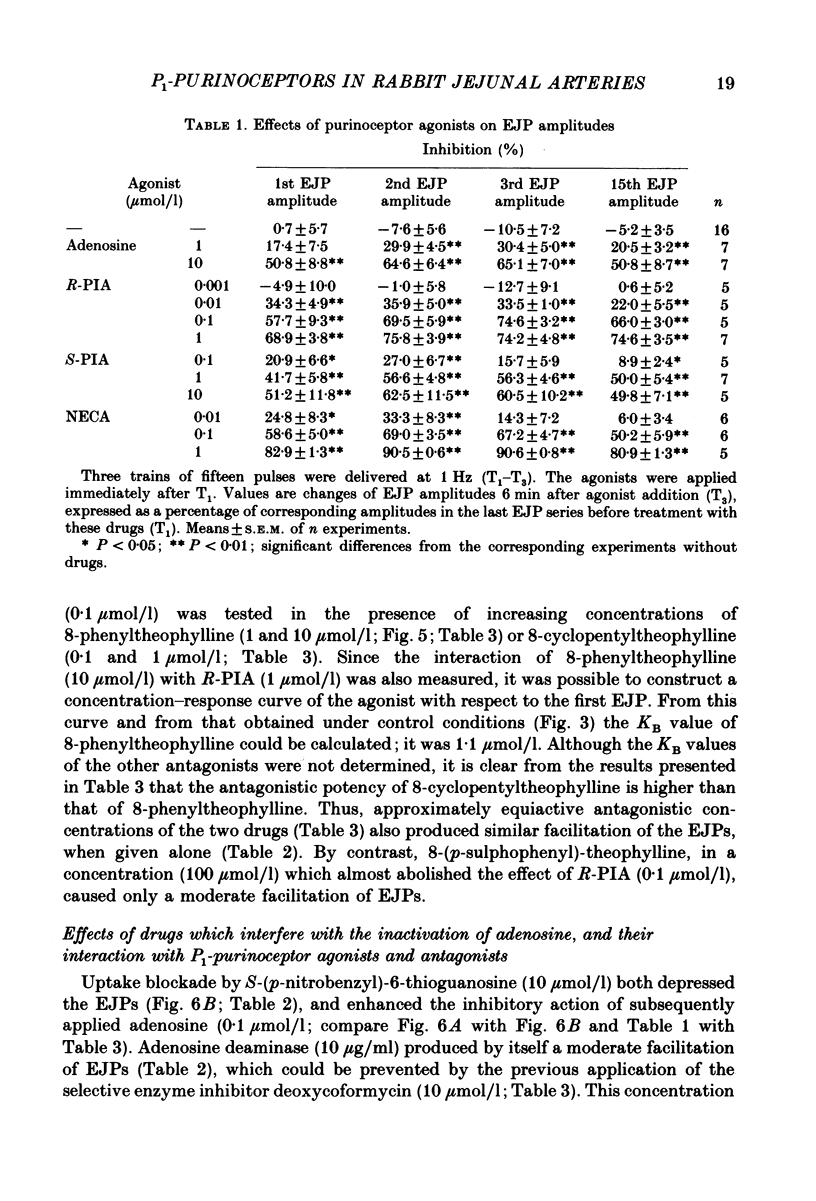
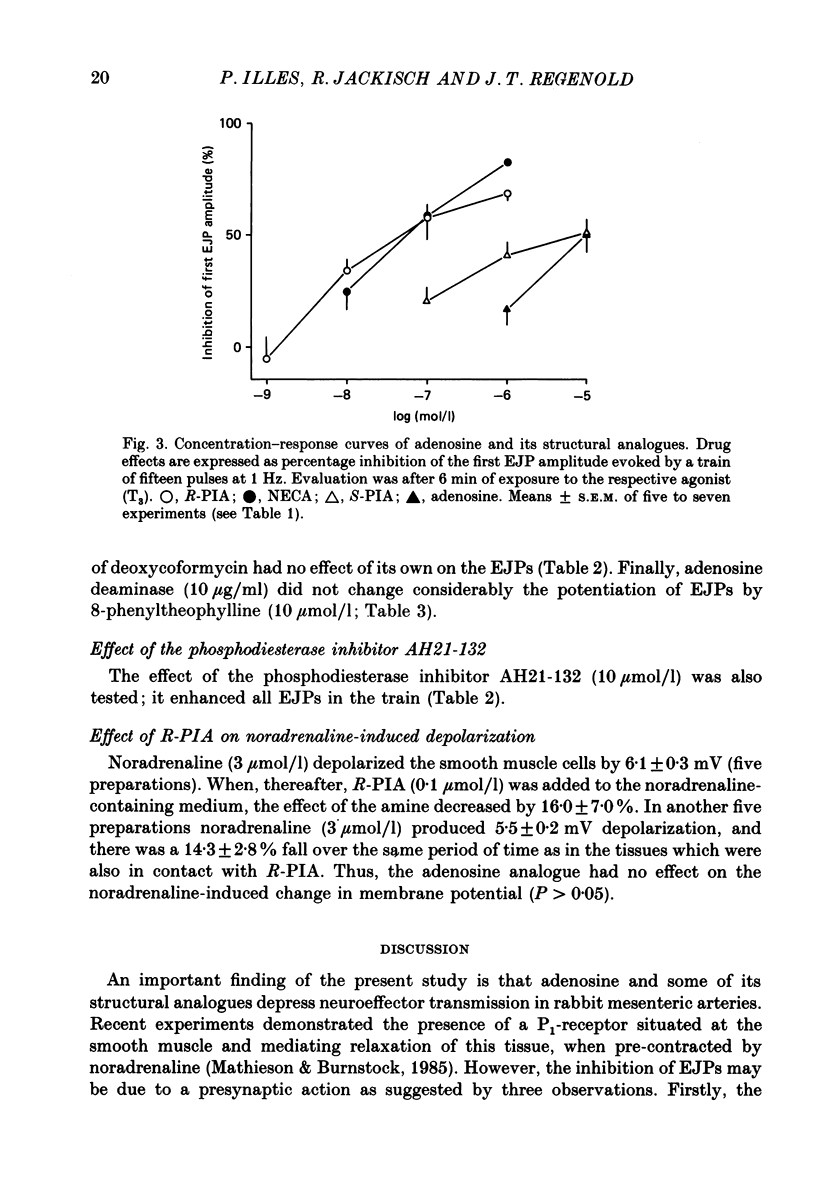
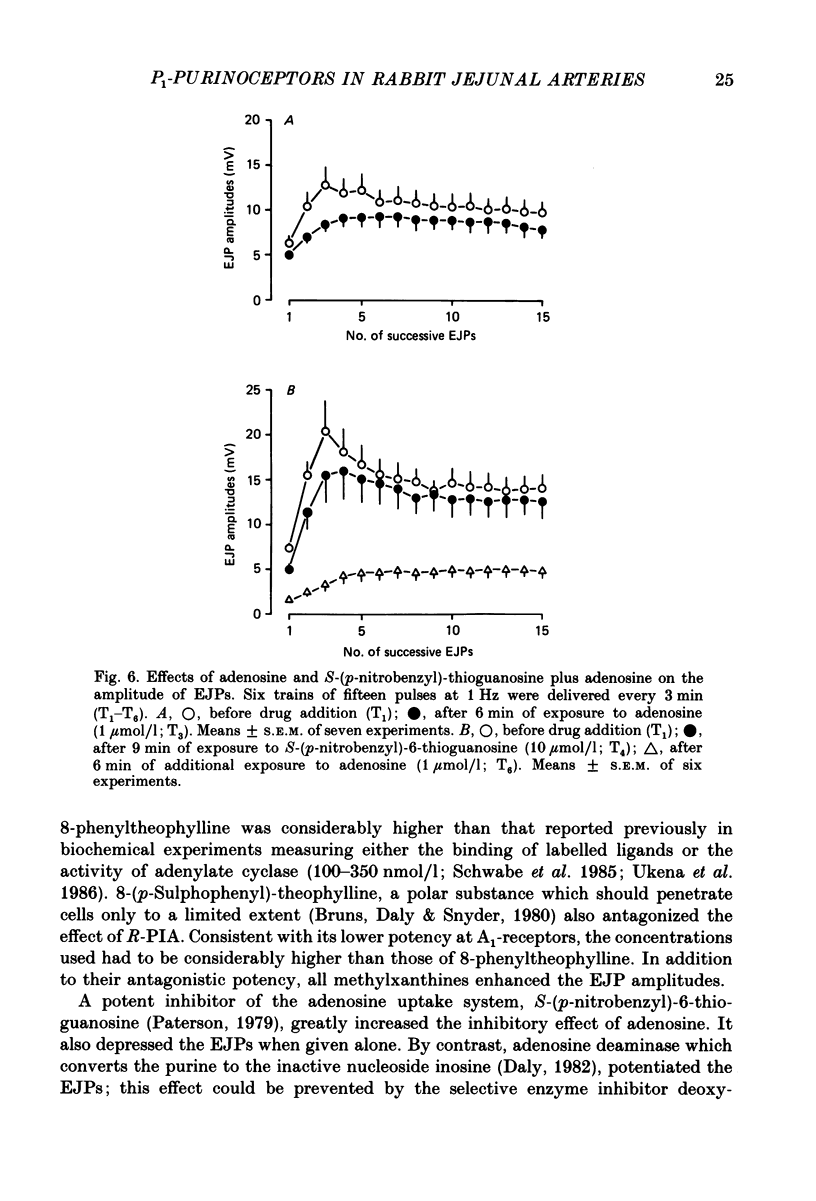
1. Excitatory junction potentials (EJPs) evoked by nerve stimulation with fifteen pulses at 1 Hz were recorded from smooth muscle cells of the rabbit isolated mesenteric artery. The effects of P1-purinoceptor agonists and antagonists, as well as of substances which interfere with the inactivation of endogenous adenosine, were tested. 2. Adenosine and its analogues depressed the EJPs in the train in a concentration-dependent manner. The percentage inhibition of the first EJP and that of the later ones was similar; some early EJPs, however, were inhibited more markedly. The rank order of potency of the agonists was (-)-N6-(R-phenylisopropyl)-adenosine (R-PIA) congruent to 5'-N-ethylcarboxamidoadenosine (NECA) greater than (+)-N6-(S-phenylisopropyl)-adenosine (S-PIA) greater than adenosine. The respective IC40 values (the concentrations producing 40% inhibition of the first EJP in the train) were 0.018, 0.028, 0.83 and 4.7 mumol/l. 3. Three methylxanthines, namely 8-phenyltheophylline (1, 10 mumol/l), 8-cyclopentyltheophylline (0.1, 1 mumol/l) and 8-(p-sulphophenyl)-theophylline (100 mumol/l), antagonized the effect of R-PIA (0.1 mumol/l). When given alone they