Abstract
1. Several studies have hypothesized that alanine decreases plasma ketone body levels by increasing availability of oxaloacetate, thus allowing acetyl groups to enter the tricarboxylic acid cycle and releasing co-enzyme A (CoA). 2. Four, fasted adult males exercised at 50% of their maximal oxygen consumption for 1.5 h, then ingested 100 g of either glucose or alanine 2 h into recovery. 3. Post-exercise ketosis had developed at 2 h into recovery, as shown by a significantly elevated concentration of beta-hydroxybutyrate in the plasma. At this time plasma free fatty acids were elevated above resting levels while plasma free carnitine concentrations had fallen below resting values. 4. After either alanine or glucose ingestion beta-hydroxybutyrate concentrations fell to the same extent. After the alanine load free carnitine increased above that seen in the glucose trial. Following either alanine or glucose ingestion free fatty acid levels fell; they remained at resting levels in the alanine trial but decreased below rest in the glucose trial. 5. We assume that plasma carnitine concentrations largely reflect the hepatic carnitine pools; therefore, elevations in the plasma free carnitine are probably the result of an increased utilization of acetyl CoA. The significant elevation in plasma free carnitine concentration found after alanine ingestion is consistent with the hypothesis that alanine increases the oxidation of acetyl CoA by providing oxaloacetate for the tricarboxylic acid cycle.
