Abstract
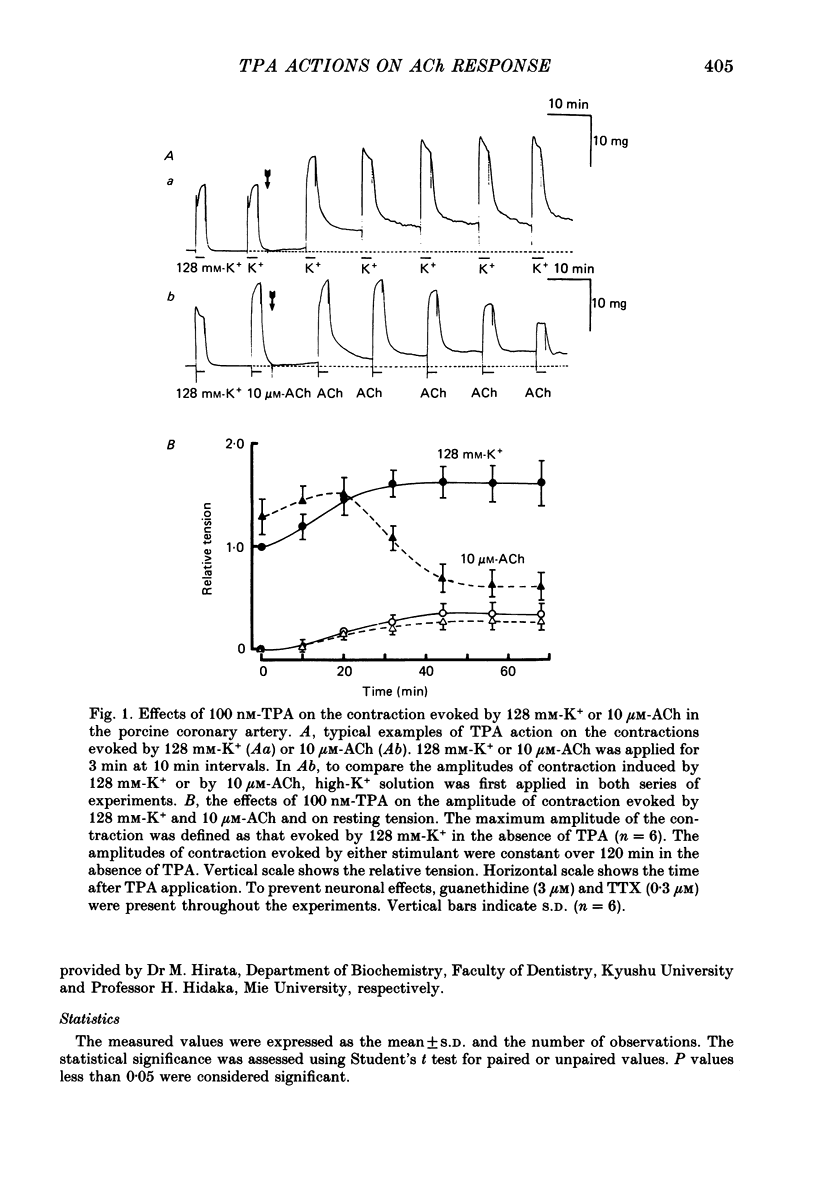
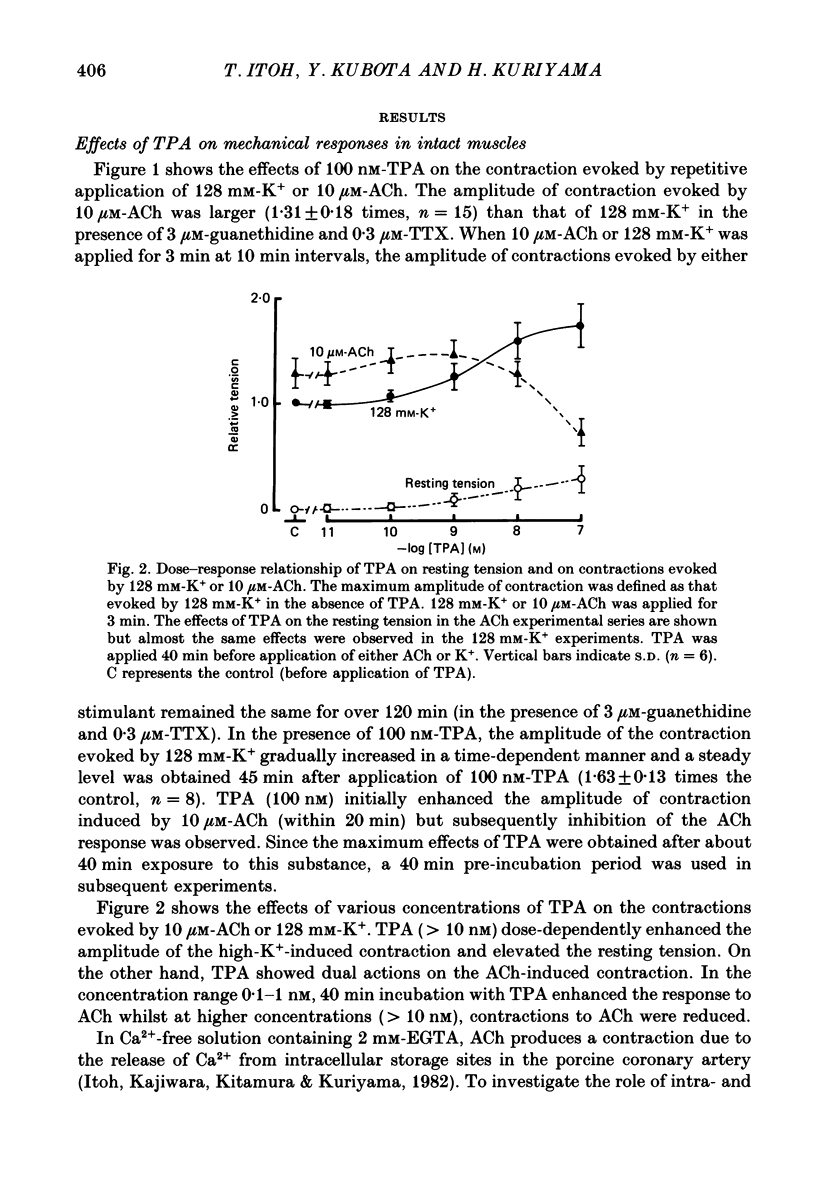
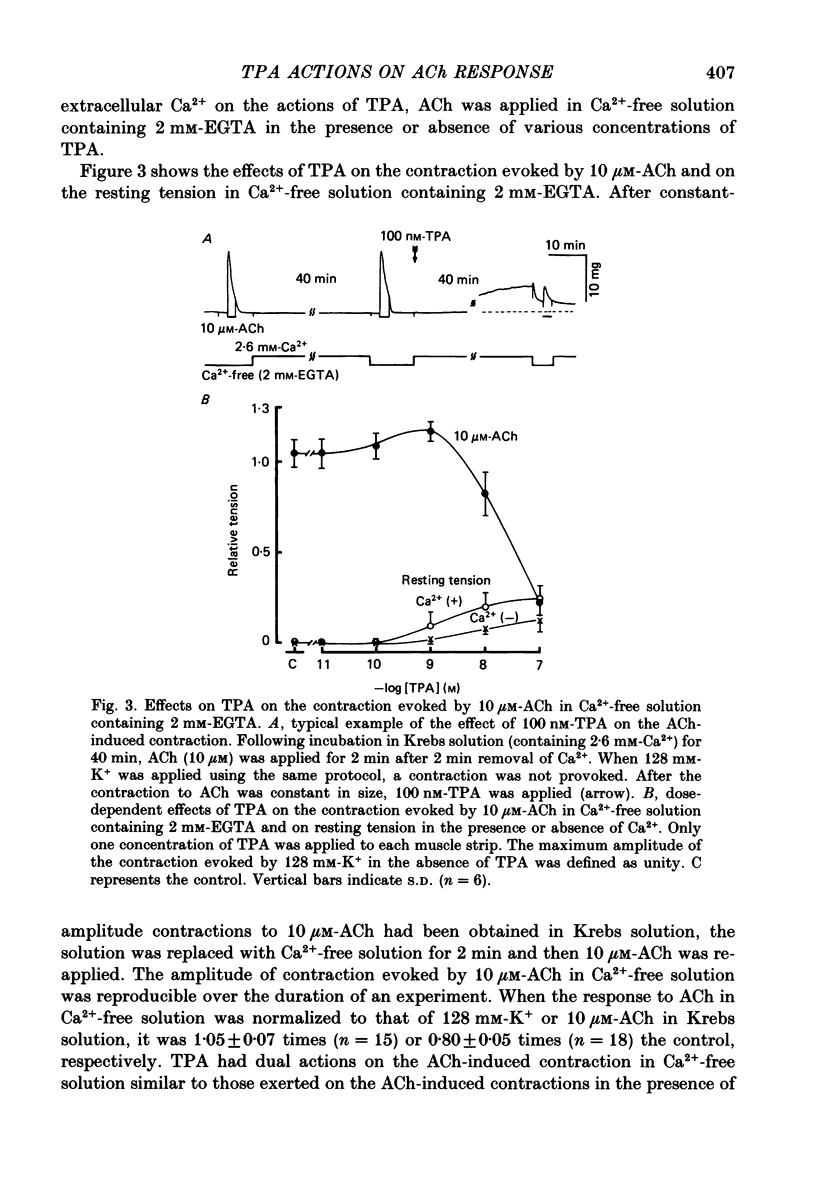
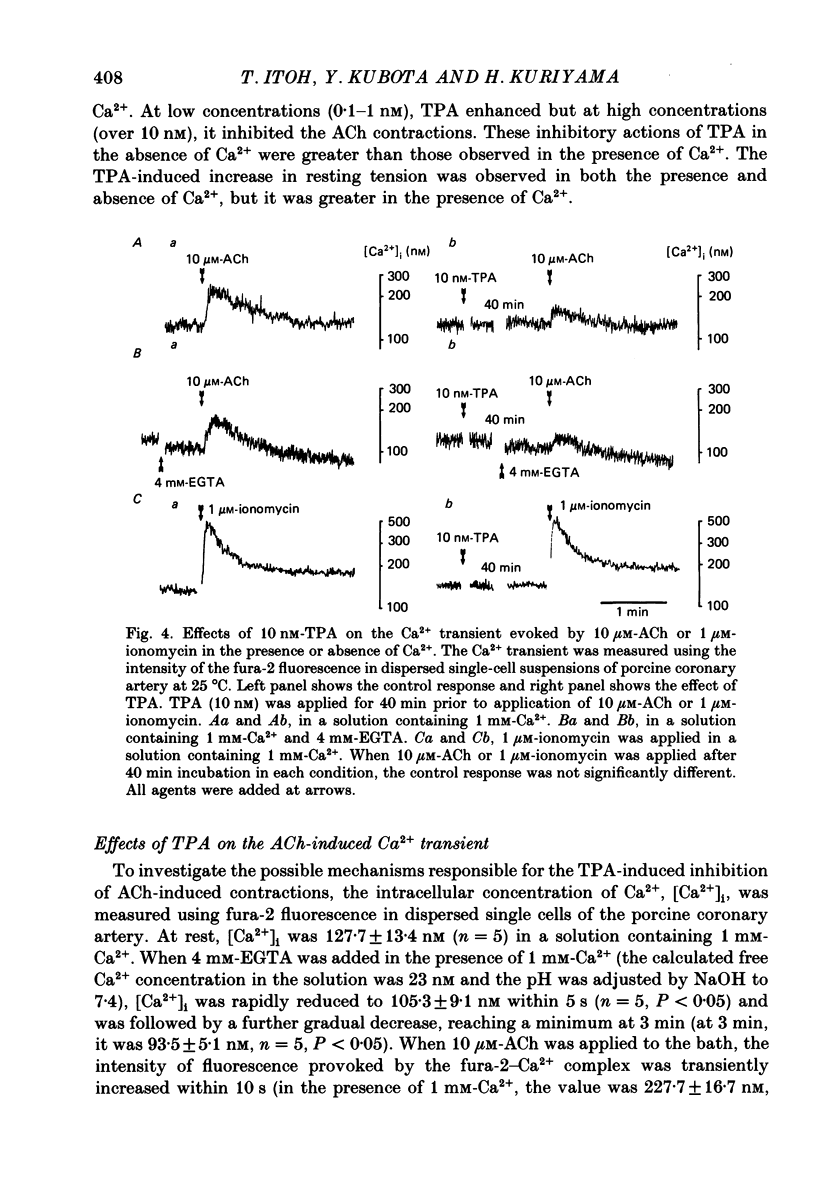
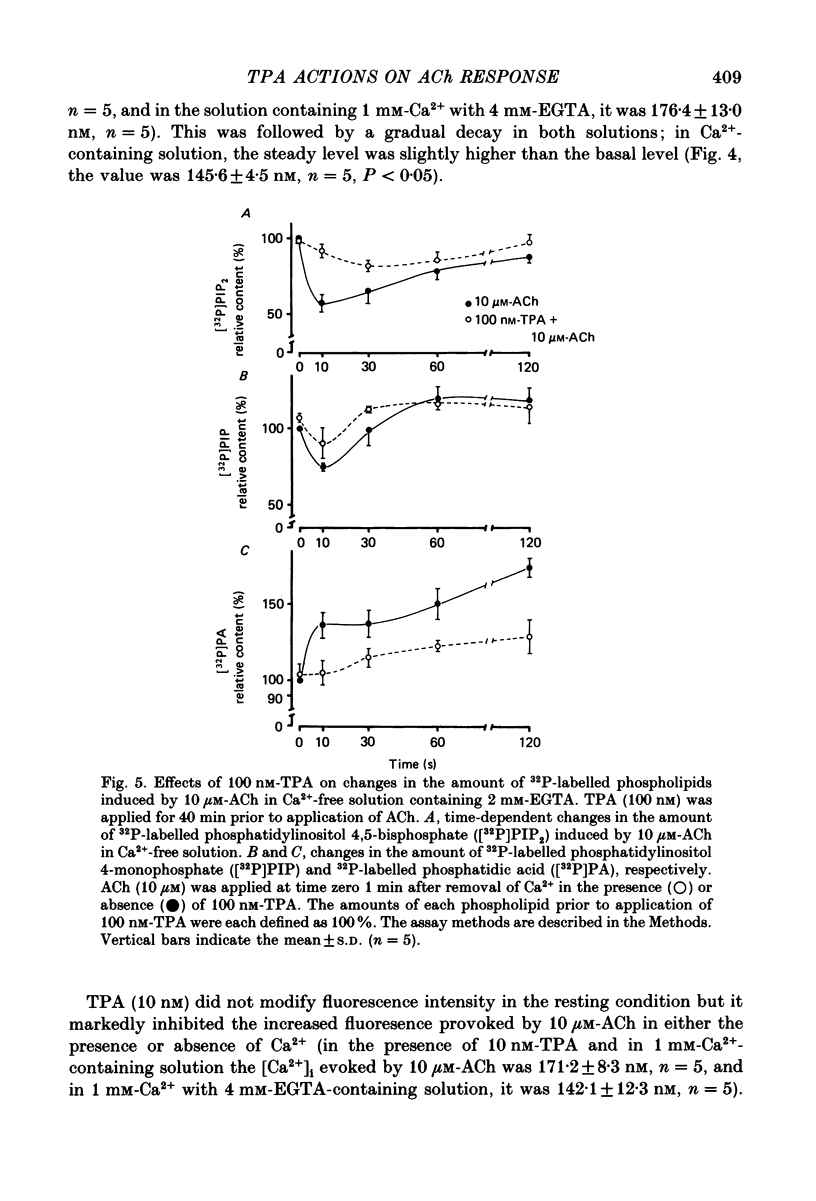
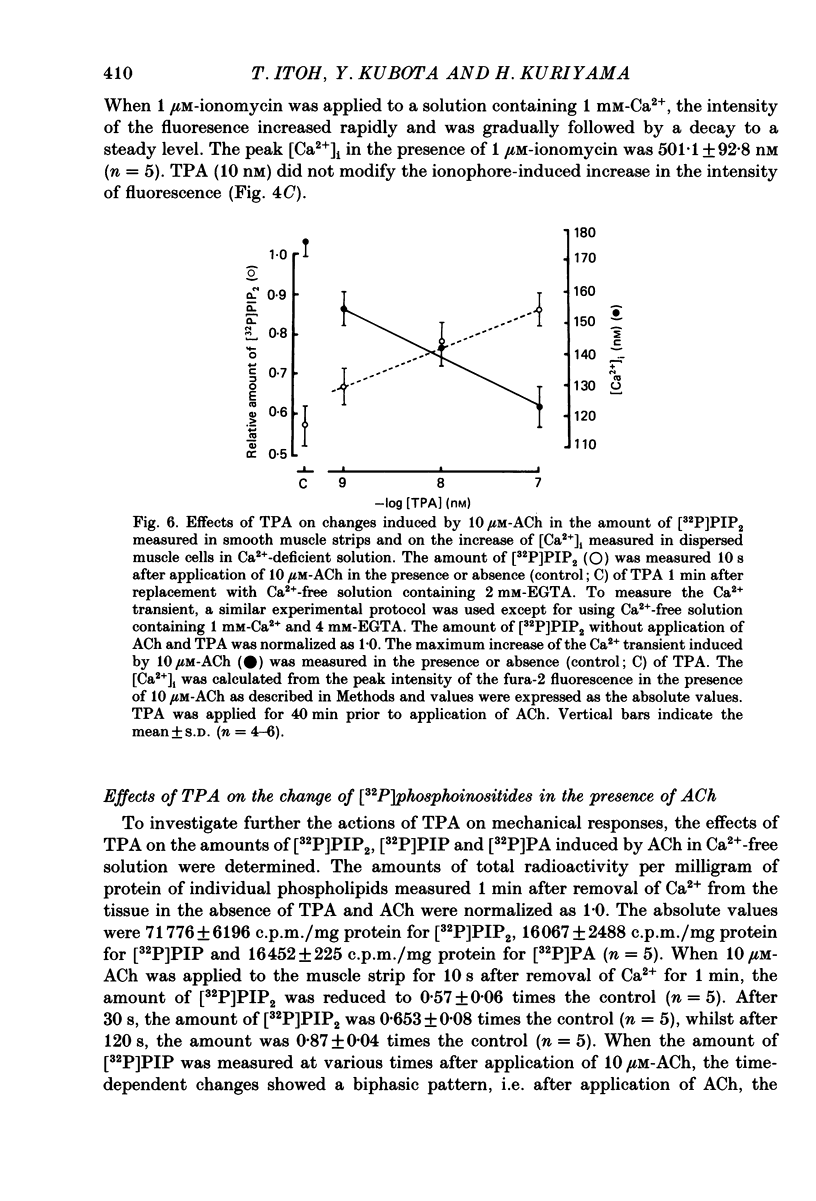
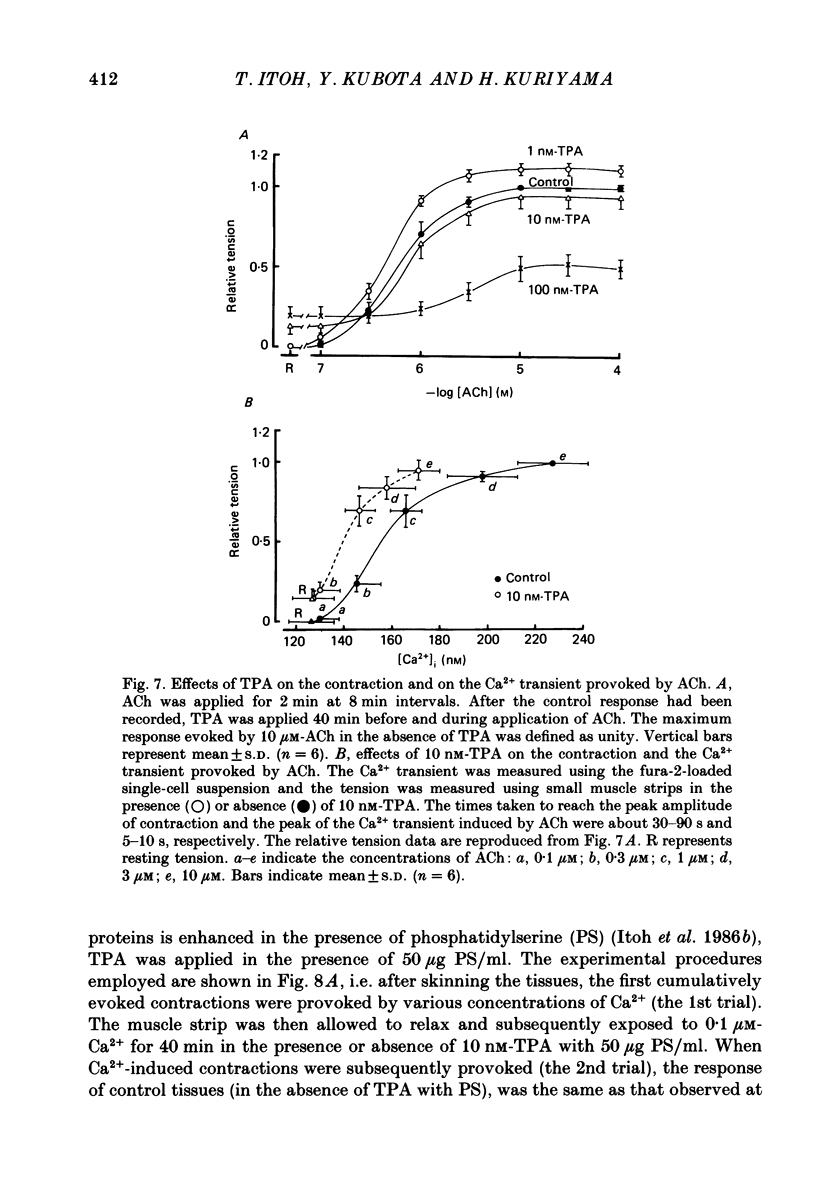
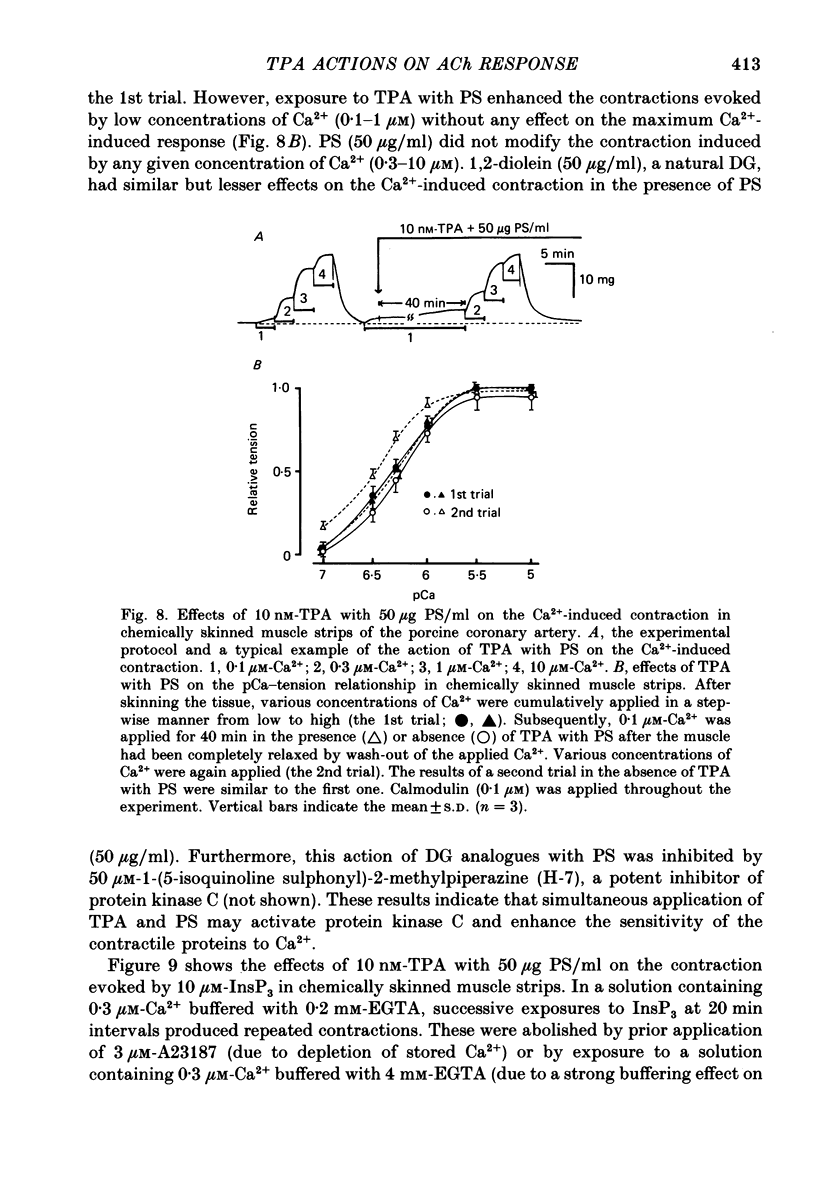
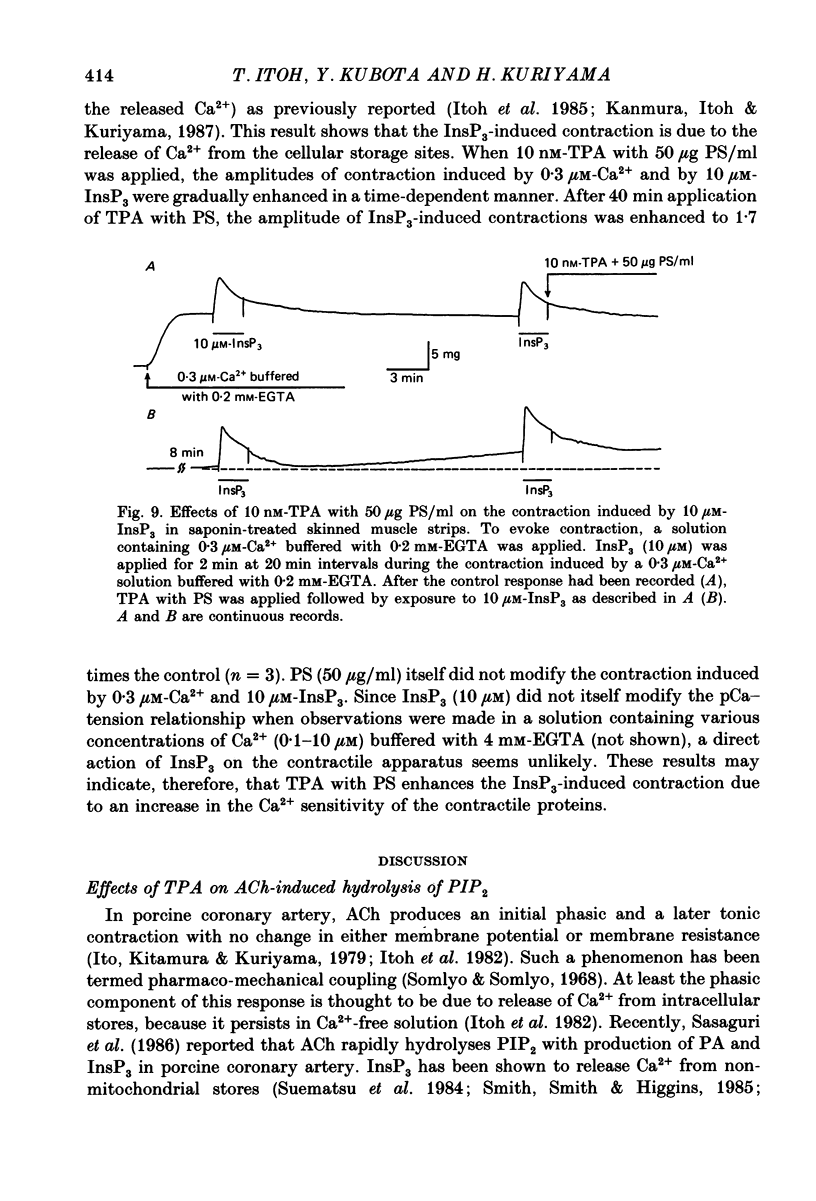
1. The effects of 12-O-tetradecanoylphorbol-13-acetate (TPA), an activator of protein kinase C, have been investigated on intact and chemically skinned muscle strips of the porcine coronary artery. 2. In the presence or absence of extracellular Ca2+, TPA (0.1-1 nM) slightly enhanced the amplitude of ACh (10 microM)-induced contractions but at 100 nM, inhibited the contractions by approximately 50%. 3. ACh (10 microM) reduced the amount of [32P]phosphatidylinositol 4,5-bisphosphate (PIP2) and increased the amount of [32P]phosphatidic acid (PA) in the presence or absence of Ca2+. TPA (over 1 nM) dose-dependently inhibited the hydrolysis of PIP2 induced by ACh. 4. ACh (over 0.1 microM) dose-dependently increased the intensity of fura-2 fluorescence in dispersed single-cell suspensions. TPA (over 1 nM) dose-dependently inhibited the increase of the Ca2+ transient evoked by ACh, but it did not modify the ionomycin-induced Ca2+ transient or the resting fluorescence. These inhibitory