Abstract
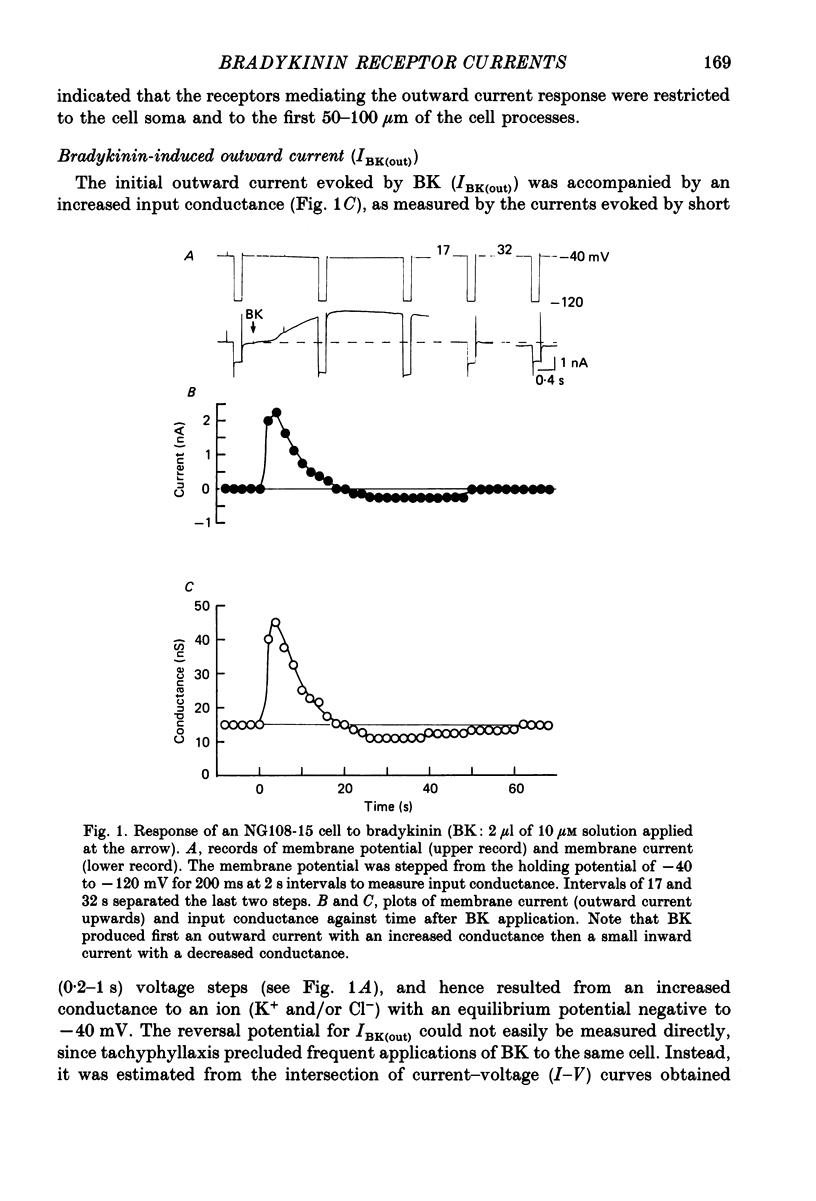
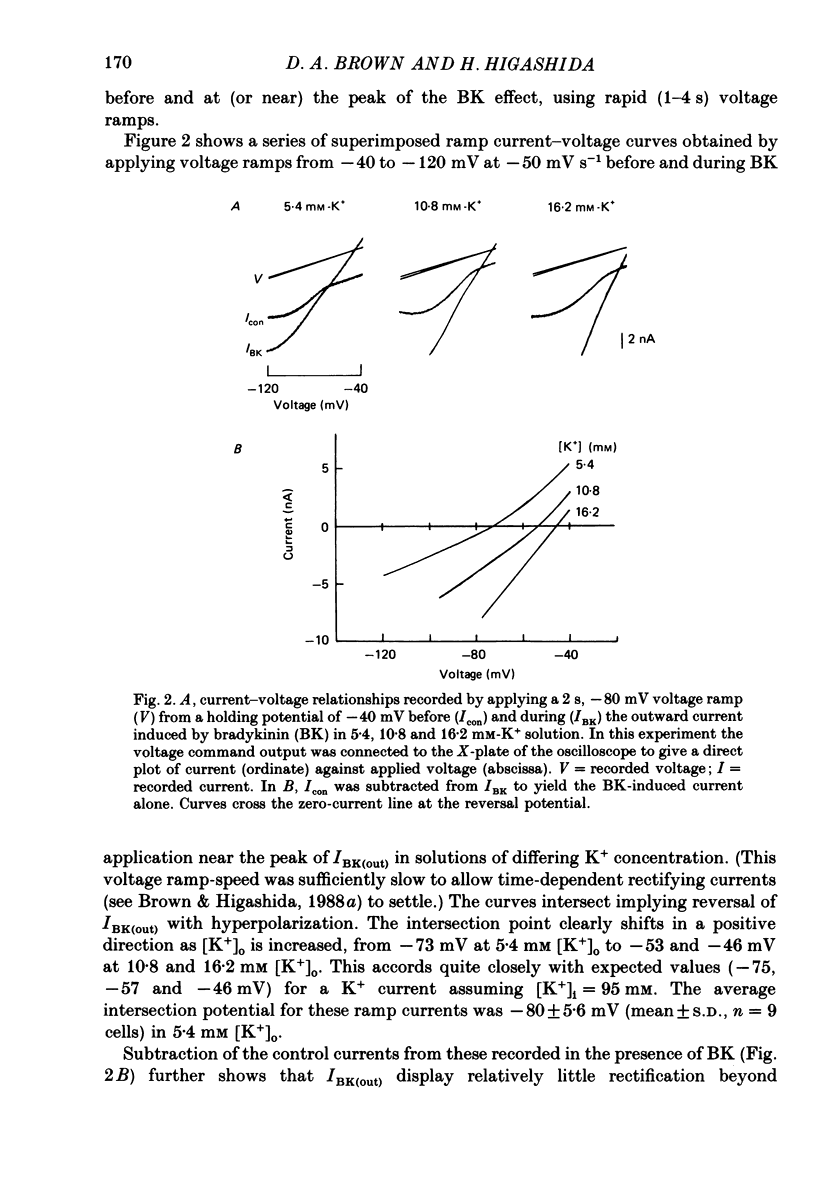
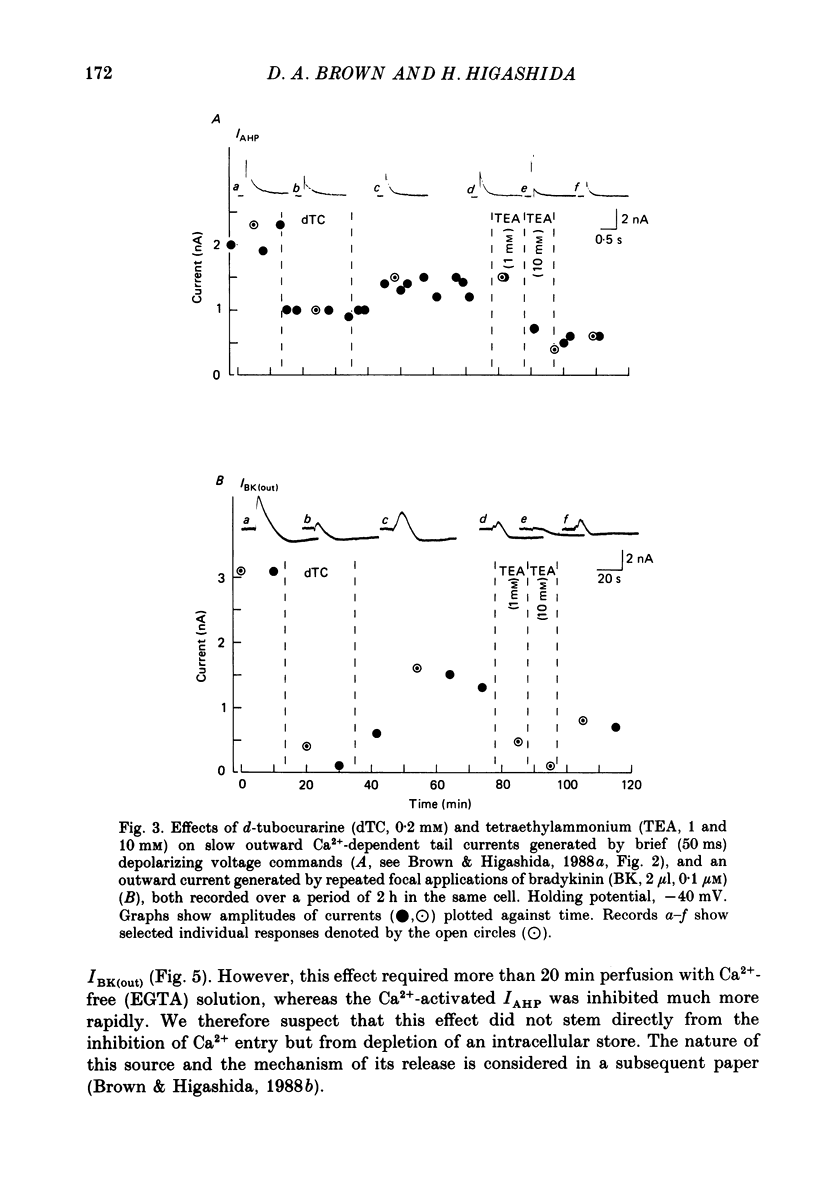
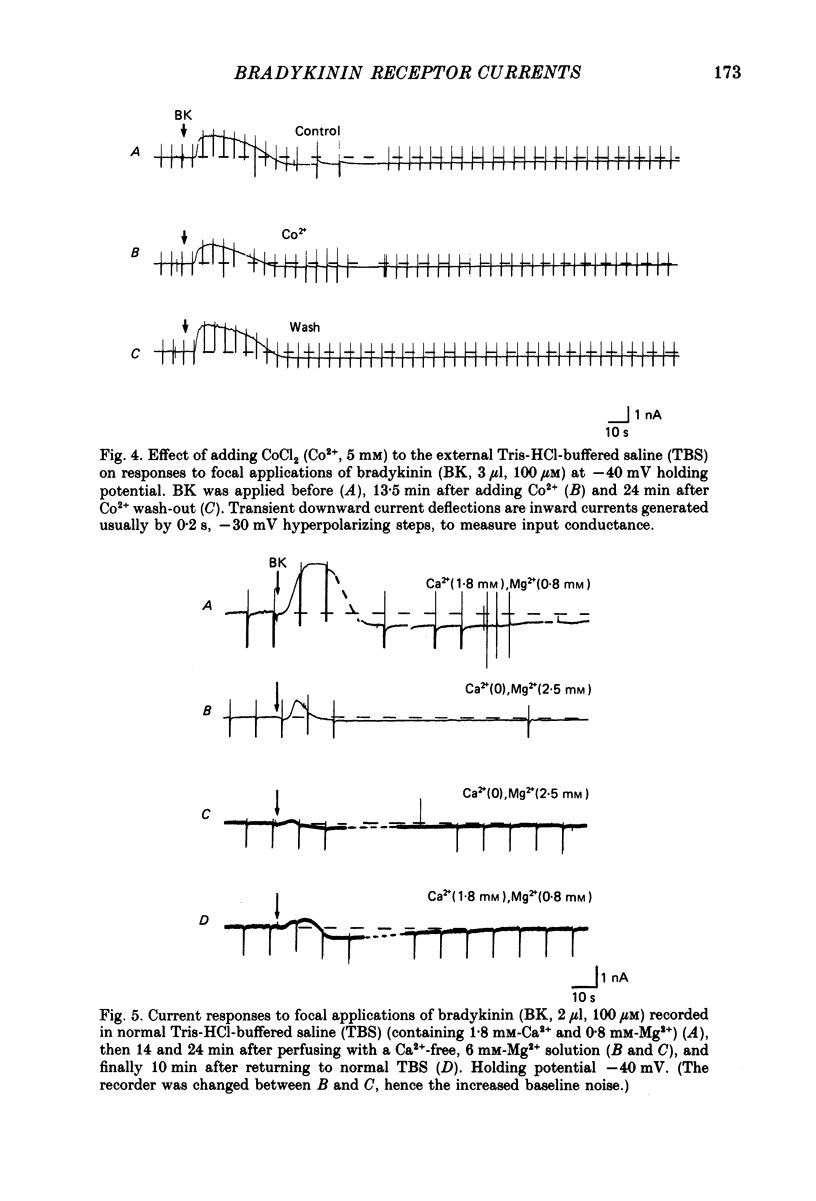
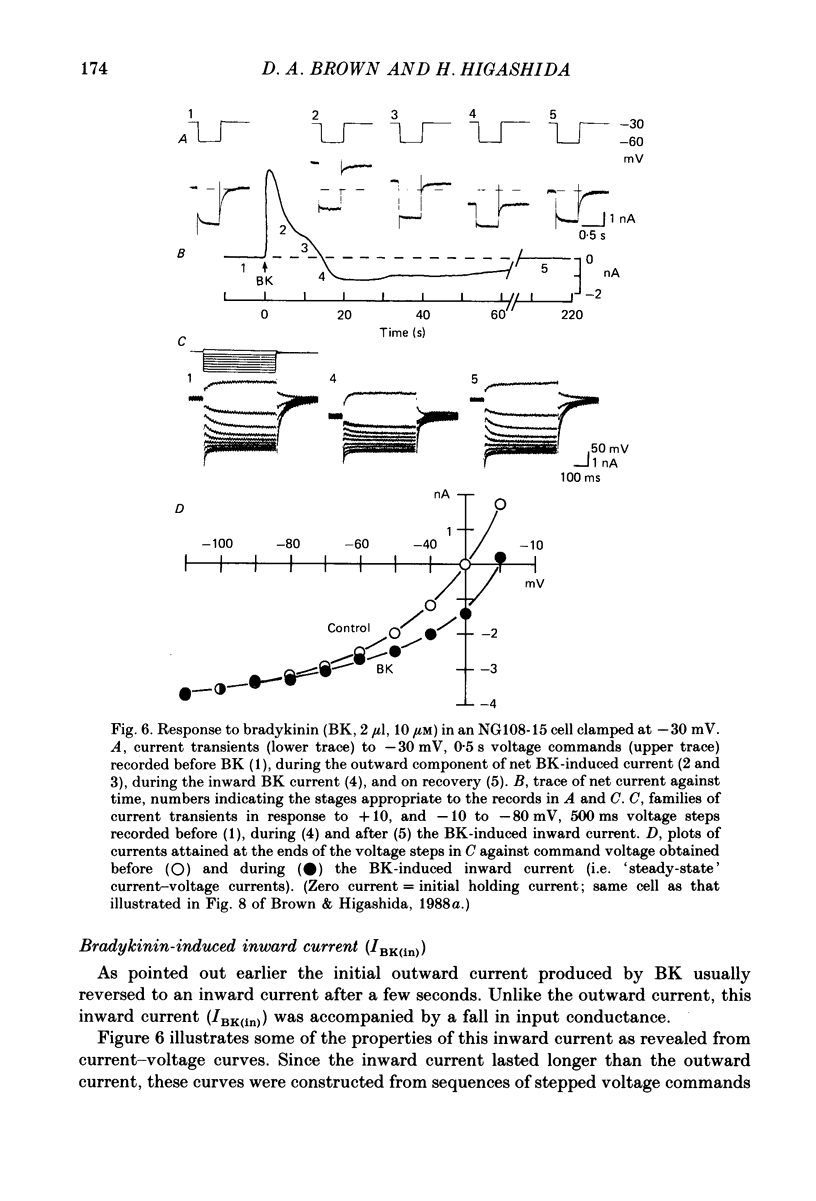
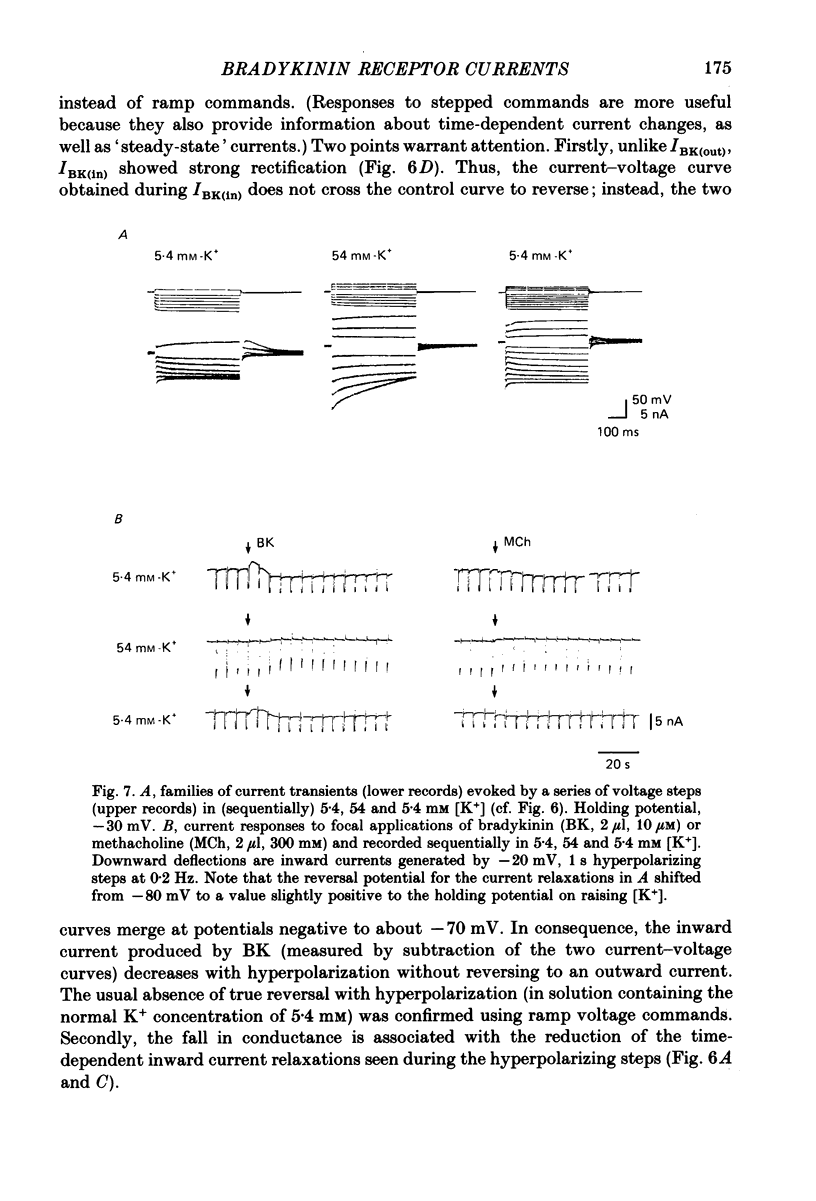
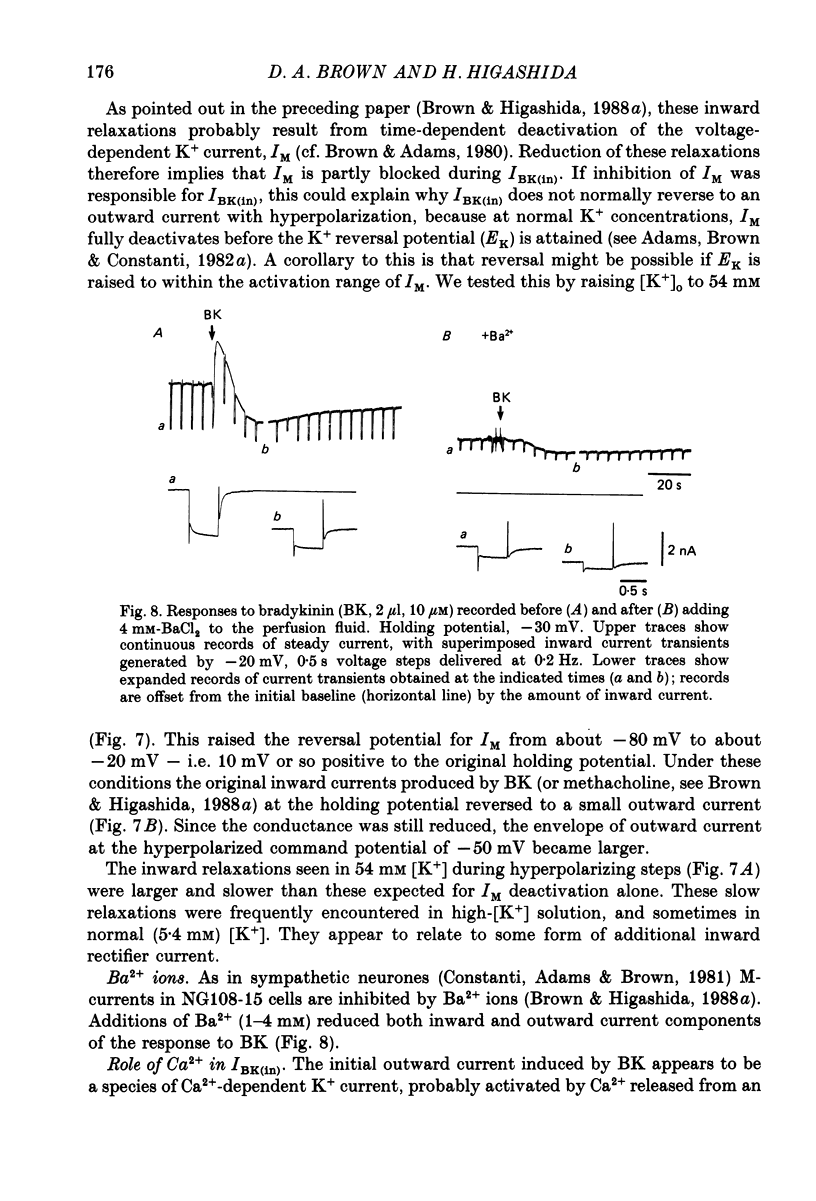
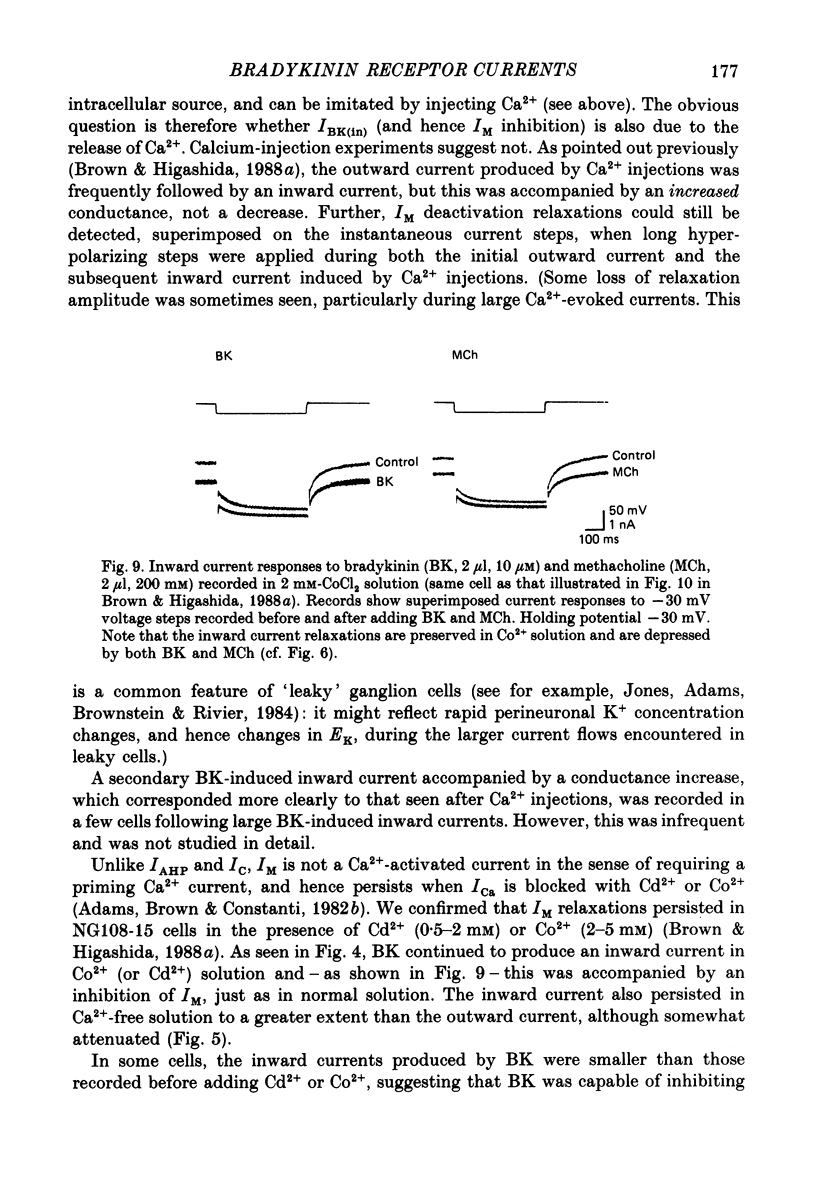
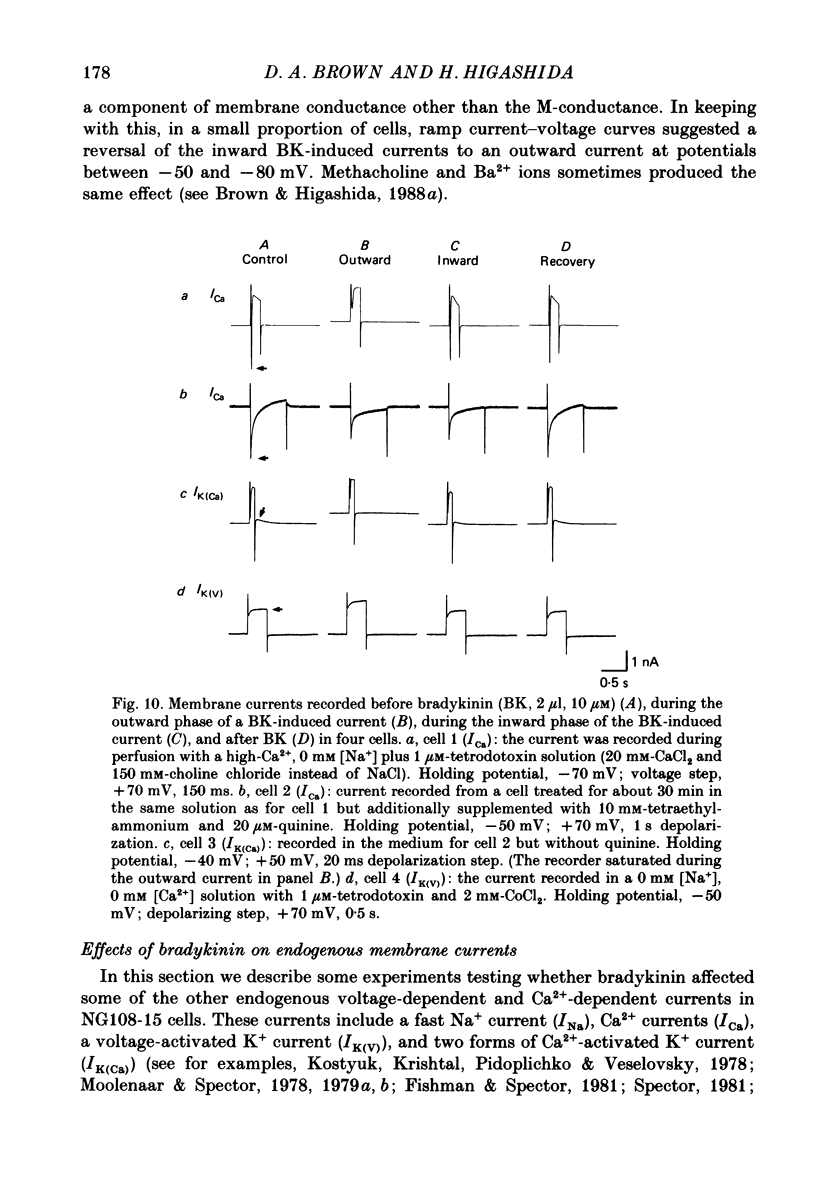
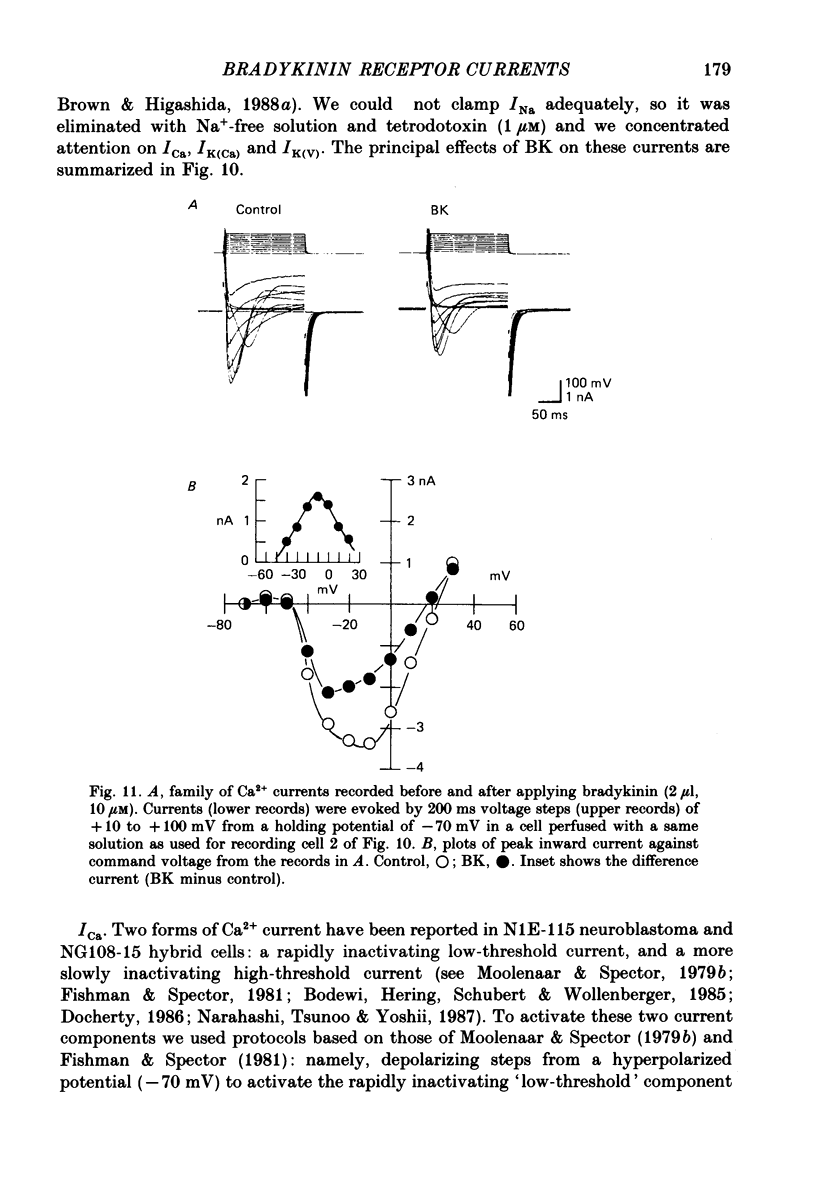
1. Membrane current responses to focal application of bradykinin (BK) were recorded in voltage-clamped NG108-15 neuroblastoma x glioma hybrid cells. 2. BK produced sequential outward and inward currents at clamp potentials between -60 and -30 mV, designated IBK(out) and IBK(in), respectively. 3. The outward current IBK(out) was accompanied by an increased membrane conductance. Ramp current-voltage (I-V) curves yielded a reversal potential (VBK) of -80 +/- 5.6 mV (mean +/- S.D., n = 9) in 5.4 mM [K+]o. VBK showed a positive shift on raising [K+]o, compatible with a primary increase in K+ conductance. Subtracted I-V curves indicated that the underlying conductance