Full text
PDF

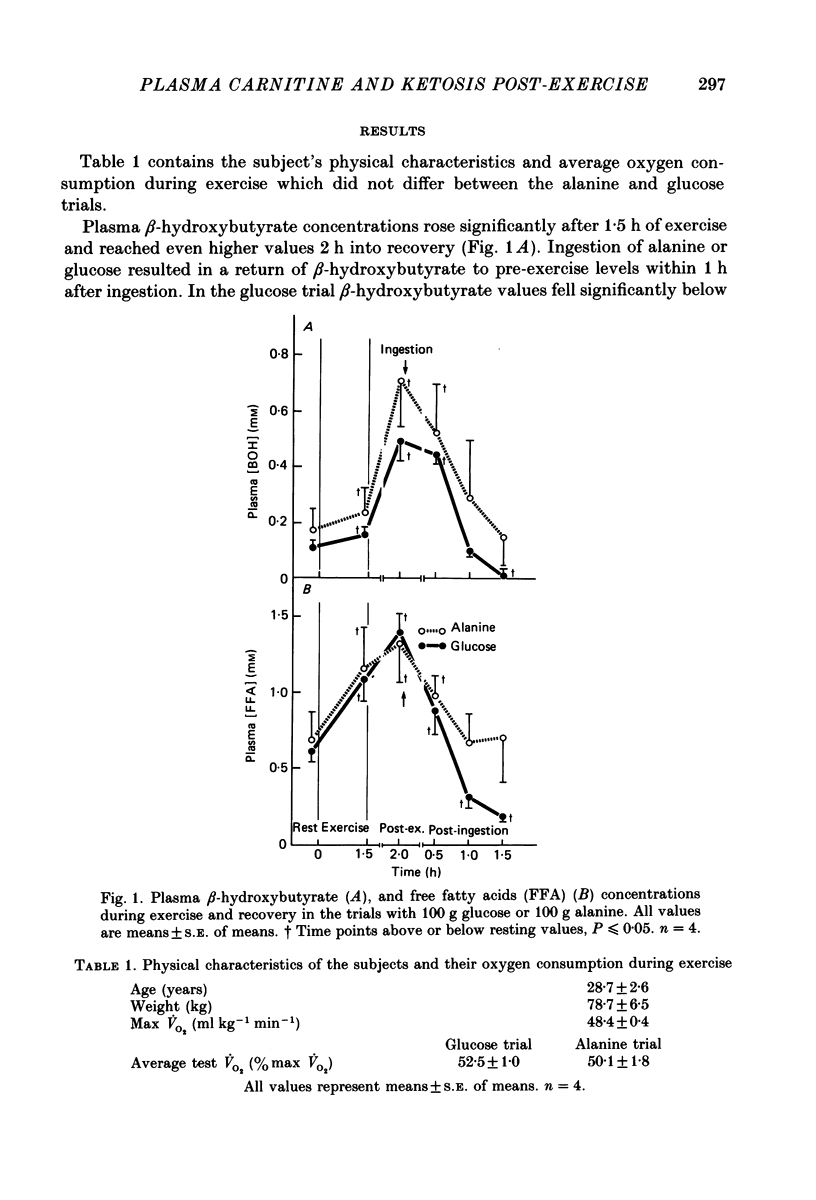
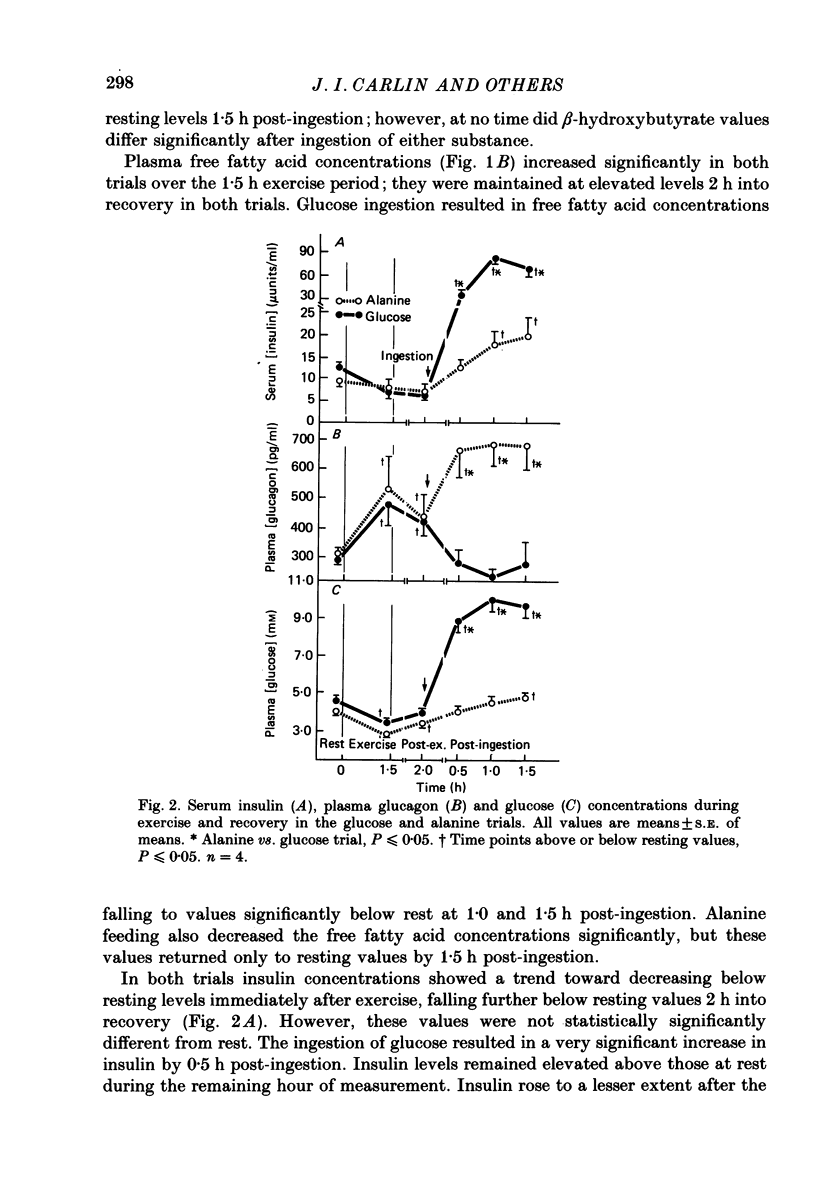
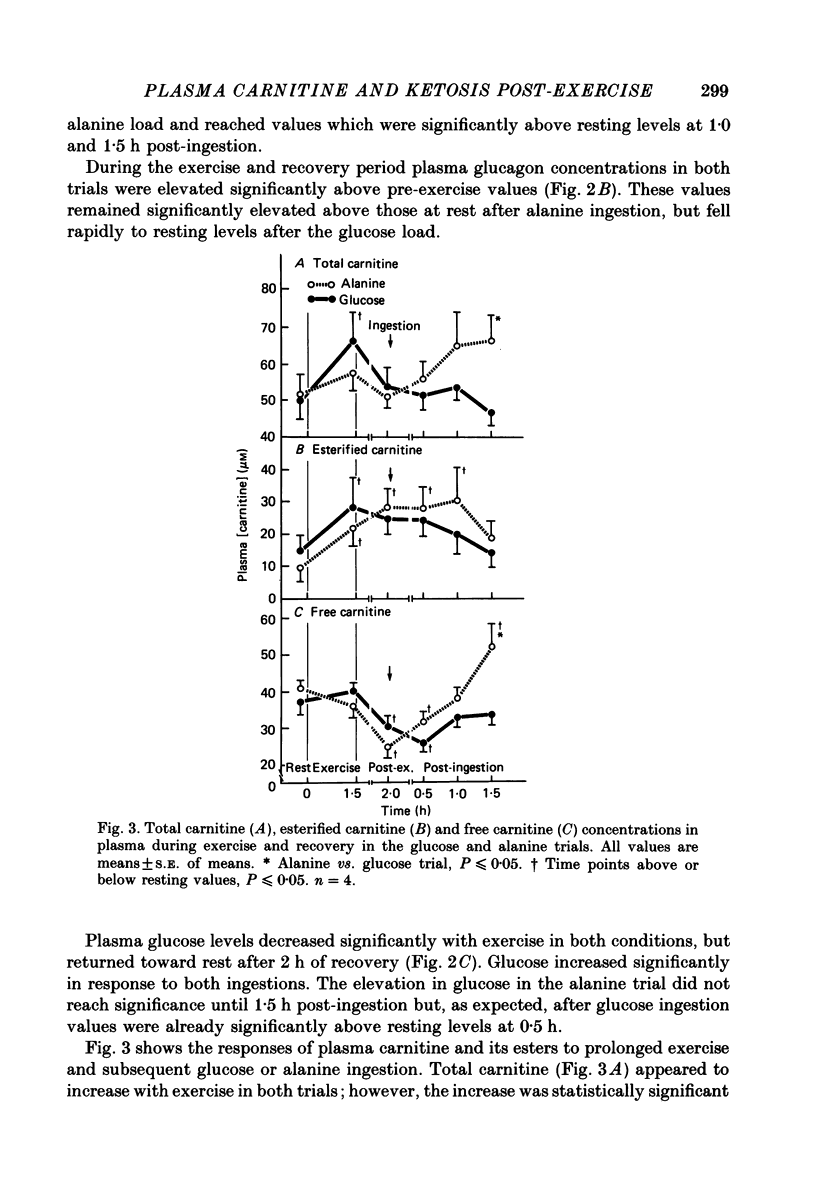
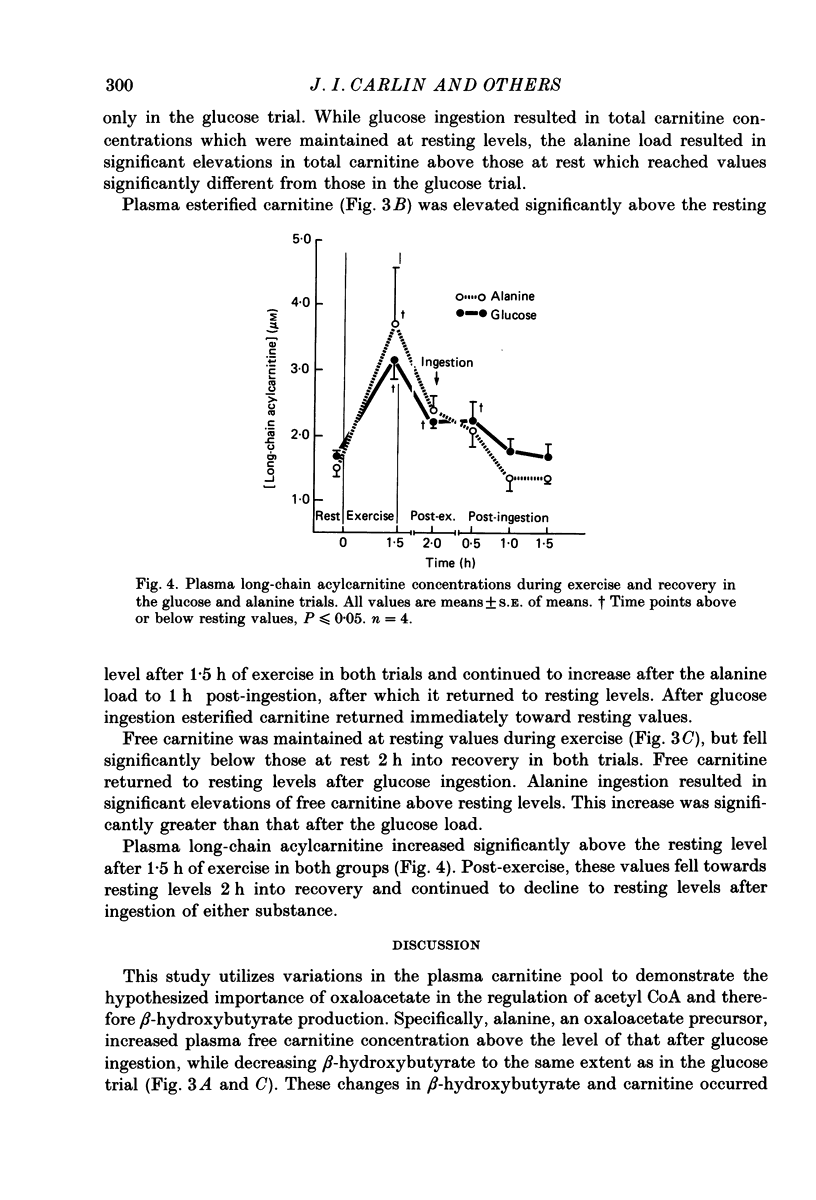



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell F. P., DeLucia A. J. Plasma and liver carnitine (free and esterified) levels and their interrelationships in moderately hypercholesterolemic monkeys (Macaca arctoides). Can J Biochem Cell Biol. 1983 Jun;61(6):328–332. doi: 10.1139/o83-045. [DOI] [PubMed] [Google Scholar]
- Brass E. P., Hoppel C. L. Relationship between acid-soluble carnitine and coenzyme A pools in vivo. Biochem J. 1980 Sep 15;190(3):495–504. doi: 10.1042/bj1900495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983 Oct;63(4):1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Brooks D. E., McIntosh J. E. Turnover of carnitine by rat tissues. Biochem J. 1975 Jun;148(3):439–445. doi: 10.1042/bj1480439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeslag J. H., Noakes T. D., Sloan A. W. The effects of alanine, glucose and starch ingestion on the ketosis produced by exercise and by starvation. J Physiol. 