Abstract
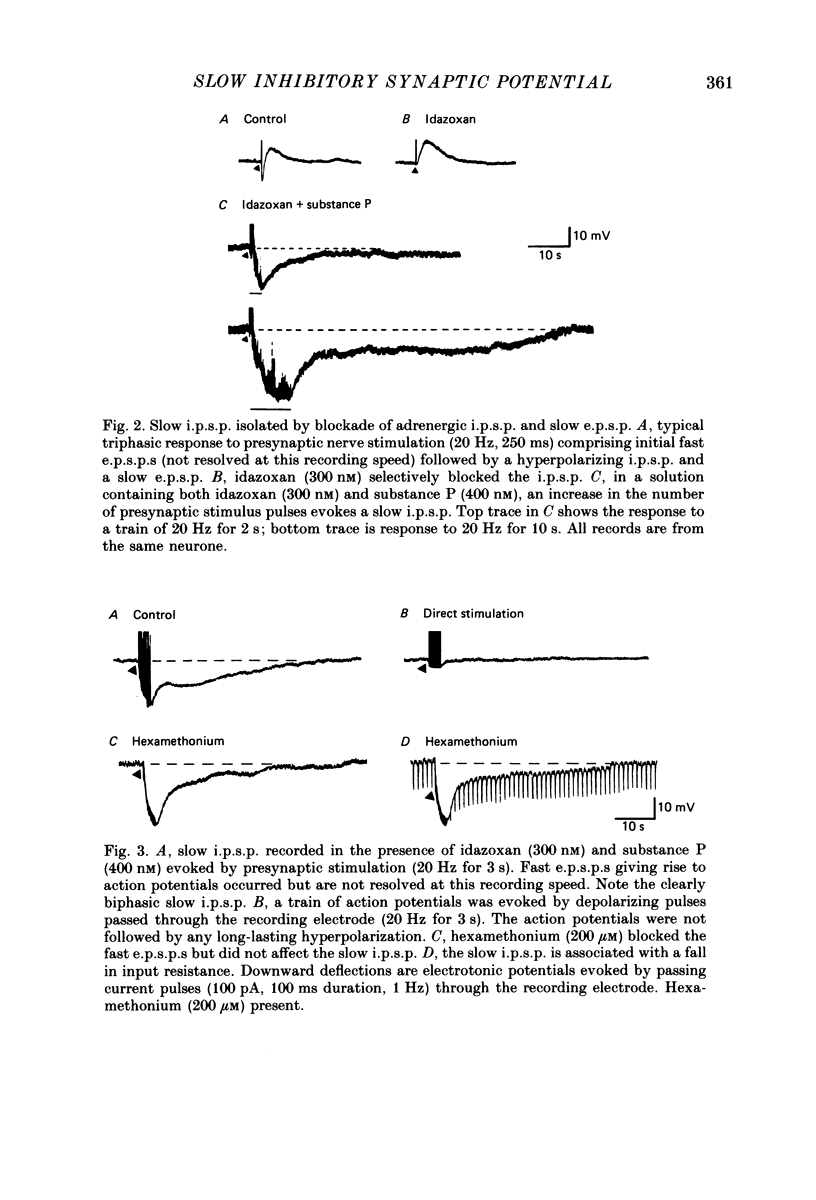
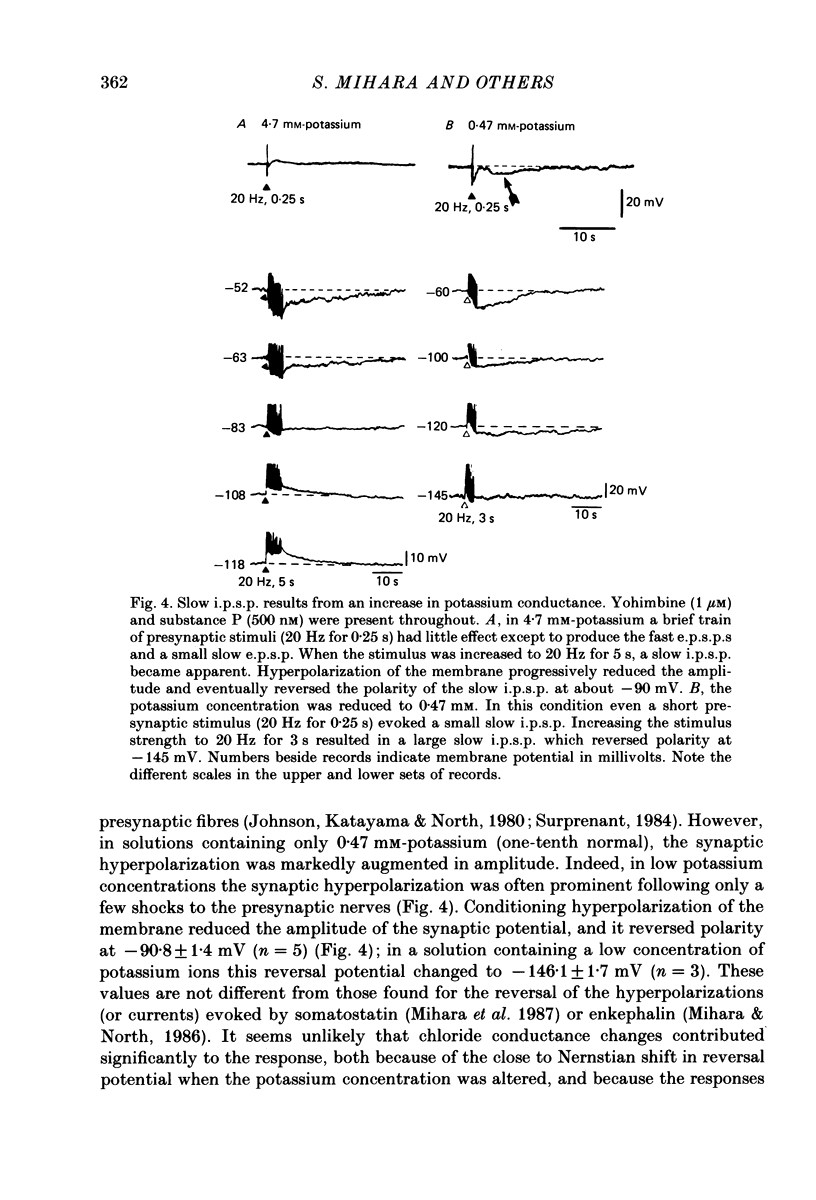
1. Intracellular recordings were made from neurones in the submucous plexus of guinea-pig ileum and caecum. The responses to electrical stimulation of fibre strands entering the nodes of the plexus were studied. 2. Stimuli comprising trains of pulses (20 Hz, 1-5 s) produced nicotinic excitatory post-synaptic potentials (fast e.p.s.p.s), an adrenergic inhibitory post-synaptic potential (i.p.s.p.), a slow excitatory post-synaptic potential (slow e.p.s.p.) and a fourth, hitherto unnoticed, slow hyperpolarization which followed the slow e.p.s.p. All these responses were abolished by tetrodotoxin or solutions containing a low calcium concentration. 3. The slow hyperpolarization (slow i.p.s.p.) was examined in the presence of blockers of the nicotinic and adrenergic responses, and in conditions in which the slow e.p.s.p. was prevented by desensitizing concentrations of substance P or vasoactive intestinal polypeptide. The slow i.p.s.p. was unaffected by prazosin (0.1-1 microM), propranolol (0.1-1 microM), atropine (1 microM) or naloxone (1 microM). 4. The amplitude and duration of the slow i.p.s.p. increased with increasing numbers of stimulus pulses; it had an amplitude of 17 mV and a duration of 70 s when evoked by a stimulus of 20 Hz for 3 s. 5. The slow i.p.s.p. was associated with a decrease in the input resistance of the cell. It reversed polarity at -90 mV in 4.7 mM-potassium and the extrapolated reversal potential in 0.47 mM-potassium was -145 mV; these findings indicate that the slow i.p.s.p. results from an increase in membrane potassium conductance. 6. The slow i.p.s.p. could still be recorded from submucous plexus neurones in segments of ileum which had been extrinsically denervated 6-11 days previously.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


