Abstract
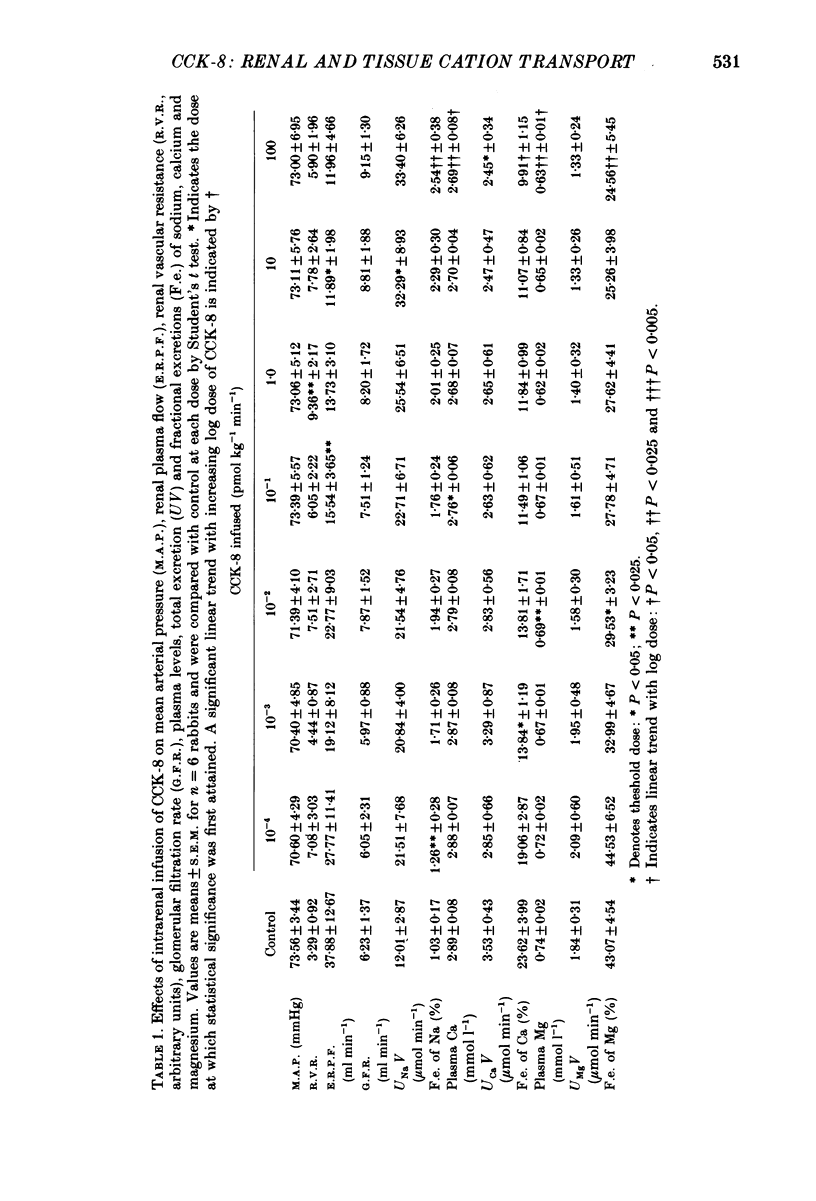
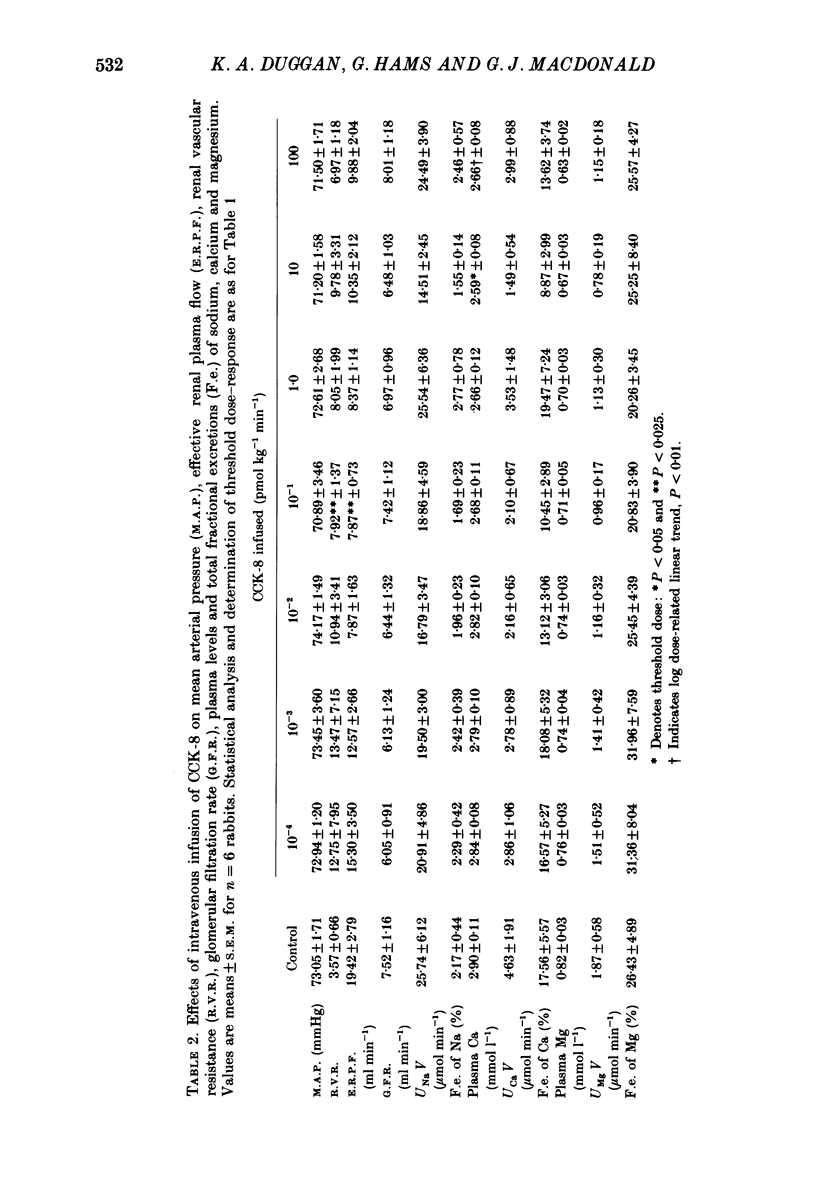
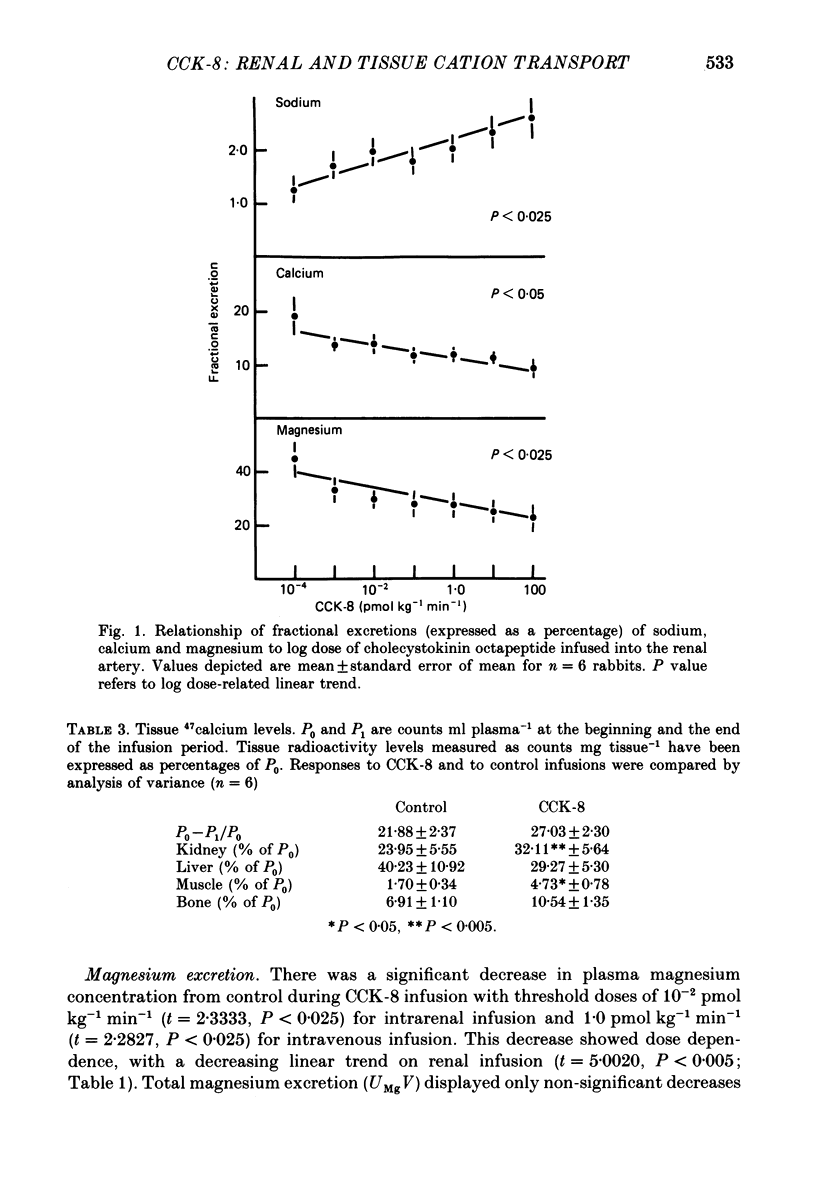
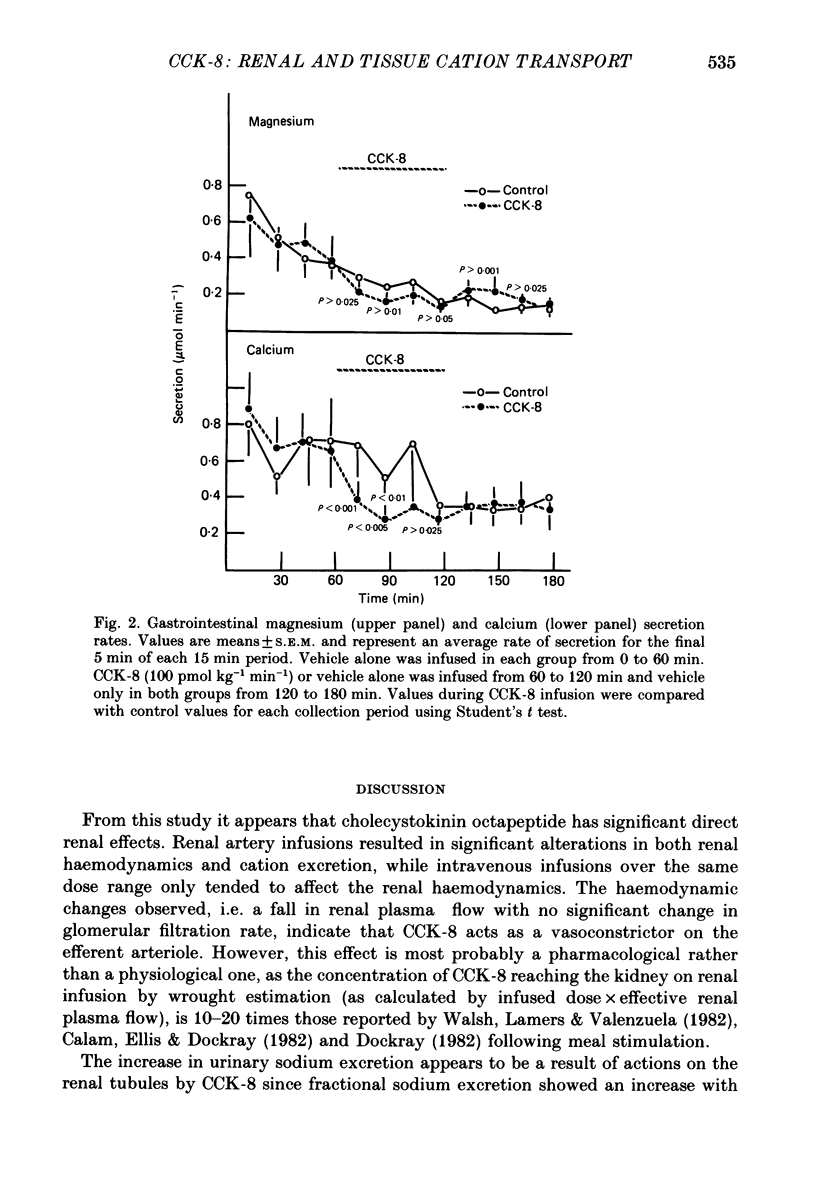
1. Reports that gastric sodium loads cause a greater natriuresis than those administered intravenously, suggest that a gastric or portal sodium monitor exists which releases a humoral natriuretic factor. To determine whether cholecystokinin octapeptide (CCK-8) had direct renal natriuretic effects (and was therefore a candidate for this gut-derived natriuretic factor) we compared the natriuretic response to CCK-8 infused intravenously with that infused directly into the renal artery of six conscious male rabbits. 2. CCK-8 produced a significant log dose-dependent decrease in the fractional excretions of calcium (P less than 0.05) and magnesium (P less than 0.005) and a log dose-dependent increase in fractional sodium excretion (P less than 0.025). The significant decreases in the fractional excretions of calcium and magnesium were accompanied by log dose-dependent falls in their plasma levels (calcium, P less than 0.05, and magnesium, P less than 0.005), indicating movement of calcium and magnesium to extravascular sites. Studies of tissue calcium and magnesium levels in response to CCK-8 infusion showed that calcium accumulated in kidney and skeletal muscle. 