Abstract
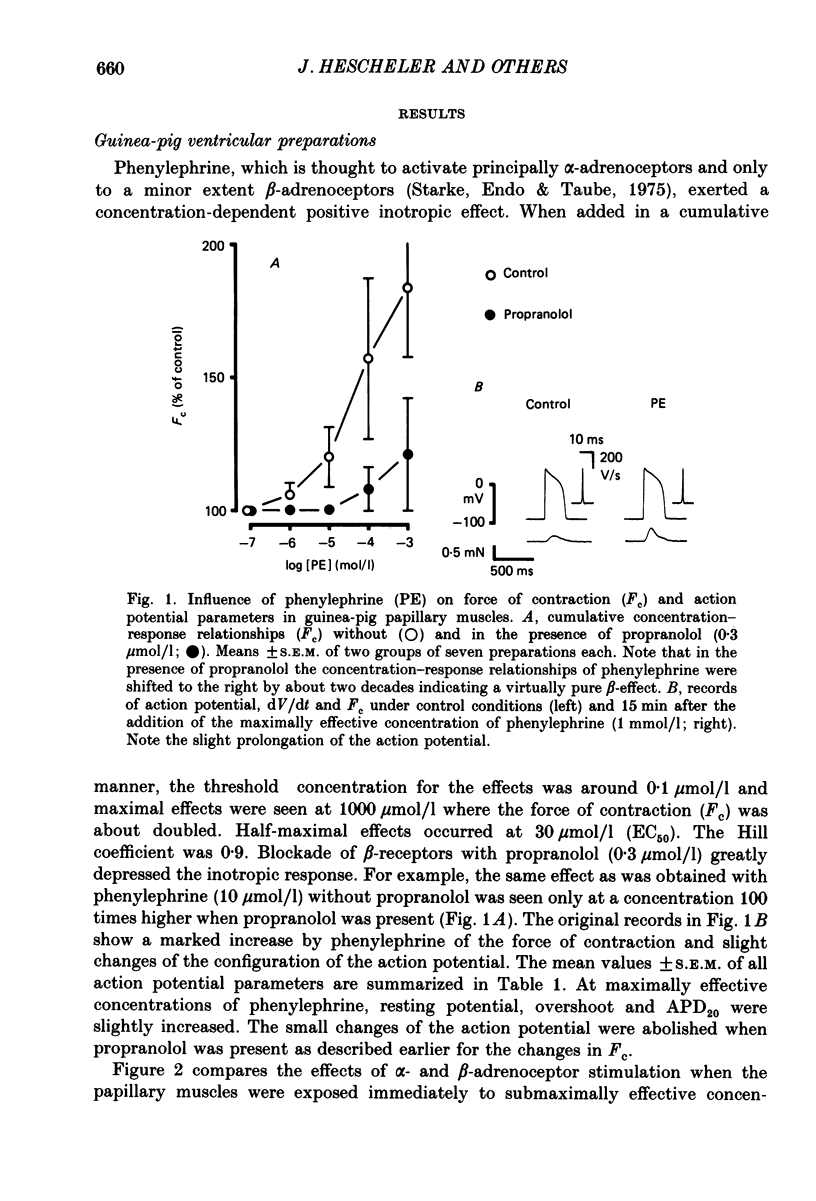
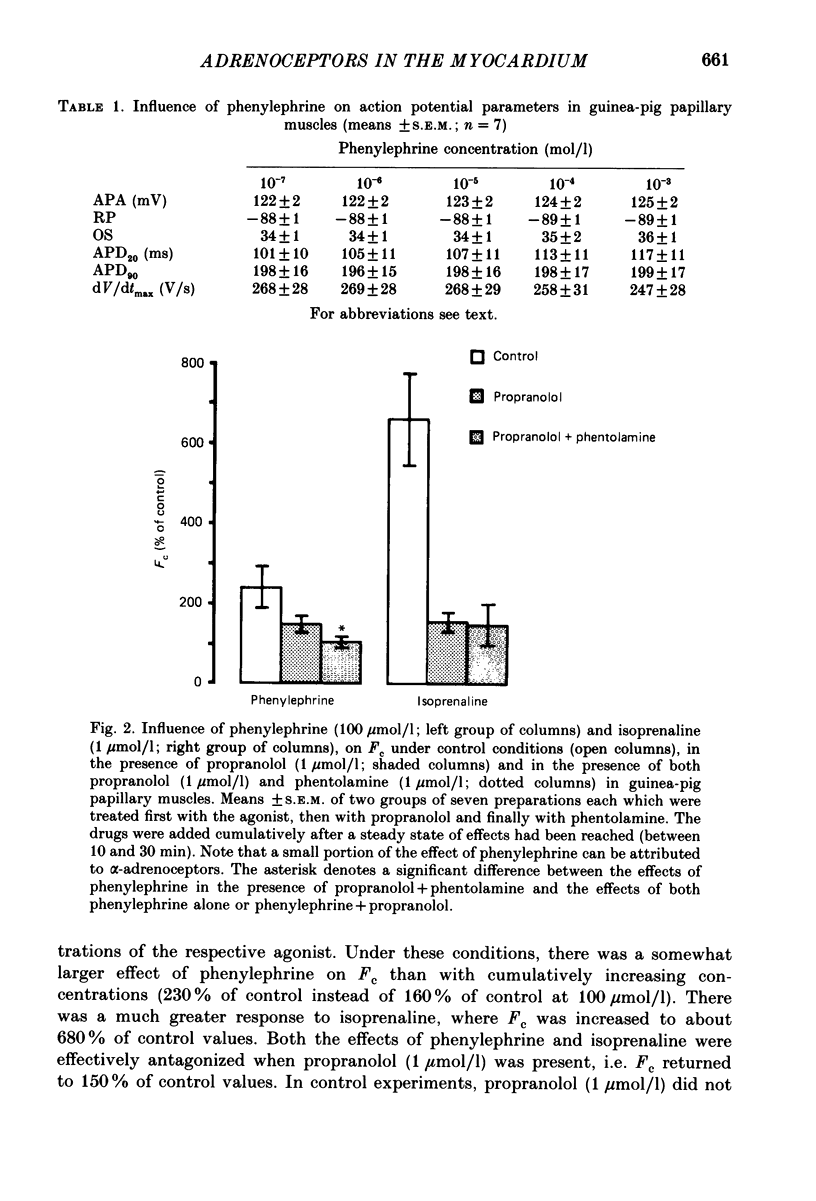
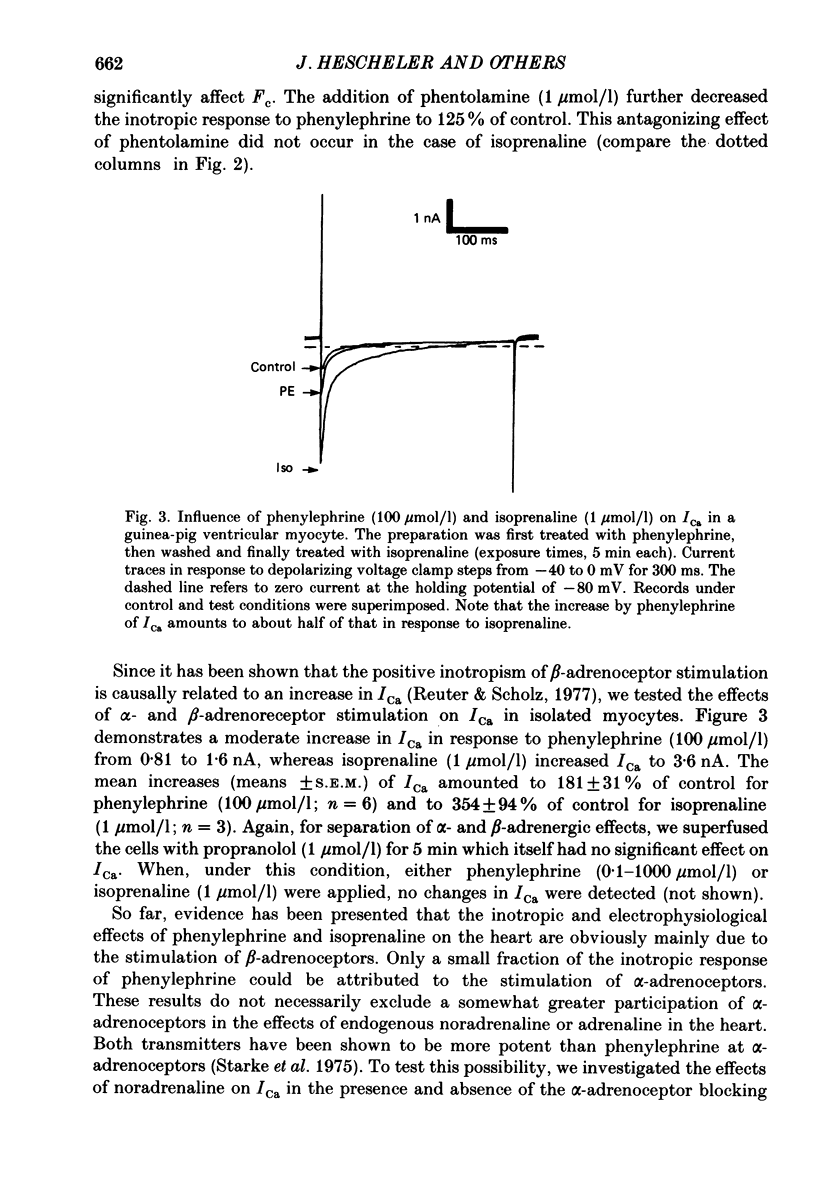
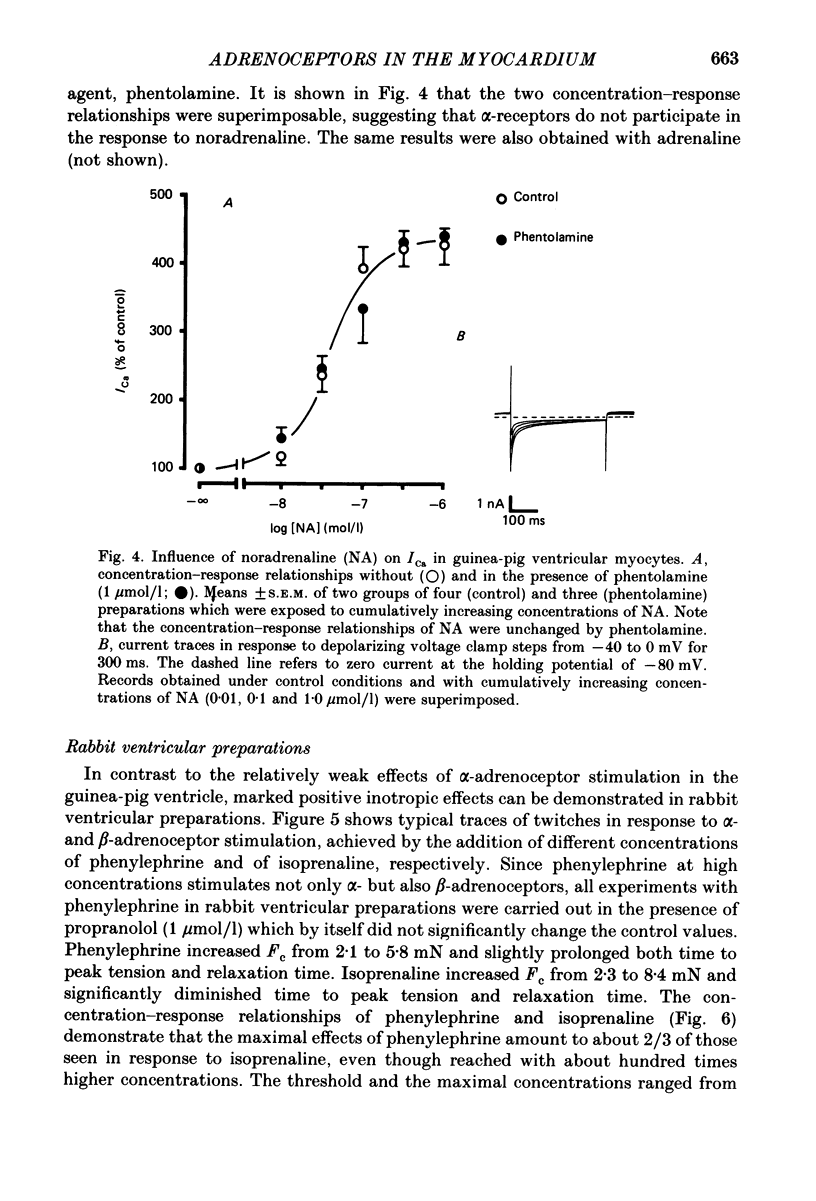
1. The influence of alpha-adrenoceptor stimulation on mechanical and electrophysiological parameters was investigated in ventricular preparations from guinea-pigs and rabbits. Action potential and force of contraction were measured in papillary muscles and ionic currents were measured in isolated myocytes. 2. The effects of alpha-adrenoceptor stimulation were compared with those of beta-adrenoceptor stimulation. 3. In the guinea-pig the stimulation of alpha-adrenoceptors caused a small increase in the force of contraction (less than 10% of the response to beta-adrenoceptor stimulation) which was not accompanied by any increase of the slow calcium inward current. beta-Adrenoceptor stimulation produced large increases in both force of contraction and slow inward calcium current. The noradrenaline-induced increase in the slow inward calcium current was not affected by phentolamine. 