also enhanced the amplitudes of all EJPs in the train. The percentage facilitation of the first EJP and that of the later ones was similar. Some early EJPs, however, were potentiated more markedly. 8-Phenyltheophylline was less potent than 8-cyclopentyltheophylline both in preventing the action of R-PIA and in enhancing the EJPs. A concentration (100 mumol/l) of 8-(p-sulphophenyl)-theophylline, which strongly antagonized the R-PIA effect, produced only a moderate facilitation of EJPs. 4. S-(p-nitrobenzyl)-6-thioguanosine (10 mumol/l) both depressed the EJPs, and enhanced the inhibitory effect of adenosine. Adenosine deaminase (10 micrograms/ml) caused some potentiation of EJPs; this action was prevented by a concentration (10 mumol/l) of deoxycoformycin, which had no effect of its own. AH21-132 (10 mumol/l) enhanced all EJPs in the train. 5. None of the above substances influenced the resting membrane potential of the smooth muscle cells. In addition, R-PIA (0.1 mumol/l) did not change the depolarization induced by noradrenaline (3 mumol/l). 6. We suggest that the axon terminals of postganglionic sympathetic neurones in the rabbit mesenteric artery possess P1-purinoceptors of the A1-type. The activation of these presynaptic receptors by endogenous adenosine may inhibit the release of the main neuroeffector transmitter, which is probably ATP.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