1982 Apr;325:363–376. doi: 10.1113/jphysiol.1982.sp014155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGee J., Allen K. G. Preparation of methyl esters from the saponifiable fatty acids in small biological specimens for gas-liquid chromatographic analysis. J Chromatogr. 1974 Nov 13;100(1):35–42. doi: 10.1016/s0021-9673(00)86037-9. [DOI] [PubMed] [Google Scholar]
- Maehlum S., Felig P., Wahren J. Splanchnic glucose and muscle glycogen metabolism after glucose feeding during postexercise recovery. Am J Physiol. 1978 Sep;235(3):E255–E260. doi: 10.1152/ajpendo.1978.235.3.E255. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. In support of the roles of malonyl-CoA and carnitine acyltransferase I in the regulation of hepatic fatty acid oxidation and ketogenesis. J Biol Chem. 1979 Sep 10;254(17):8163–8168. [PubMed] [Google Scholar]
- Nosadini R., Alberti K. G., Johnston D. G., Del Prato S., Marescotti C., Duner E. The antiketogenic effect of alanine in normal man: evidence for an alanine-ketone body cycle. Metabolism. 1981 Jun;30(6):563–567. doi: 10.1016/0026-0495(81)90131-1. [DOI] [PubMed] [Google Scholar]
- Parvin R., Pande S. V. Enhancement of mitochondrial carnitine and carnitine acylcarnitine translocase-mediated transport of fatty acids into liver mitochondria under ketogenic conditions. J Biol Chem. 1979 Jun 25;254(12):5423–5429. [PubMed] [Google Scholar]