4. In the rabbit, alpha-adrenoceptor stimulation produced large increases in the force of contraction (about two thirds of those seen in response to beta-adrenoceptor stimulation). Whereas beta-adrenoceptor stimulation also produced large increases in both maximal upstroke velocity of slow-response action potentials and slow inward calcium current, there was almost no change of both parameters in response to alpha-adrenoceptor stimulation. 5. We conclude that, first, the contribution of alpha-adrenoceptors to adrenoceptor-mediated changes of force of contraction is minimal in the guinea-pig ventricle, and second, the pronounced changes of force of contraction in the rabbit ventricle in response to alpha-adrenoceptor stimulation are unrelated to changes in the slow inward calcium current.
Full text
PDF


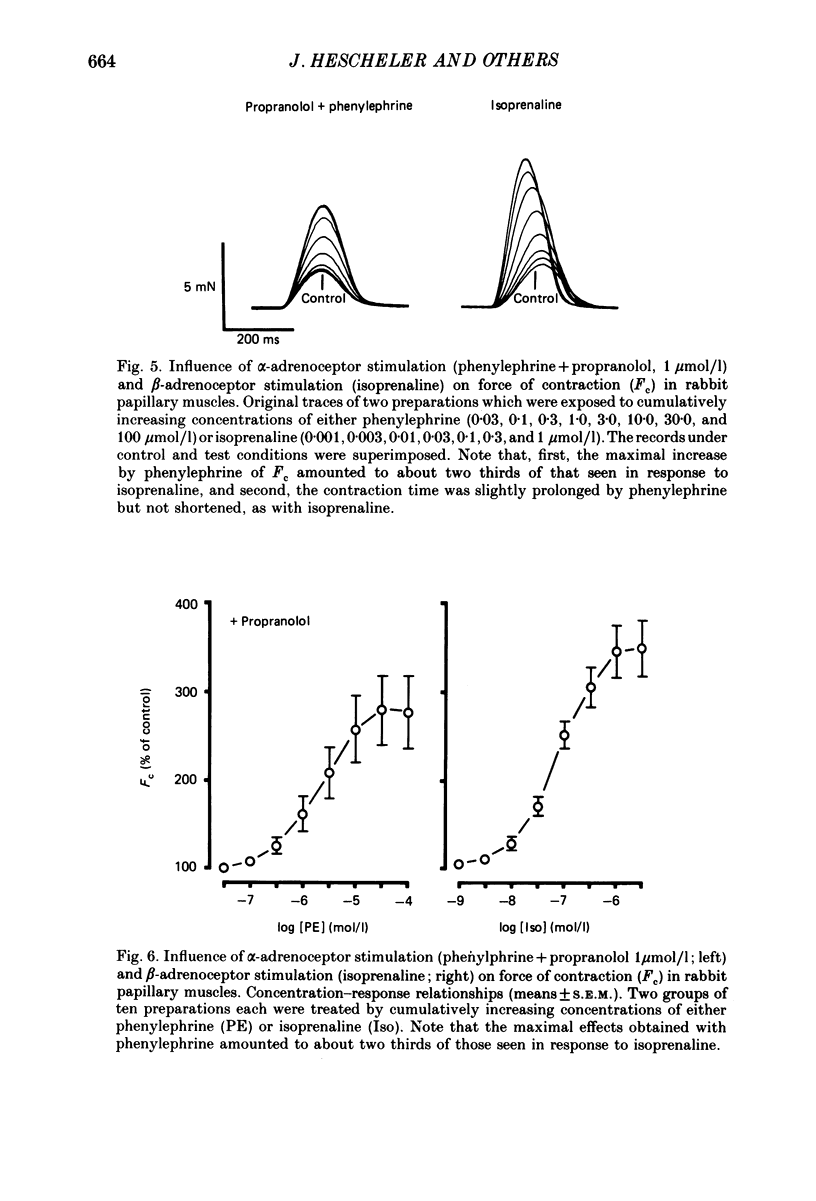
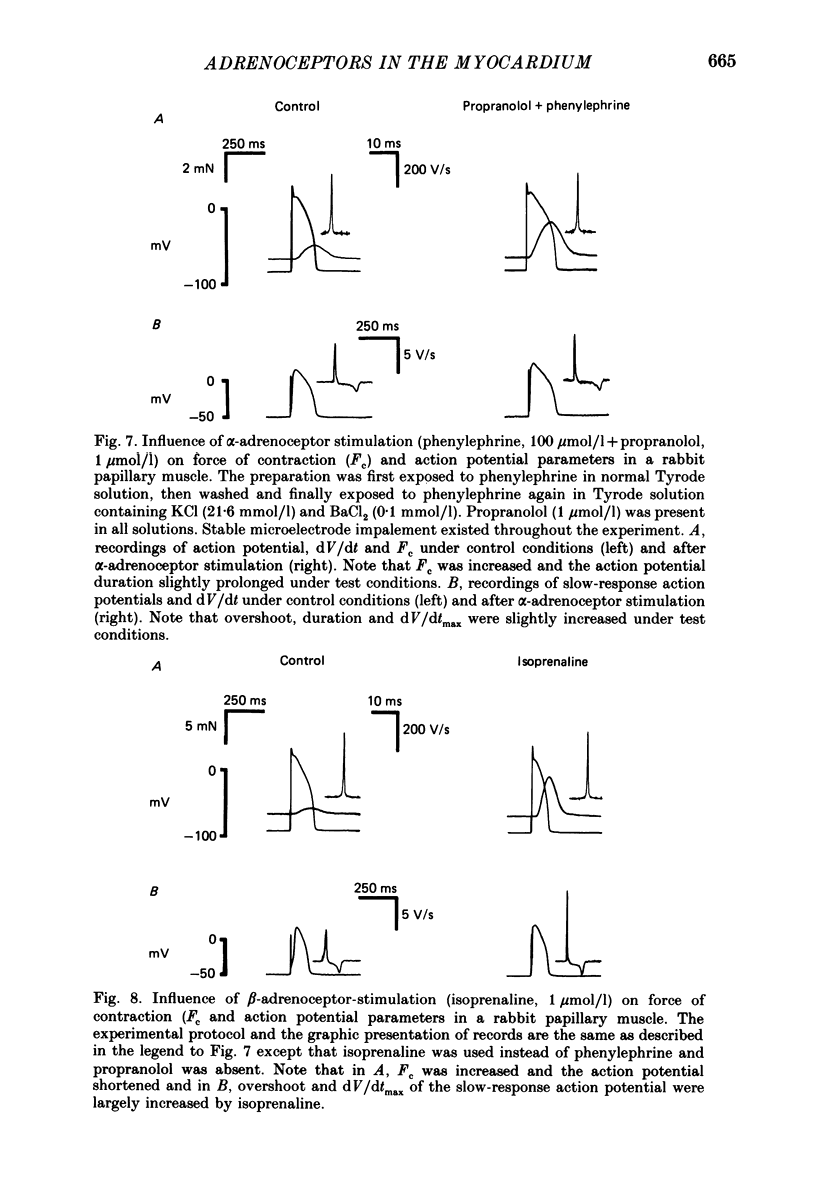