was not strongly voltage dependent between -120 and -40 mV. 4. IBK(out) was inhibited by d-tubocurarine (dTC, 0.1-0.5 mM) but was insensitive to tetraethylammonium (TEA) below 5 mM. 5. The inward current IBK(in) was accompanied by a fall in membrane conductance. This was associated with the inhibition of a time- and voltage-dependent K+ current, IM. In consequence, IBK(in) was strongly voltage dependent and dissipated, usually without reversal, on hyperpolarizing the cell beyond -70 mV in 5.4 mM [K+]o. Reversal to an outward current negative to -40 mV could be obtained on raising [K+]o to 54 mM. 5. Both IBK(in) and IBK(out) persisted when ICa was blocked with Co2+ or Cd2+. IBK(out) slowly diminished in Ca2+-free, Mg2+-substituted solution. 6. The Ca2+ spike current ICa and the Ca2+-activated K+ current IAHP were inhibited during IBK(out) or after Ca2+ injections. BK did not affect the voltage-activated K+ current IK(V) recorded in Co2+ solution. 7. It is concluded that the dual response to BK results from opposing effects on two different species of K+ current. IBK(out) results from activation of a Ca2+-dependent, voltage-insensitive K+ conductance, probably mediated by a transient rise in intracellular Ca2+. It is suggested that the Ca2+ is released from an intracellular store. IBK(in) results primarily from inhibition of the Ca2+-independent, voltage-gated K+ current, IM. This effect is not replicated by a rise of intracellular Ca2+ and must therefore be generated by another mechanism.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Bodewei R., Hering S., Schubert B., Wollenberger A. Sodium and calcium currents in neuroblastoma x glioma hybrid cells before and after morphological differentiation by dibutyryl cyclic AMP. Gen Physiol Biophys. 1985 Apr;4(2):113–127. [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A., Adams P. R. Ca-activated potassium current in vertebrate sympathetic neurons. Cell Calcium. 1983 Dec;4(5-6):407–420. doi: 10.1016/0143-4160(83)90017-9. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Inositol 1,4,5-trisphosphate and diacylglycerol mimic bradykinin effects on mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:185–207. doi: 10.1113/jphysiol.1988.sp016995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Voltage- and calcium-activated potassium currents in mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:149–165. doi: 10.1113/jphysiol.1988.sp016993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Selyanko A. A. Two components of muscarine-sensitive membrane current in rat sympathetic neurones. J Physiol. 1985 Jan;358:335–363. doi: 10.1113/jphysiol.1985.sp015554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Adams P. R., Brown D. A. Who do barium ions imitate acetylcholine? Brain Res. 1981 Feb 9;206(1):244–250. doi: 10.1016/0006-8993(81)90125-6. [DOI] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Fishman M. C., Spector I. Potassium current suppression by quinidine reveals additional calcium currents in neuroblastoma cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5245–5249. doi: 10.1073/pnas.78.8.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman S. B., Dawson G., Villereal M. L., Miller R. J. Identification and characterization of voltage-sensitive calcium channels in neuronal clonal cell lines. J Neurosci. 1984 Jun;4(6):1453–1467. doi: 10.1523/JNEUROSCI.04-06-01453.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya K., Furuya S., Yamagishi S. Developmental time courses of Na and Ca spikes in neuroblastoma X glioma hybrid cells. Brain Res. 1983 Dec;313(2):229–234. doi: 10.1016/0165-3806(83)90220-1. [DOI] [PubMed] [Google Scholar]
- Gater P. R., Haylett D. G., Jenkinson D. H. Neuromuscular blocking agents inhibit receptor-mediated increases in the potassium permeability of intestinal smooth muscle. Br J Pharmacol. 1985 Dec;86(4):861–868. doi: 10.1111/j.1476-5381.1985.tb11108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamprecht B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Int Rev Cytol. 1977;49:99–170. doi: 10.1016/s0074-7696(08)61948-8. [DOI] [PubMed] [Google Scholar]
- Higashida H., Brown D. A. Two polyphosphatidylinositide metabolites control two K+ currents in a neuronal cell. 1986 Sep 25-Oct 1Nature. 323(6086):333–335. doi: 10.1038/323333a0. [DOI] [PubMed] [Google Scholar]