effects of TPA occurred over a similar dose range to that which inhibited ACh-induced PIP2 break-down. 5. When the relationship between ACh-induced contraction amplitude and Ca2+ transient was observed in the presence or absence of 10 nM-TPA, TPA greatly reduced the Ca2+ transient but did not modify the amplitude of contraction. 6. In saponin-treated skinned muscle strips, TPA (10 nM) or 1,2-diolein (50 micrograms/ml) with phosphatidylserine (PS; 50 micrograms/ml) increased the amplitude of contraction evoked by various concentrations of Ca2+ (0.1-1.0 microM) without any change in the maximum amplitude of the Ca2+-induced contraction. 7. TPA (10 nM) with PS (50 micrograms/ml) increased the amplitude of contraction evoked by 10 microM-inositol 1,4,5-trisphosphate in chemically skinned muscle strips. 8. It is concluded that TPA inhibits the ACh-induced [Ca2+]i increase by inhibiting the hydrolysis of PIP2, but that it enhances the Ca2+ sensitivity of the contractile proteins. These results indicate that ACh-induced contractions are controlled by negative feed-back regulation of PIP2 hydrolysis together with a positive feed-back regulation of the Ca2+ sensitivity of the contractile proteins. This may depend on the on-going level of protein kinase C activation.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Baraban J. M., Gould R. J., Peroutka S. J., Snyder S. H. Phorbol ester effects on neurotransmission: interaction with neurotransmitters and calcium in smooth muscle. Proc Natl Acad Sci U S A. 1985 Jan;82(2):604–607. doi: 10.1073/pnas.82.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., May W. S., Jr, LeVine H., 3rd, Cragoe E. J., Jr, Cuatrecasas P. Amiloride inhibits phorbol ester-stimulated Na+/H+ exchange and protein kinase C. An amiloride analog selectively inhibits Na+/H+ exchange. J Biol Chem. 1985 Jan 25;260(2):1155–1159. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- Brock T. A., Rittenhouse S. E., Powers C. W., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Phorbol ester and 1-oleoyl-2-acetylglycerol inhibit angiotensin activation of phospholipase C in cultured vascular smooth muscle cells. J Biol Chem. 1985 Nov 15;260(26):14158–14162. [PubMed] [Google Scholar]
- Chatterjee M., Tejada M. Phorbol ester-induced contraction in chemically skinned vascular smooth muscle. Am J Physiol. 1986 Sep;251(3 Pt 1):C356–C361. doi: 10.1152/ajpcell.1986.251.3.C356. [DOI] [PubMed] [Google Scholar]
- Colucci W. S., Gimbrone M. A., Jr, Alexander R. W. Phorbol diester modulates alpha-adrenergic receptor-coupled calcium efflux and alpha-adrenergic receptor number in cultured vascular smooth muscle cells. Circ Res. 1986 Mar;58(3):393–398. doi: 10.1161/01.res.58.3.393. [DOI] [PubMed] [Google Scholar]