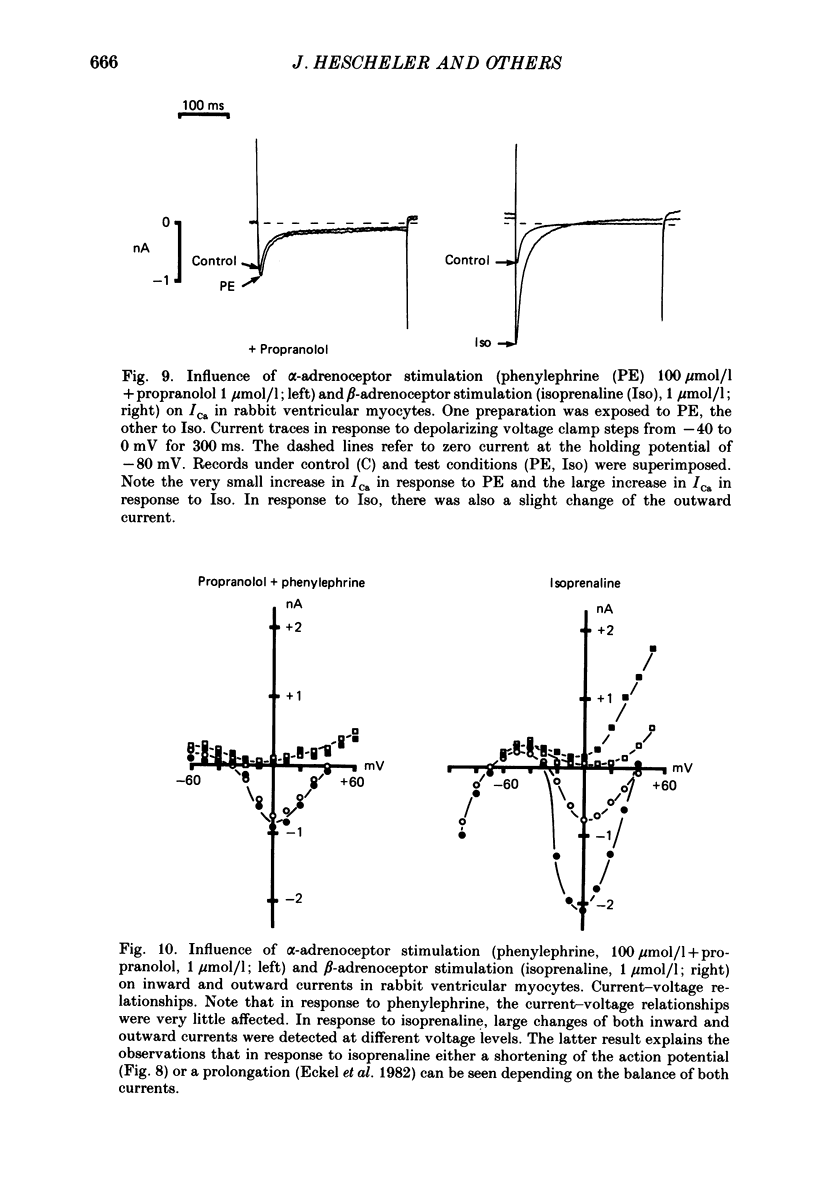




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Benfey B. G. Theophylline and phenylephrine effects on cardiac relaxation. Br J Pharmacol. 1977 Jan;59(1):75–81. doi: 10.1111/j.1476-5381.1977.tb06979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brodde O. E., Motomura S., Endoh M., Schümann H. J. Lack of correlation between the positive inotropic effect evoked by alpha-adrenoceptor stimulation and the levels of cyclic AMP and/or cyclic GMP in the isolated ventricle strip of the rabbit. J Mol Cell Cardiol. 1978 Mar;10(3):207–219. doi: 10.1016/0022-2828(78)90344-9. [DOI] [PubMed] [Google Scholar]
- Brückner R., Mügge A., Scholz H. Existence and functional role of alpha 1-adrenoceptors in the mammalian heart. J Mol Cell Cardiol. 1985 Jul;17(7):639–645. doi: 10.1016/s0022-2828(85)80063-8. [DOI] [PubMed] [Google Scholar]