- Higashida H., Streaty R. A., Klee W., Nirenberg M. Bradykinin-activated transmembrane signals are coupled via No or Ni to production of inositol 1,4,5-trisphosphate, a second messenger in NG108-15 neuroblastoma-glioma hybrid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):942–946. doi: 10.1073/pnas.83.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Adams P. R., Brownstein M. J., Rivier J. E. Teleost luteinizing hormone-releasing hormone: action on bullfrog sympathetic ganglia is consistent with role as neurotransmitter. J Neurosci. 1984 Feb;4(2):420–429. doi: 10.1523/JNEUROSCI.04-02-00420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I., Veselovsky N. S. Ionic currents in the neuroblastoma cell membrane. Neuroscience. 1978;3(3):327–332. doi: 10.1016/0306-4522(78)90080-5. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Ionic currents in cultured mouse neuroblastoma cells under voltage-clamp conditions. J Physiol. 1978 May;278:265–286. doi: 10.1113/jphysiol.1978.sp012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. The calcium action potential and a prolonged calcium dependent after-hyperpolarization in mouse neuroblastoma cells. J Physiol. 1979 Jul;292:297–306. doi: 10.1113/jphysiol.1979.sp012851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. The calcium current and the activation of a slow potassium conductance in voltage-clamped mouse neuroblastoma cells. J Physiol. 1979 Jul;292:307–323. doi: 10.1113/jphysiol.1979.sp012852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg M., Wilson S., Higashida H., Rotter A., Krueger K., Busis N., Ray R., Kenimer J. G., Adler M. Modulation of synapse formation by cyclic adenosine monophosphate. Science. 1983 Nov 18;222(4625):794–799. doi: 10.1126/science.6314503. [DOI] [PubMed] [Google Scholar]
- Osugi T., Uchida S., Imaizumi T., Yoshida H. Bradykinin-induced intracellular Ca2+ elevation in neuroblastoma X glioma hybrid NG108-15 cells; relationship to the action of inositol phospholipids metabolites. Brain Res. 1986 Jul 30;379(1):84–89. doi: 10.1016/0006-8993(86)90258-1. [DOI] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt F. N., Narahashi T. Isolation and kinetic analysis of inward currents in neuroblastoma cells. Neuroscience. 1984 Sep;13(1):249–262. doi: 10.1016/0306-4522(84)90275-6. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J., Park W. K. Receptors for bradykinin in rabbit aortae. Can J Physiol Pharmacol. 1977 Aug;55(4):855–867. doi: 10.1139/y77-115. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Reiser G., Hamprecht B. Bradykinin causes a transient rise of intracellular Ca2+-activity in cultured neural cells. Pflugers Arch. 1985 Oct;405(3):260–264. doi: 10.1007/BF00582570. [DOI] [PubMed] [Google Scholar]
- Reiser G., Hamprecht B. Bradykinin induces hyperpolarizations in rat glioma cells and in neuroblastoma X glioma hybrid cells. Brain Res. 1982 May 6;239(1):191–199. doi: 10.1016/0006-8993(82)90841-1. [DOI] [PubMed] [Google Scholar]
- Yano K., Higashida H., Inoue R., Nozawa Y. Bradykinin-induced rapid breakdown of phosphatidylinositol 4,5-bisphosphate in neuroblastoma X glioma hybrid NG108-15 cells. J Biol Chem. 1984 Aug 25;259(16):10201–10207. [PubMed] [Google Scholar]