3. We conclude that CCK-8 has direct renal natriuretic effects at the tubular level and could be the gut-derived natriuretic factor. In addition to its effects on sodium excretion, CCK-8 causes renal retention and increased gut absorption of calcium and magnesium with movement of these ions to extravascular sites.
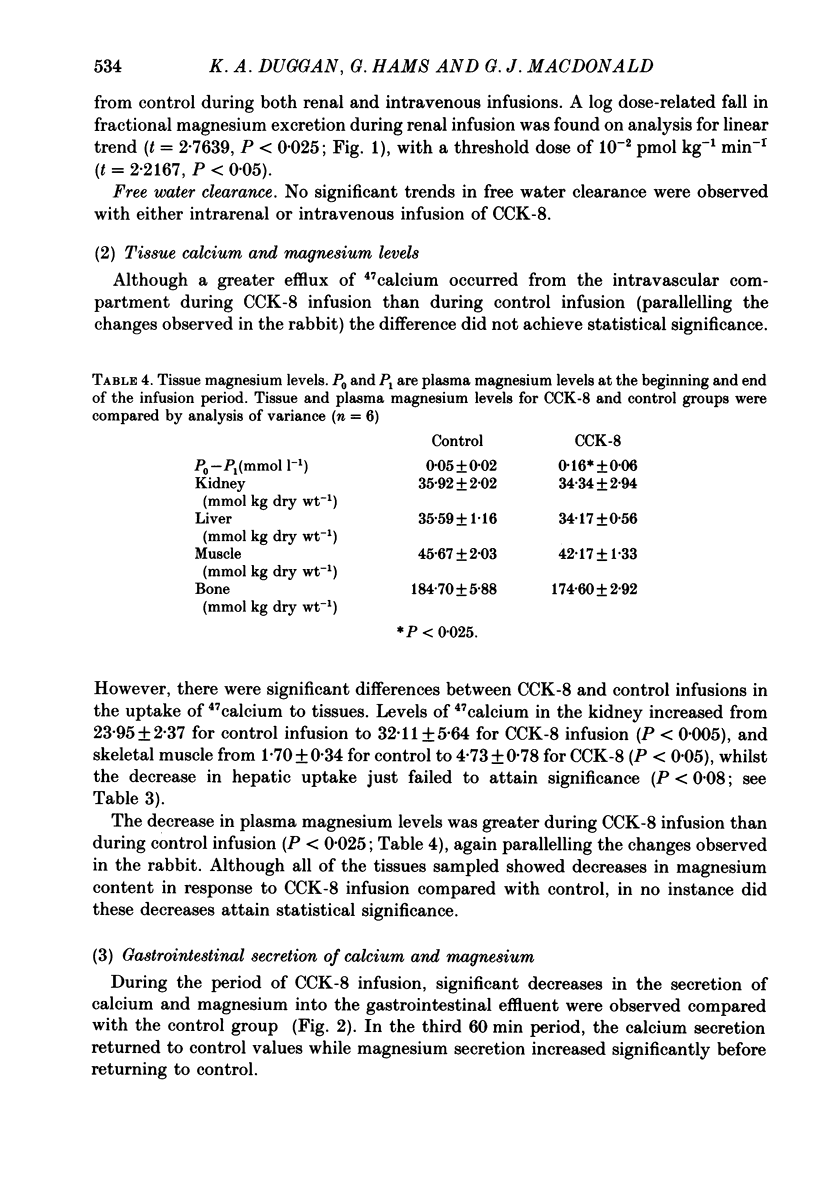
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourdeau J. E., Burg M. B. Voltage dependence of calcium transport in the thick ascending limb of Henle's loop. Am J Physiol. 1979 Apr;236(4):F357–F364. doi: 10.1152/ajprenal.1979.236.4.F357. [DOI] [PubMed] [Google Scholar]
- Brunette M. G., Vigneault N., Carriere S. Micropuncture study of magnesium transport along the nephron in the young rat. Am J Physiol. 1974 Oct;227(4):891–896. doi: 10.1152/ajplegacy.1974.227.4.891. [DOI] [PubMed] [Google Scholar]
- Byrnes D. J., Borody T., Daskalopoulos G., Boyle M., Benn I. Cholecystokinin and gallbladder contraction: effect of CCK infusion. Peptides. 1981;2 (Suppl 2):259–262. doi: 10.1016/0196-9781(81)90041-3. [DOI] [PubMed] [Google Scholar]