- Brückner R., Scholz H. Effects of alpha-adrenoceptor stimulation with phenylephrine in the presence of propranolol on force of contraction, slow inward current and cyclic AMP content in the bovine heart. Br J Pharmacol. 1984 May;82(1):223–232. doi: 10.1111/j.1476-5381.1984.tb16462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel L., Gristwood R. W., Nawrath H., Owen D. A., Satter P. Inotropic and electrophysiological effects of histamine on human ventricular heart muscle. J Physiol. 1982 Sep;330:111–123. doi: 10.1113/jphysiol.1982.sp014332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Motomura S. Differentiation by cholinergic stimulation of positive inotropic actions mediated via alpha- and beta-adrenoceptors in the rabbit heart. Life Sci. 1979 Aug 27;25(9):759–768. doi: 10.1016/0024-3205(79)90520-4. [DOI] [PubMed] [Google Scholar]
- Endoh M., Schümann H. J. Frequency-dependence of the positive inotropic effect of methoxamine and naphazoline mediated by alpha-Adrenoceptors in the isolated rabbit papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1975;287(4):377–389. doi: 10.1007/BF00500039. [DOI] [PubMed] [Google Scholar]
- Endoh M., Yamashita S. Adenosine antagonizes the positive inotropic action mediated via beta-, but not alpha-adrenoceptors in the rabbit papillary muscle. Eur J Pharmacol. 1980 Aug 8;65(4):445–448. doi: 10.1016/0014-2999(80)90352-0. [DOI] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F., Pelzer D., Cavalié A., Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986 Sep 4;323(6083):66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibres: the slow inward current component under the influence of modified [Ca2+]i. Pflugers Arch. 1977 Oct 19;371(1-2):61–69. doi: 10.1007/BF00580773. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Kunos G., Nickerson M. Temperature-induced interconversion of alpha-and beta-adrenoceptors in the frog heart. J Physiol. 