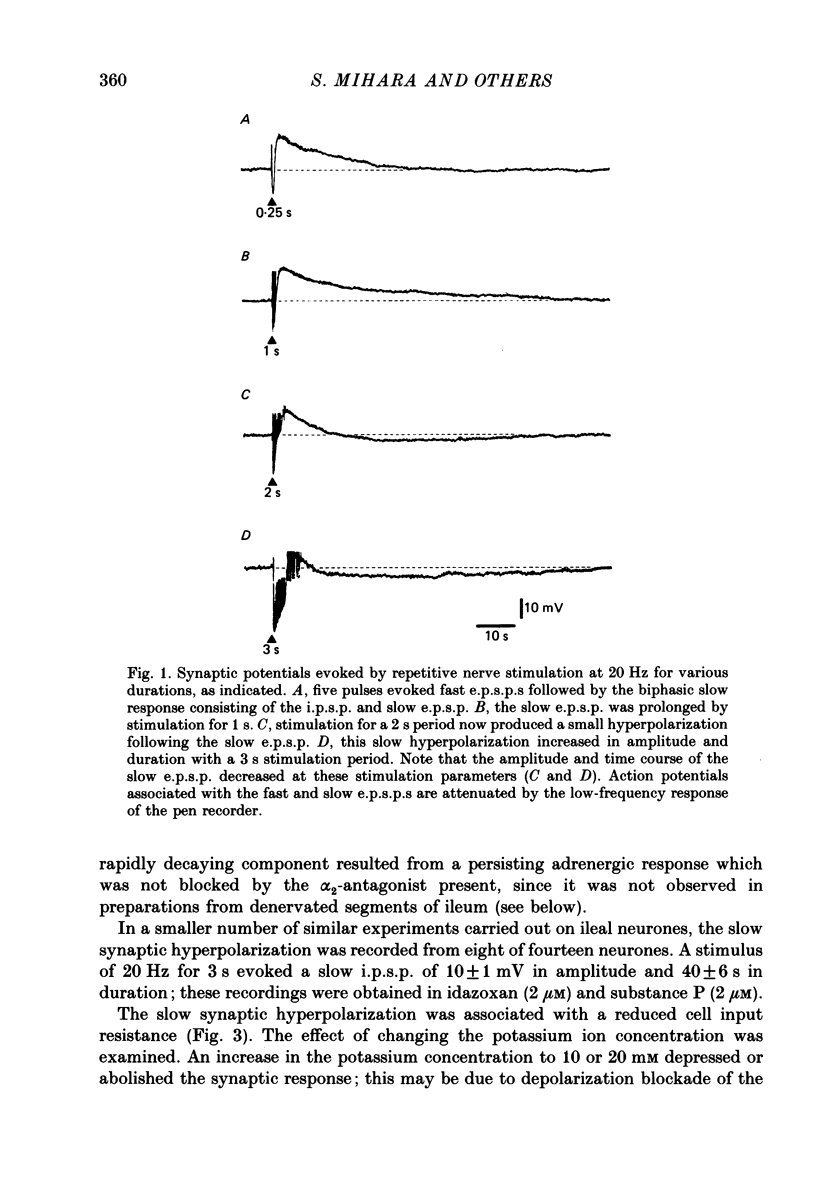



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costa M., Furness J. B., Smith I. J., Davies B., Oliver J. An immunohistochemical study of the projections of somatostatin-containing neurons in the guinea-pig intestine. Neuroscience. 1980;5(5):841–852. doi: 10.1016/0306-4522(80)90153-0. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B. Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience. 1984 Nov;13(3):911–919. doi: 10.1016/0306-4522(84)90105-2. [DOI] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Distribution of intrinsic nerve cell bodies and axons which take up aromatic amines and their precursors in the small intestine of the guinea-pig. Cell Tissue Res. 1978 Apr 28;188(3):527–543. doi: 10.1007/BF00219790. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Gibbins I. L., Llewellyn-Smith I. J., Oliver J. R. Neurochemically similar myenteric and submucous neurons directly traced to the mucosa of the small intestine. Cell Tissue Res. 1985;241(1):155–163. doi: 10.1007/BF00214637. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Keast J. R. Choline acetyltransferase- and peptide immunoreactivity of submucous neurons in the small intestine of the guinea-pig. Cell Tissue Res. 1984;237(2):329–336. doi: 10.1007/BF00217152. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Wilson A. J. Water-stable fluorophores, produced by reaction with aldehyde solutions, for the histochemical localization of catechol- and indolethylamines. Histochemistry. 1977 May 20;52(2):159–170. doi: 10.1007/BF00492292. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., McKirdy H. C. Synaptic potentials recorded from neurones of the submucous plexus of guinea-pig small intestine. J Physiol. 1975 Jul;249(2):369–385. doi: 10.1113/jphysiol.1975.sp011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Silinsky E. M. Some effects of 5-hydroxytryptamine, dopamine and noradrenaline on neurones in the submucous plexus of guinea-pig small intestine. J Physiol. 