Abstract
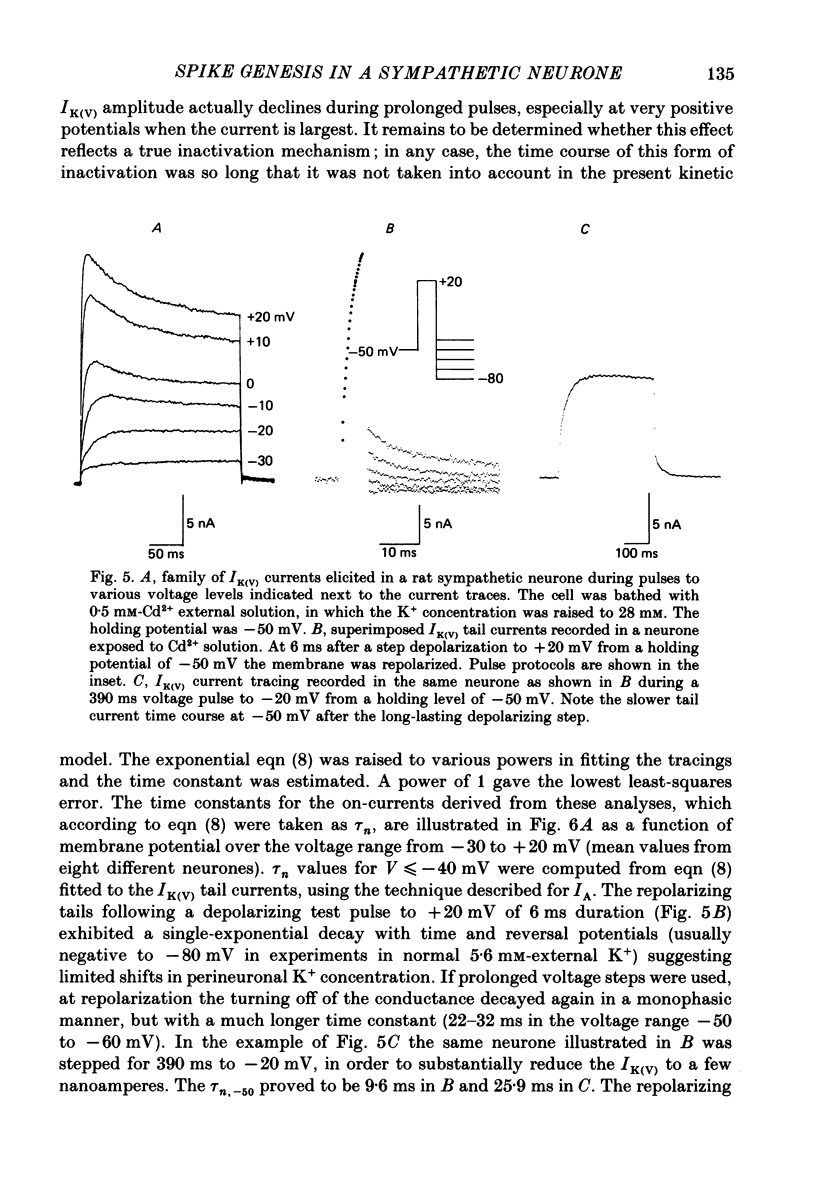
1. Membrane conductance parameters for the rat sympathetic neurone in vitro at 37 degrees C have been determined by two-electrode voltage-clamp analysis. The activation kinetics of two ionic currents, IA and IK(V), has been considered. Data for both currents are expressed in terms of Hodgkin-Huxley equations. 2. The isolated IA developed following third-order kinetics. The activation time constant, tau a, was estimated from the current time-to-peak and, for V less than or equal to -40 mV, from the IA tail current analysis upon membrane repolarization to various potentials. The maximum tau a occurred at -55 mV and varied from 0.26 to 0.82 ms in the range of potentials between -100 and +10 mV. The steady-state value of the variable a, corrected for inactivation, was evaluated in the voltage range from -60 to 0 mV; 14.4 mV are required to change a infinity e-fold. Steady-state gA was voltage dependent, increasing with depolarization to a maximum of 1.40 microS at +10 mV. 3. IK(V) was similarly analysed in isolation. The current proved to develop as a first-order process. tau n was determined by fitting a single exponential to the IK(V) rising phase and to the tail currents at the end of short depolarizing pulses. The bell-shaped voltage dependence of tau n exhibited a maximum (25.5 ms) at -30 mV, becoming minimal (1.8 ms) at -80 and +20 mV. The n infinity curve was obtained (n infinity = 0.5 at -6.54 mV; k = 8.91 mV). The mean maximum conductance, gK(V), was 0.33 microS per neurone at +10 mV. 4. Single spikes have been elicited by brief current pulses at membrane potentials from -40 to -100 mV under two-electrode current-clamp conditions in normal saline and in the presence of blockers of the ICa-IK(Ca) (Cd2+) and/or IK(V) (TEA, tetraethylammonium) systems. Spike repolarization was affected by the suppression of either current in the depolarized neurone, but was insensitive to both treatments when the spike arose from holding levels negative to -75 to -80 mV, indicating that at these membrane potentials the IA current mainly, if not exclusively, contributes to the action potential falling phase. 5. The basic features of the sympathetic neurone action potential were reconstructed by simulations based on present and previous voltage-clamp characterization of the IA, IK(V) and INa conductances.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belluzzi O., Sacchi O. A quantitative description of the sodium current in the rat sympathetic neurone. J Physiol. 1986 Nov;380:275–291. doi: 10.1113/jphysiol.1986.sp016285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. Identification of delayed potassium and calcium currents in the rat sympathetic neurone under voltage clamp. J Physiol. 1985 Jan;358:109–129. doi: 10.1113/jphysiol.1985.sp015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Chiu S. Y., Gray P. T., Ritchie J. M. The presence of voltage-gated sodium, potassium and chloride channels in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):299–313. doi: 10.1098/rspb.1985.0063. [DOI] [PubMed] [Google Scholar]