- Danthuluri N. R., Deth R. C. Phorbol ester-induced contraction of arterial smooth muscle and inhibition of alpha-adrenergic response. Biochem Biophys Res Commun. 1984 Dec 28;125(3):1103–1109. doi: 10.1016/0006-291x(84)91397-4. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Halenda S. P., Feinstein M. B. Phorbol myristate acetate stimulates formation of phosphatidyl inositol 4-phosphate and phosphatidyl inositol 4,5-bisphosphate in human platelets. Biochem Biophys Res Commun. 1984 Oct 30;124(2):507–513. doi: 10.1016/0006-291x(84)91583-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M., Kawamoto S., Hidaka H. Serotonin secretion from human platelets may be modified by Ca2+-activated, phospholipid-dependent myosin phosphorylation. J Biol Chem. 1984 Dec 10;259(23):14321–14323. [PubMed] [Google Scholar]
- Ito Y., Kitamura K., Kuriyama H. Effects of acetylcholine and catecholamines on the smooth muscle cell of the porcine coronary artery. J Physiol. 1979 Sep;294:595–611. doi: 10.1113/jphysiol.1979.sp012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. A23187 increases calcium permeability of store sites more than of surface membranes in the rabbit mesenteric artery. J Physiol. 1985 Feb;359:467–484. doi: 10.1113/jphysiol.1985.sp015597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. Inorganic phosphate regulates the contraction-relaxation cycle in skinned muscles of the rabbit mesenteric artery. J Physiol. 