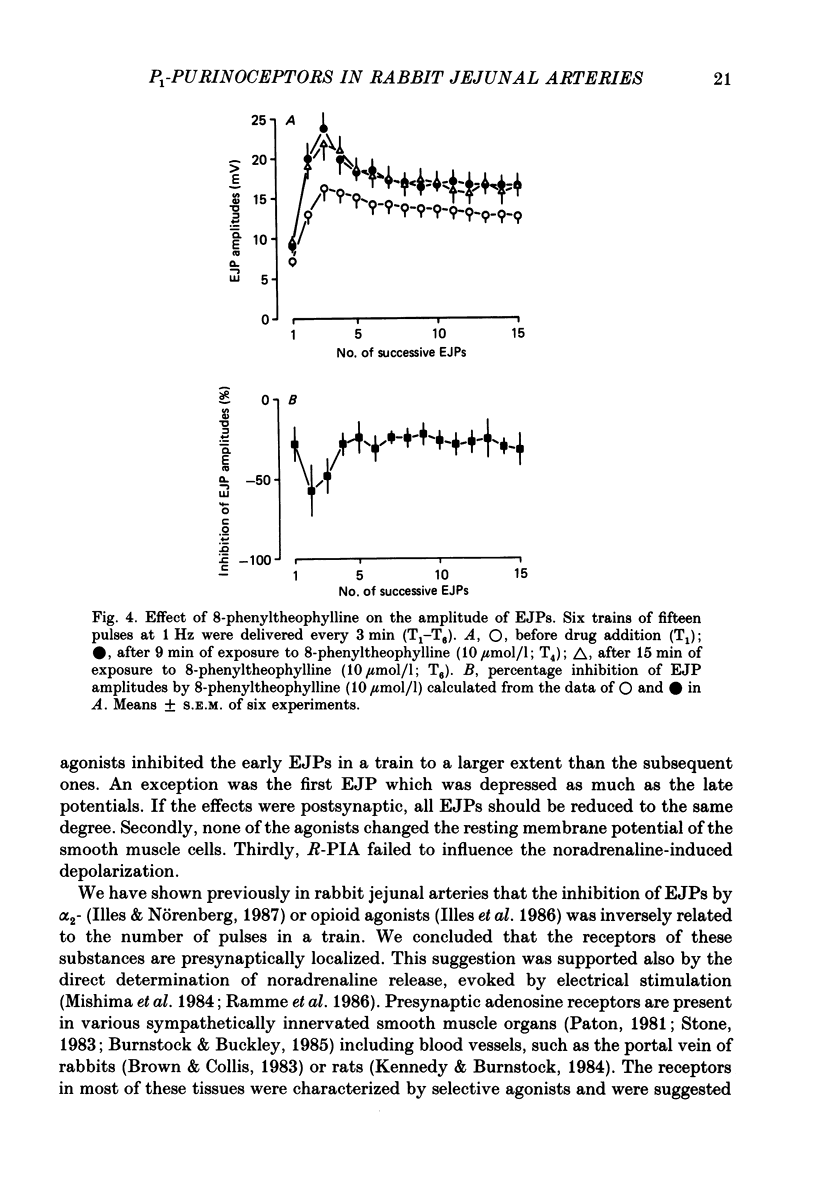
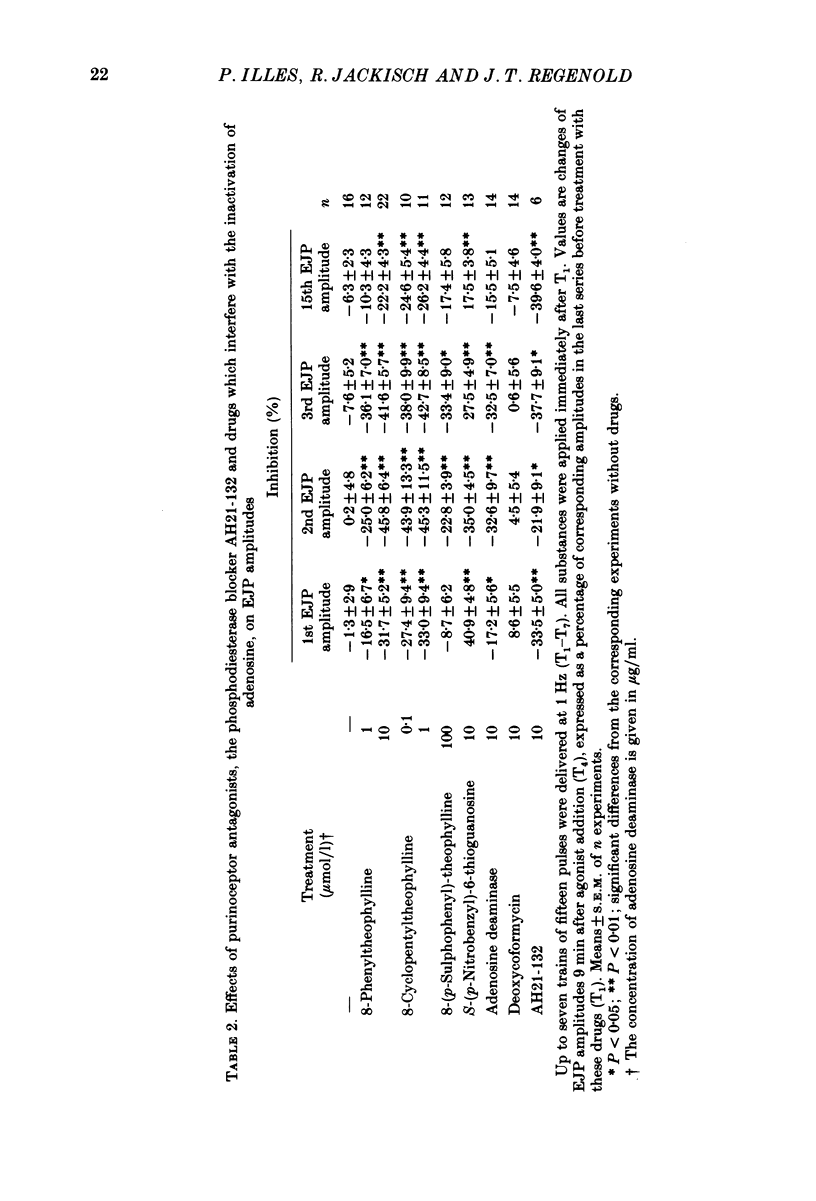
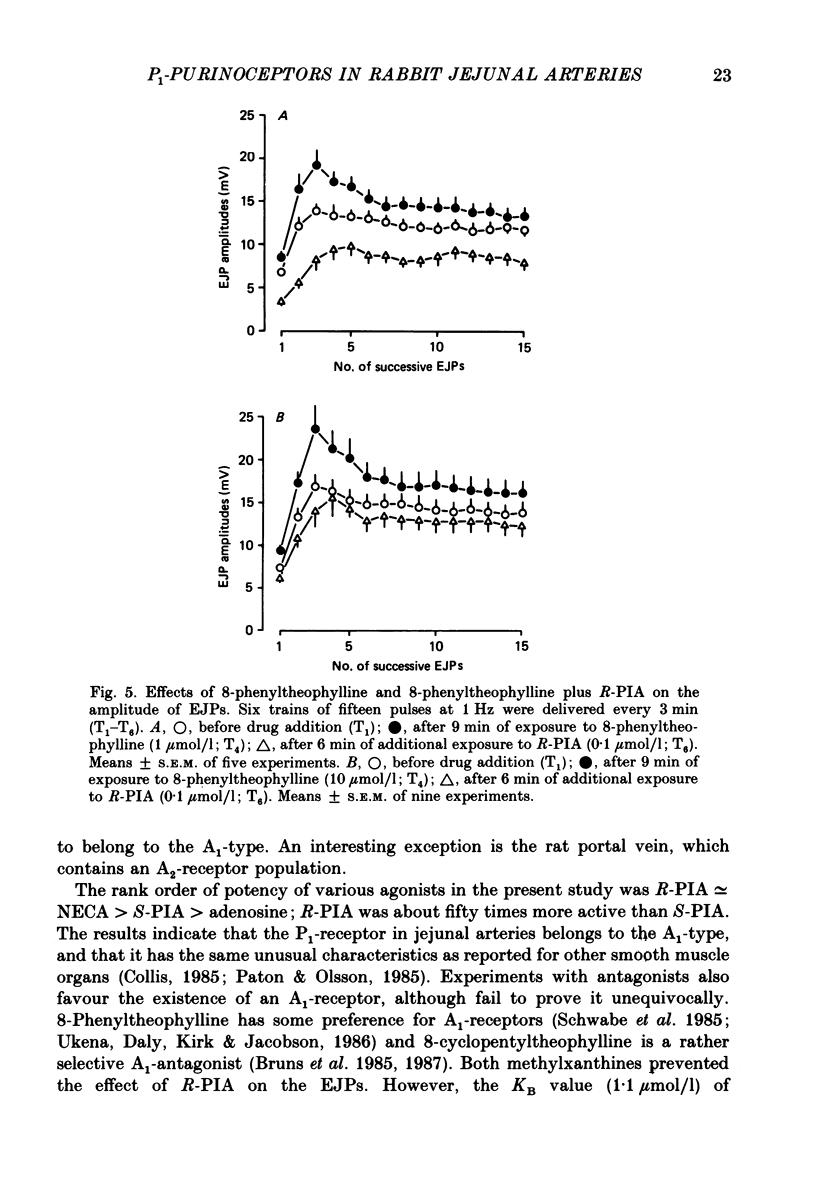
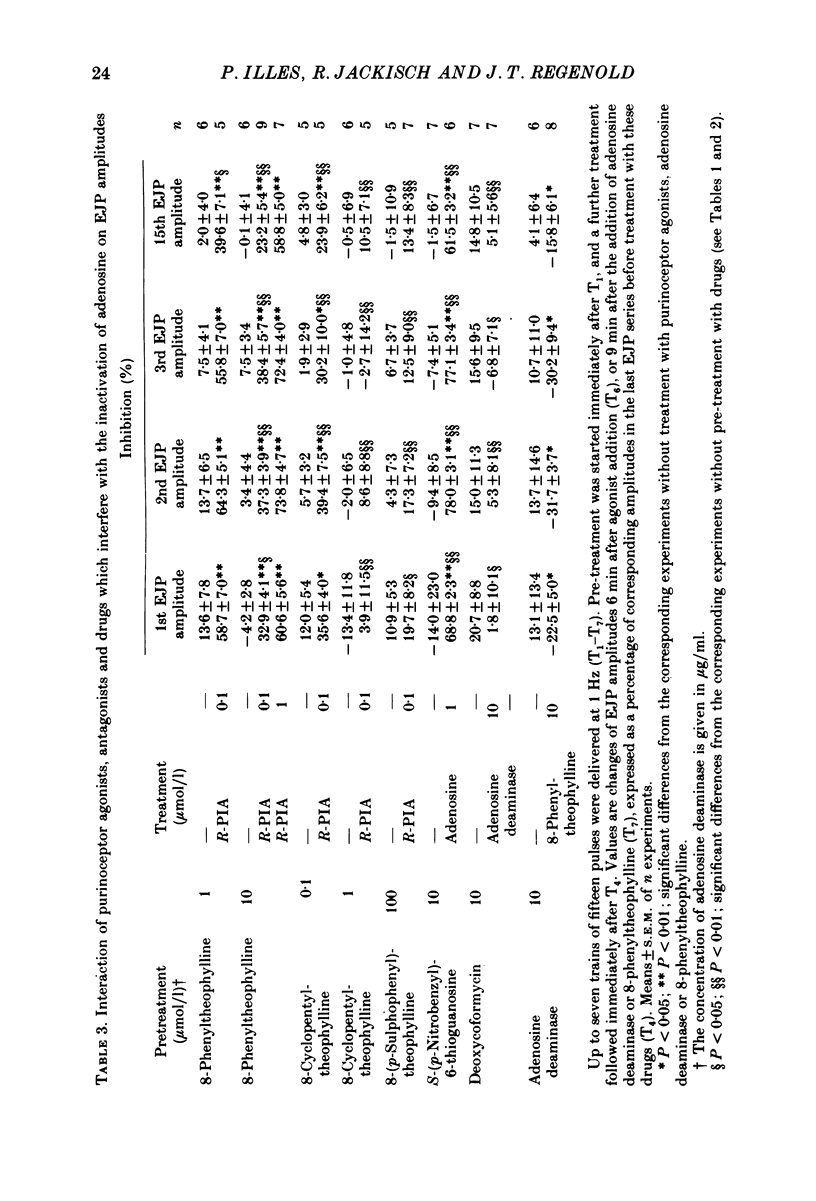




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Spector T., Parks R. E., Jr Tight-binding inhibitors--IV. Inhibition of adenosine deaminases by various inhibitors. Biochem Pharmacol. 1977 Mar 1;26(5):359–367. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Collis M. G. Adenosine A1 receptor mediated inhibition of nerve stimulation-induced contractions of the rabbit portal vein. Eur J Pharmacol. 1983 Sep 30;93(3-4):277–282. doi: 10.1016/0014-2999(83)90148-6. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Fergus J. H., Badger E. W., Bristol J. A., Santay L. A., Hartman J. D., Hays S. J., Huang C. C. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. A dual function for adenosine 5'-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986 Mar;58(3):319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- Enero M. A., Saidman B. Q. Possible feed-back inhibition of noradrenaline release by purine compounds. Naunyn Schmiedebergs Arch Pharmacol. 1977 Mar;297(1):39–46. doi: 10.1007/BF00508808. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol. 1980 Jun 15;29(12):1635–1643. doi: 10.1016/0006-2952(80)90117-3. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Sollevi A. The release of adenosine and inosine from canine subcutaneous adipose tissue by nerve stimulation and noradrenaline. J Physiol. 1981;313:351–367. doi: 10.1113/jphysiol.1981.sp013670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedqvist P., Fredholm B. B., Olundh S. Antagonistic effects of theophylline and adenosine on adrenergic neuroeffector transmission in the rabbit kidney. Circ Res. 1978 Oct;43(4):592–598. doi: 10.1161/01.res.43.4.592. [DOI] [PubMed] [Google Scholar]
- Illes P., Ramme D., Starke K. Presynaptic opioid delta-receptors in the rabbit mesenteric artery. J Physiol. 1986 Oct;379:217–228. doi: 10.1113/jphysiol.1986.sp016249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S. Actions of ATP and alpha, beta-methylene ATP on neuromuscular transmission and smooth muscle membrane of the rabbit and guinea-pig mesenteric arteries. Br J Pharmacol. 1985 Dec;86(4):777–787. doi: 10.1111/j.1476-5381.1985.tb11099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackisch R., Fehr R., Hertting G. Adenosine: an endogenous modulator of hippocampal noradrenaline release. Neuropharmacology. 1985 Jun;24(6):499–507. doi: 10.1016/0028-3908(85)90055-3. [DOI] [PubMed] [Google Scholar]