- Byrne J. H. Quantitative aspects of ionic conductance mechanisms contributing to firing pattern of motor cells mediating inking behavior in Aplysia californica. J Neurophysiol. 1980 Mar;43(3):651–668. doi: 10.1152/jn.1980.43.3.651. [DOI] [PubMed] [Google Scholar]
- Cassell J. F., McLachlan E. M. The effect of a transient outward current (IA) on synaptic potentials in sympathetic ganglion cells of the guinea-pig. J Physiol. 1986 May;374:273–288. doi: 10.1113/jphysiol.1986.sp016079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R. Potassium ion accumulation slows the closing rate of potassium channels in squid axons. Biophys J. 1986 Jul;50(1):197–200. doi: 10.1016/S0006-3495(86)83452-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971 Feb;213(1):31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Shrier A. Single-channel analysis of fast transient potassium currents from rat nodose neurones. J Physiol. 1985 Dec;369:199–208. doi: 10.1113/jphysiol.1985.sp015896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J. Modulation of the excitatory synaptic response by fast transient K+ current in snail neurones. Nat New Biol. 1973 Dec 19;246(155):193–196. doi: 10.1038/newbio246193a0. [DOI] [PubMed] [Google Scholar]
- Freschi J. E. Membrane currents of cultured rat sympathetic neurons under voltage clamp. J Neurophysiol. 1983 Dec;50(6):1460–1478. doi: 10.1152/jn.1983.50.6.1460. [DOI] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The effect of external potassium on the removal of sodium inactivation in squid giant axons. J Physiol. 1981 Jun;315:493–514. doi: 10.1113/jphysiol.1981.sp013760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Kameyama M., Yamaguchi K., Fukuda J. Single transient K channels in mammalian sensory neurons. Biophys J. 1986 Jun;49(6):1243–1247. doi: 10.1016/S0006-3495(86)83754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Kimura J. E. Kinetics of activation of the sodium conductance in the squid giant axon. J Physiol. 1983 Mar;336:621–634. doi: 10.1113/jphysiol.1983.sp014601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Ramon F. On numerical integration of the Hodgkin and Huxley equations for a membrane action potential. J Theor Biol. 1974 May;45(1):249–273. doi: 10.1016/0022-5193(74)90054-x. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Rogawski M. A., Barker J. L. A transient potassium conductance regulates the excitability of cultured hippocampal and spinal neurons. J Neurosci. 1984 Feb;4(2):604–609. doi: 10.1523/JNEUROSCI.04-02-00604.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P., Chiu S. Y., Ritchie J. M. Voltage-dependent sodium and potassium channels in mammalian cultured Schwann cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):948–952. doi: 10.1073/pnas.82.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Jr, Armstrong C. M. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 1981 Jun 4;291(5814):427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]