1976 Mar;256(1):23–40. doi: 10.1113/jphysiol.1976.sp011309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann J. P. Alpha-adrenergic stimulation of sarcolemmal protein phosphorylation and slow responses in intact myocardium. J Biol Chem. 1986 Apr 15;261(11):4860–4867. [PubMed] [Google Scholar]
- Maisel A. S., Motulsky H. J., Insel P. A. Life cycles of cardiac alpha 1- and beta-adrenergic receptors. Biochem Pharmacol. 1987 Jan 1;36(1):1–6. doi: 10.1016/0006-2952(87)90375-3. [DOI] [PubMed] [Google Scholar]
- Miura Y., Inui J., Imamura H. Alpha-adrenoceptor-mediated restoration of calcium-dependent potential in the partially depolarized rabbit papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1978 Jan-Feb;301(3):201–205. doi: 10.1007/BF00507038. [DOI] [PubMed] [Google Scholar]
- Morad M., Rolett E. L. Relaxing effects of catecholamines on mammalian heart. J Physiol. 1972 Aug;224(3):537–558. doi: 10.1113/jphysiol.1972.sp009912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M., Maeda K., Sekiya A., Hagino Y. Effect of hypothyroid status on myocardial responses to sympathomimetic drugs. Jpn J Pharmacol. 1971 Dec;21(6):819–825. doi: 10.1254/jjp.21.819. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Sulpice J. C., Vassort G. Inositol phosphate production following alpha 1-adrenergic, muscarinic or electrical stimulation in isolated rat heart. FEBS Lett. 1986 Oct 6;206(2):292–298. doi: 10.1016/0014-5793(86)80999-1. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Simpson P., Bishopric N., Coughlin S., Karliner J., Ordahl C., Starksen N., Tsao T., White N., Williams L. Dual trophic effects of the alpha 1-adrenergic receptor in cultured neonatal rat heart muscle cells. J Mol Cell Cardiol. 1986 Nov;18 (Suppl 5):45–58. doi: 10.1016/s0022-2828(86)80460-6. [DOI] [PubMed] [Google Scholar]
- Wagner J., Brodde O. E. On the presence and distribution of alpha-adrenoceptors in the heart of various mammalian species. Naunyn Schmiedebergs Arch Pharmacol. 1978 May;302(3):239–254. doi: 10.1007/BF00508293. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]


