Abstract
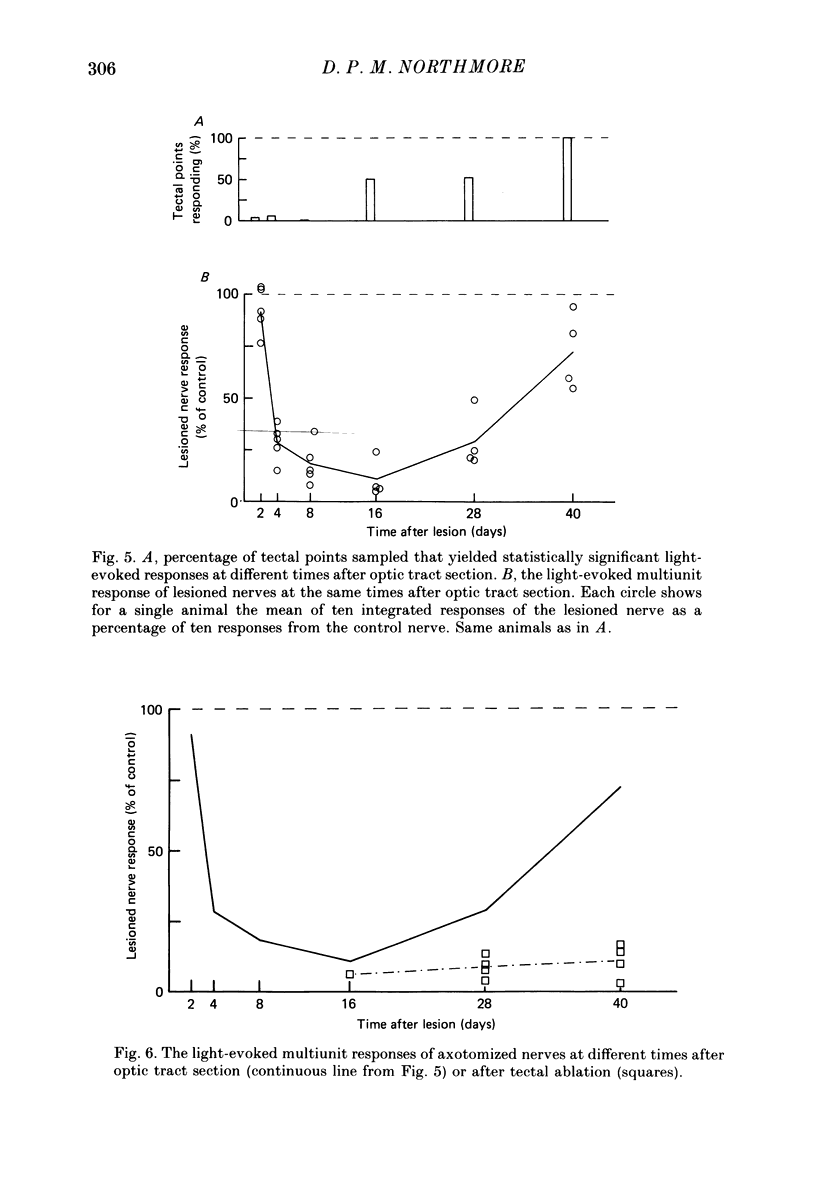
1. Retinal ganglion cells of one eye were axotomized in goldfish either by sectioning the contralateral optic tract or by ablating the contralateral lobe of the optic tectum. Between 2 and 40 days later, multiunit activity in response to diffuse light flashes was recorded from the axotomized and normal optic nerves, and from the optic tectum. 2. Two days after tract section, the amplitude of the integrated multiunit response of the axotomized nerve was normal. By 16 days it had fallen to 15% of control values, at which time visual responses carried by the regenerating tract were first recorded in tectum. Activity in the axotomized nerve then recovered gradually. 3. After ablation of one tectal lobe, multiunit responses in the axotomized nerve had not recovered by 40 days. 4. Integrated spontaneous activity in the axotomized nerve was depressed with a similar time course to the depression of light-evoked activity, both after tract section and tectal ablation. 5. Retinal ganglion cell nuclear size, a morphological indicator of the cell body reaction, varied inversely with evoked activity, whether axotomy was by tract section or by tectal ablation. 6. Electrically evoked compound action potentials of normal amplitude could be recorded from an axotomized nerve despite depressed responses to light flashes. 7. It is concluded that optic nerve axotomy in goldfish reduces the number of optic fibres carrying impulses and/or the frequency of their discharge. The effect is closely linked to morphological changes occurring in the retinal ganglion cell bodies. Recovery of impulse activity and morphology depends upon the regenerating optic fibres innervating an appropriate target.