- Katsuragi T., Su C. Augmentation by theophylline of [3H]purine release from vascular adrenergic nerves: evidence for presynaptic autoinhibition. J Pharmacol Exp Ther. 1982 Jan;220(1):152–156. [PubMed] [Google Scholar]
- Kennedy C., Burnstock G. Evidence for an inhibitory prejunctional P1-purinoceptor in the rat portal vein with characteristics of the A2 rather than of the A1 subtype. Eur J Pharmacol. 1984 May 4;100(3-4):363–368. doi: 10.1016/0014-2999(84)90014-1. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Makita Y. The presynaptic regulation of noradrenaline release differs in mesenteric arteries of the rabbit and guinea-pig. J Physiol. 1984 Jun;351:379–396. doi: 10.1113/jphysiol.1984.sp015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980 Dec;32(4):337–362. [PubMed] [Google Scholar]
- Levitt B., Westfall D. P. Factors influencing the release of purines and norepinephrine in the rabbit portal vein. Blood Vessels. 1982;19(1):30–40. doi: 10.1159/000158371. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchelli-Fortis M. A., Fredholm B. B., Langer S. Z. Release of radioactive purines from cat nictitating membrane labeled with 3H-adenine. Eur J Pharmacol. 1979 Oct 15;58(4):389–397. doi: 10.1016/0014-2999(79)90309-1. [DOI] [PubMed] [Google Scholar]