1986 Jul;376:231–252. doi: 10.1113/jphysiol.1986.sp016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H., Sumimoto K. A phorbol ester has dual actions on the mechanical response in the rabbit mesenteric and porcine coronary arteries. J Physiol. 1986 Jun;375:515–534. doi: 10.1113/jphysiol.1986.sp016131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmura Y., Itoh T., Kuriyama H. Mechanisms of vasoconstriction induced by 9,11-epithio-11,12-methano-thromboxane A2 in the rabbit coronary artery. Circ Res. 1987 Mar;60(3):402–409. doi: 10.1161/01.res.60.3.402. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan M., Chernow B., Roth B. L. Phorbol esters inhibit alpha 1-adrenergic receptor-stimulated phosphoinositide hydrolysis and contraction in rat aorta: evidence for a link between vascular contraction and phosphoinositide turnover. Biochem Biophys Res Commun. 1986 Jan 29;134(2):970–974. doi: 10.1016/s0006-291x(86)80515-0. [DOI] [PubMed] [Google Scholar]
- Miller J. R., Hawkins D. J., Wells J. N. Phorbol diesters alter the contractile responses of porcine coronary artery. J Pharmacol Exp Ther. 1986 Oct;239(1):38–42. [PubMed] [Google Scholar]
- Minakuchi R., Takai Y., Yu B., Nishizuka Y. Widespread occurrence of calcium-activated, phospholipid-dependent protein kinase in mammalian tissues. J Biochem. 1981 May;89(5):1651–1654. doi: 10.1093/oxfordjournals.jbchem.a133362. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Phorbol ester and diacylglycerol mimic growth factors in raising cytoplasmic pH. Nature. 1984 Nov 22;312(5992):371–374. doi: 10.1038/312371a0. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabika T., Velletri P. A., Lovenberg W., Beaven M. A. Increase in cytosolic calcium and phosphoinositide metabolism induced by angiotensin II and [Arg]vasopressin in vascular smooth muscle cells. J Biol Chem. 1985 Apr 25;260(8):4661–4670. [PubMed] [Google Scholar]
- Nishikawa M., Sellers J. R., Adelstein R. S., Hidaka H. Protein kinase C modulates in vitro phosphorylation of the smooth muscle heavy meromyosin by myosin light chain kinase. J Biol Chem. 1984 Jul 25;259(14):8808–8814. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Park S., Rasmussen H. Carbachol-induced protein phosphorylation changes in bovine tracheal smooth muscle. J Biol Chem. 1986 Nov 25;261(33):15734–15739. [PubMed] [Google Scholar]
- Rasmussen H., Forder J., Kojima I., Scriabine A. TPA-induced contraction of isolated rabbit vascular smooth muscle. Biochem Biophys Res Commun. 1984 Jul 31;122(2):776–784. doi: 10.1016/s0006-291x(84)80101-1. [DOI] [PubMed] [Google Scholar]