- Calam J., Ellis A., Dockray G. J. Identification and measurement of molecular variants of cholecystokinin in duodenal mucosa and plasma. Diminished concentrations in patients with celiac disease. J Clin Invest. 1982 Jan;69(1):218–225. doi: 10.1172/JCI110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R. M. Evidence for a splanchnic sodium input monitor regulating renal sodium excretion in man. Lack of dependence upon aldosterone. Circ Res. 1978 Jul;43(1):19–23. doi: 10.1161/01.res.43.1.19. [DOI] [PubMed] [Google Scholar]
- Carey R. M., Smith J. R., Ortt E. M. Gastrointestinal control of sodium excretion in sodium-depleted conscious rabbits. Am J Physiol. 1976 Jun;230(6):1504–1508. doi: 10.1152/ajplegacy.1976.230.6.1504. [DOI] [PubMed] [Google Scholar]
- Crawford R. J., Wells J. R. Short half-life precursor of globin messenger RNA from chicken erythroblasts. Biochemistry. 1978 May 2;17(9):1591–1596. doi: 10.1021/bi00602a002. [DOI] [PubMed] [Google Scholar]
- Daly J. J., Roe J. W., Horrocks P. A comparison of sodium excretion following the infusion of saline into systemic and portal veins in the dog: evidence for a hepatic role in the control of sodium excretion. Clin Sci. 1967 Dec;33(3):481–487. [PubMed] [Google Scholar]