- Markstein R., Digges K., Marshall N. R., Starke K. Forskolin and the release of noradrenaline in cerebrocortical slices. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jan;325(1):17–24. doi: 10.1007/BF00507049. [DOI] [PubMed] [Google Scholar]
- Mathieson J. J., Burnstock G. Purine-mediated relaxation and constriction of isolated rabbit mesenteric artery are not endothelium-dependent. Eur J Pharmacol. 1985 Dec 3;118(3):221–229. doi: 10.1016/0014-2999(85)90132-3. [DOI] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Mishima S., Miyahara H., Suzuki H. Transmitter release modulated by alpha-adrenoceptor antagonists in the rabbit mesenteric artery: a comparison between noradrenaline outflow and electrical activity. Br J Pharmacol. 1984 Oct;83(2):537–547. doi: 10.1111/j.1476-5381.1984.tb16518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu I., Fujiwara M., Miura A., Sakakibara Y. Possible involvement of adenine nucleotides in sympathetic neuroeffector mechanisms of dog basilar artery. J Pharmacol Exp Ther. 1981 Feb;216(2):401–409. [PubMed] [Google Scholar]
- Nimit Y., Skolnick P., Daly J. W. Adenosine and cyclic AMP in rat cerebral cortical slices: effects of adenosine uptake inhibitors and adenosine deaminase inhibitors. J Neurochem. 1981 Mar;36(3):908–912. doi: 10.1111/j.1471-4159.1981.tb01680.x. [DOI] [PubMed] [Google Scholar]
- Paton D. M. Structure-activity relations for presynaptic inhibition of noradrenergic and cholinergic transmission by adenosine: evidence for action on A1 receptors. J Auton Pharmacol. 1981 Sep;1(4):287–290. doi: 10.1111/j.1474-8673.1981.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Ramme D., Illes P., Späth L., Starke K. Blockade of alpha 2-adrenoceptors permits the operation of otherwise silent opioid kappa-receptors at the sympathetic axons of rabbit jejunal arteries. Naunyn Schmiedebergs Arch Pharmacol. 1986 Sep;334(1):48–55. doi: 10.1007/BF00498739. [DOI] [PubMed] [Google Scholar]
- Ramme D., Regenold J. T., Starke K., Busse R., Illes P. Identification of the neuroeffector transmitter in jejunal branches of the rabbit mesenteric artery. Naunyn Schmiedebergs Arch Pharmacol. 1987 Sep;336(3):267–273. doi: 10.1007/BF00172677. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ukena D., Lohse M. J. Xanthine derivatives as antagonists at A1 and A2 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1985 Sep;330(3):212–221. doi: 10.1007/BF00572436. [DOI] [PubMed] [Google Scholar]