- Roth B. L., Nakaki T., Chuang D. M., Costa E. 5-Hydroxytryptamine2 receptors coupled to phospholipase C in rat aorta: modulation of phosphoinositide turnover by phorbol ester. J Pharmacol Exp Ther. 1986 Aug;238(2):480–485. [PubMed] [Google Scholar]
- Sasaguri T., Hirata M., Itoh T., Koga T., Kuriyama H. Guanine nucleotide binding protein involved in muscarinic responses in the pig coronary artery is insensitive to islet-activating protein. Biochem J. 1986 Nov 1;239(3):567–574. doi: 10.1042/bj2390567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Smith L., Higgins B. L. Temperature and nucleotide dependence of calcium release by myo-inositol 1,4,5-trisphosphate in cultured vascular smooth muscle cells. J Biol Chem. 1985 Nov 25;260(27):14413–14416. [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- Sumimoto K., Kuriyama H. Mobilization of free Ca2+ measured during contraction-relaxation cycles in smooth muscle cells of the porcine coronary artery using quin2. Pflugers Arch. 1986 Feb;406(2):173–180. doi: 10.1007/BF00586679. [DOI] [PubMed] [Google Scholar]
- Swann K., Whitaker M. Stimulation of the Na/H exchanger of sea urchin eggs by phorbol ester. Nature. 1985 Mar 21;314(6008):274–277. doi: 10.1038/314274a0. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Takuwa N., Rasmussen H. Carbachol induces a rapid and sustained hydrolysis of polyphosphoinositide in bovine tracheal smooth muscle measurements of the mass of polyphosphoinositides, 1,2-diacylglycerol, and phosphatidic acid. J Biol Chem. 1986 Nov 5;261(31):14670–14675. [PubMed] [Google Scholar]