- Dockray G. J. The physiology of cholecystokinin in brain and gut. Br Med Bull. 1982 Sep;38(3):253–258. doi: 10.1093/oxfordjournals.bmb.a071769. [DOI] [PubMed] [Google Scholar]
- Jamison R. L., Frey N. R., Lacy F. B. Calcium reabsorption in the thin loop of Henle. Am J Physiol. 1974 Sep;227(3):745–751. doi: 10.1152/ajplegacy.1974.227.3.745. [DOI] [PubMed] [Google Scholar]
- Le Grimellec C., Roinel N., Morel F. Simultaneous Mg, Ca, P, K, Na and Cl analysis in rat tubular fluid. 3. During acute Ca plasma loading. Pflugers Arch. 1974;346(3):171–188. doi: 10.1007/BF00595705. [DOI] [PubMed] [Google Scholar]
- Le Grimellec C., Roinel N., Morel F. Simultaneous Mg, Ca, P,K,Na and Cl analysis in rat tubular fluid. I. During perfusion of either inulin or ferrocyanide. Pflugers Arch. 1973 May 23;340(3):181–196. doi: 10.1007/BF00586838. [DOI] [PubMed] [Google Scholar]
- Lennane R. J., Carey R. M., Goodwin T. J., Peart W. S. A comparison of natriuresis after oral and intravenous sodium loading in sodium-depleted man: evidence for a gastrointestinal or portal monitor of sodium intake. Clin Sci Mol Med. 1975 Nov;49(5):437–440. doi: 10.1042/cs0490437. [DOI] [PubMed] [Google Scholar]