- Smellie F. W., Davis C. W., Daly J. W., Wells J. N. Alkylxanthines: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979 Jun 25;24(26):2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Purine receptors in the rat anococcygeus muscle. J Physiol. 1983 Feb;335:591–608. doi: 10.1113/jphysiol.1983.sp014553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. Extracellular functions of nucleotides in heart and blood vessels. Annu Rev Physiol. 1985;47:665–676. doi: 10.1146/annurev.ph.47.030185.003313. [DOI] [PubMed] [Google Scholar]
- Su C. Purinergic neurotransmission and neuromodulation. Annu Rev Pharmacol Toxicol. 1983;23:397–411. doi: 10.1146/annurev.pa.23.040183.002145. [DOI] [PubMed] [Google Scholar]
- Ukena D., Daly J. W., Kirk K. L., Jacobson K. A. Functionalized congeners of 1,3-dipropyl-8-phenylxanthine: potent antagonists for adenosine receptors that modulate membrane adenylate cyclase in pheochromocytoma cells, platelets and fat cells. Life Sci. 1986 Mar 3;38(9):797–807. doi: 10.1016/0024-3205(86)90596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall D. P., Stitzel R. E., Rowe J. N. The postjunctional effects and neural release of purine compounds in the guinea-pig vas deferens. Eur J Pharmacol. 1978 Jul 1;50(1):27–38. doi: 10.1016/0014-2999(78)90250-9. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