- Parvin R., Pande S. V. Microdetermination of (-)carnitine and carnitine acetyltransferase activity. Anal Biochem. 1977 May 1;79(1-2):190–201. doi: 10.1016/0003-2697(77)90393-1. [DOI] [PubMed] [Google Scholar]
- Pearson D. J., Tubbs P. K. Carnitine and derivatives in rat tissues. Biochem J. 1967 Dec;105(3):953–963. doi: 10.1042/bj1050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkner K. J., Bieber L. L. Short-chain acylcarnitines of human blood and urine. Biochem Med. 1982 Oct;28(2):197–203. doi: 10.1016/0006-2944(82)90070-9. [DOI] [PubMed] [Google Scholar]
- Vranic M., Kawamori R. Essential roles of insulin and glucagon in regulating glucose fluxes during exercise in dogs. Diabetes. 1979 Jan;28 (Suppl 1):45–52. doi: 10.2337/diab.28.1.s45. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Hendler R., Ahlborg G. Glucose and amino acid metabolism during recovery after exercise. J Appl Physiol. 1973 Jun;34(6):838–845. doi: 10.1152/jappl.1973.34.6.838. [DOI] [PubMed] [Google Scholar]
- Zinman B., Murray F. T., Vranic M., Albisser A. M., Leibel B. S., Mc Clean P. A., Marliss E. B. Glucoregulation during moderate exercise in insulin treated diabetics. J Clin Endocrinol Metab. 1977 Oct;45(4):641–652. doi: 10.1210/jcem-45-4-641. [DOI] [PubMed] [Google Scholar]