- Lennane R. J., Peart W. S., Carey R. M., Shaw J. A comparison on natriuresis after oral and intravenous sodium loading in sodium-depleted rabbits: evidence for a gastrointestinal or portal monitor of sodium intake. Clin Sci Mol Med. 1975 Nov;49(5):433–436. doi: 10.1042/cs0490433. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Ahumada J. J., Coburn J. W., Kleeman C. R. Effect of MgCl2 infusion on urinary Ca and Na during reduction in their filtered loads. Am J Physiol. 1970 Oct;219(4):881–885. doi: 10.1152/ajplegacy.1970.219.4.881. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Kleeman C. R. Renal handling of magnesium in the dog. Am J Physiol. 1969 Jun;216(6):1460–1467. doi: 10.1152/ajplegacy.1969.216.6.1460. [DOI] [PubMed] [Google Scholar]
- Morel F., Roinel N., Le Grimellec C. Electron probe analysis of tubular fluid composition. Nephron. 1969;6(3):350–364. doi: 10.1159/000179738. [DOI] [PubMed] [Google Scholar]
- Passo S. S., Thornborough J. R., Rothballer A. B. Hepatic receptors in control of sodium excretion in anesthetized cats. Am J Physiol. 1973 Feb;224(2):373–375. doi: 10.1152/ajplegacy.1973.224.2.373. [DOI] [PubMed] [Google Scholar]
- Quamme G. A., Dirks J. H. Intraluminal and contraluminal magnesium on magnesium and calcium transfer in the rat nephron. Am J Physiol. 1980 Mar;238(3):F187–F198. doi: 10.1152/ajprenal.1980.238.3.F187. [DOI] [PubMed] [Google Scholar]