- Tohmatsu T., Hattori H., Nagao S., Ohki K., Nozawa Y. Reversal by protein kinase C inhibitor of suppressive actions of phorbol-12-myristate-13-acetate on polyphosphoinositide metabolism and cytosolic Ca2+ mobilization in thrombin-stimulated human platelets. Biochem Biophys Res Commun. 1986 Jan 29;134(2):868–875. doi: 10.1016/s0006-291x(86)80500-9. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Lapetina E. G. 1,2-Diacylglycerol and phorbol ester inhibit agonist-induced formation of inositol phosphates in human platelets: possible implications for negative feedback regulation of inositol phospholipid hydrolysis. Proc Natl Acad Sci U S A. 1985 May;82(9):2623–2626. doi: 10.1073/pnas.82.9.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., van Breemen C. Inositol-1,4,5-trisphosphate releases calcium from skinned cultured smooth muscle cells. Biochem Biophys Res Commun. 1985 Jul 16;130(1):270–274. doi: 10.1016/0006-291x(85)90412-7. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D., Roevens P., van Belle H. Stimulation by serotonin of 40 kDa and 20 kDa protein phosphorylation in human platelets. FEBS Lett. 1984 Jun 11;171(2):289–292. doi: 10.1016/0014-5793(84)80506-2. [DOI] [PubMed] [Google Scholar]