1975 Oct;251(3):817–832. doi: 10.1113/jphysiol.1975.sp011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., North R. A. Slow synaptic potentials in neurones of the myenteric plexus. J Physiol. 1980 Apr;301:505–516. doi: 10.1113/jphysiol.1980.sp013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S., Katayama Y., Nishi S. Slow postsynaptic potentials in neurones of submucous plexus of guinea-pig caecum and their mimicry by noradrenaline and various peptides. Neuroscience. 1985 Dec;16(4):1057–1068. doi: 10.1016/0306-4522(85)90116-2. [DOI] [PubMed] [Google Scholar]
- Mihara S., North R. A. Opioids increase potassium conductance in submucous neurones of guinea-pig caecum by activating delta-receptors. Br J Pharmacol. 1986 Jun;88(2):315–322. doi: 10.1111/j.1476-5381.1986.tb10207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S., North R. A., Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. J Physiol. 1987 Sep;390:335–355. doi: 10.1113/jphysiol.1987.sp016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O. Slowly-developing depolarization of neurones in the guinea-pig inferior mesenteric ganglion following repetitive stimulation of the preganglionic nerves. Brain Res. 1978 Jan 27;140(2):231–239. doi: 10.1016/0006-8993(78)90457-2. [DOI] [PubMed] [Google Scholar]
- Nishi S., Koketsu K. Early and late after discharges of amphibian sympathetic ganglion cells. J Neurophysiol. 1968 Jan;31(1):109–121. doi: 10.1152/jn.1968.31.1.109. [DOI] [PubMed] [Google Scholar]
- North R. A., Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol. 1985 Jan;358:17–33. doi: 10.1113/jphysiol.1985.sp015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. J Physiol. 1984 Jun;351:343–361. doi: 10.1113/jphysiol.1984.sp015249. [DOI] [PMC free article] [PubMed] [Google Scholar]