- Quamme G. A., Wong N. L., Dirks J. H., Roinel N., De Rouffignac C., Morel F. Magnesium handling in the dog kidney: a micropuncture study. Pflugers Arch. 1978 Oct 18;377(1):95–99. doi: 10.1007/BF00584380. [DOI] [PubMed] [Google Scholar]
- Reeder D. D., Becker H. D., Smith N. J., Rayford P. L., Thompson J. C. Measurement of endogenous release of cholecystokinin by radioimmunoassay. Ann Surg. 1973 Sep;178(3):304–310. doi: 10.1097/00000658-197309000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. H., Lamers C. B., Valenzuela J. E. Cholecystokinin-octapeptidelike immunoreactivity in human plasma. Gastroenterology. 1982 Mar;82(3):438–444. [PubMed] [Google Scholar]
- Wen S. F., Evanson R. L., Dirks J. H. Micropuncture study of renal magnesium transport in proximal and distal tubule of the dog. Am J Physiol. 1970 Sep;219(3):570–576. doi: 10.1152/ajplegacy.1970.219.3.570. [DOI] [PubMed] [Google Scholar]
- de Rouffignac C., Morel F., Moss N., Roinel N. Micropuncture study of water and electrolyte movements along the loop of Henle in psammomys with special reference to magnesium, calcium and phosphorus. Pflugers Arch. 1973 Nov 30;344(4):309–326. doi: 10.1007/BF00592784. [DOI] [PubMed] [Google Scholar]