Full text
PDF

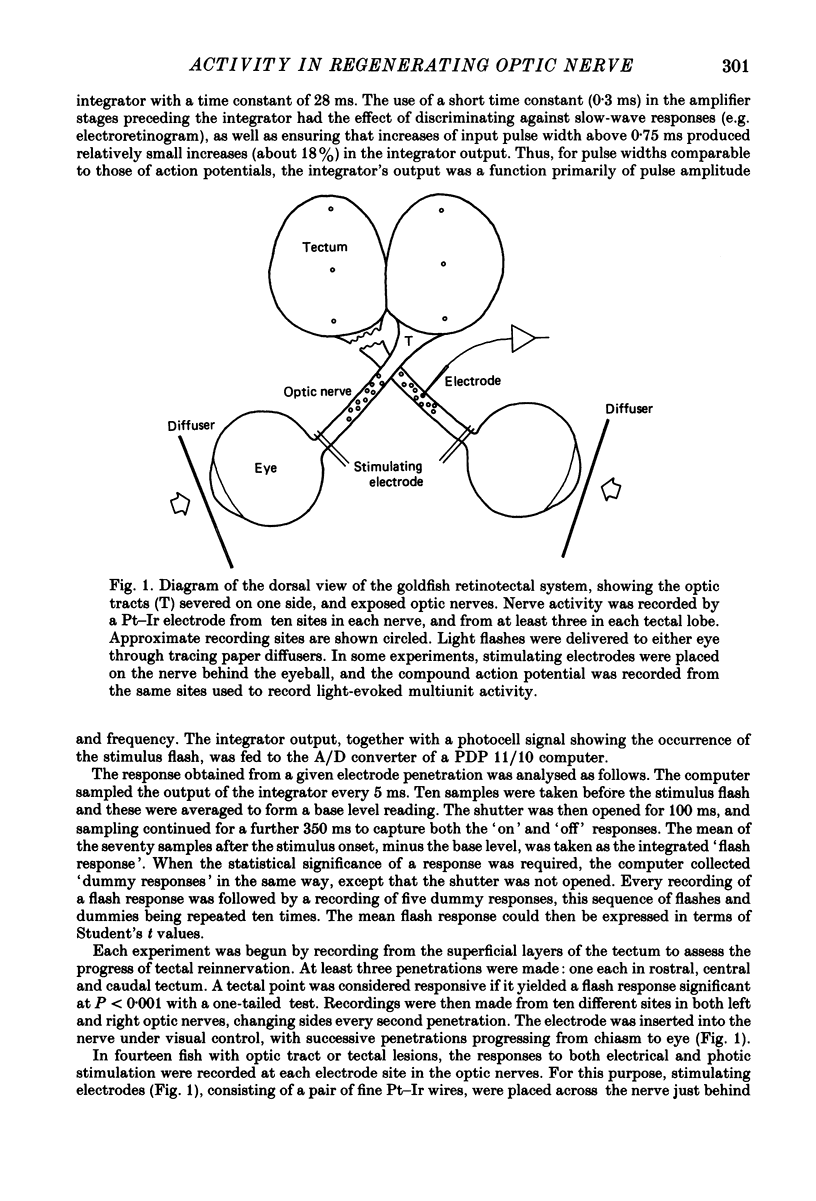

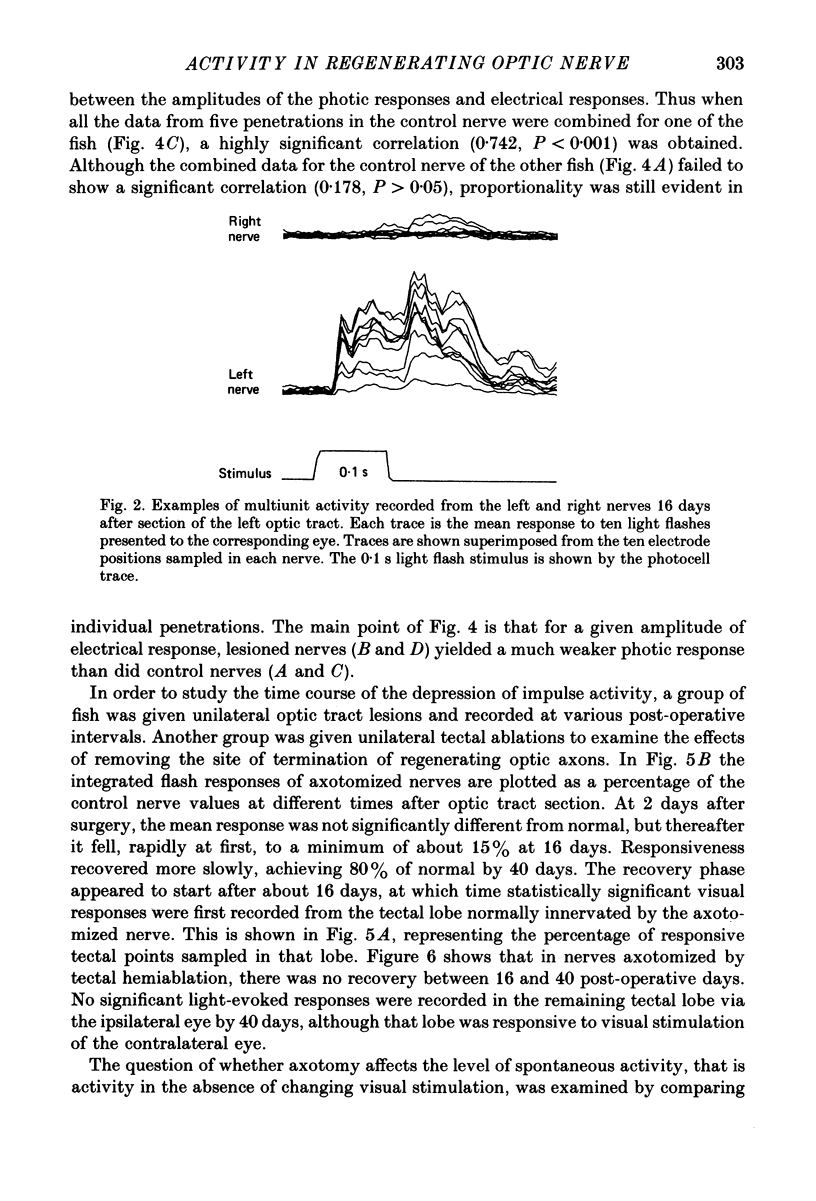
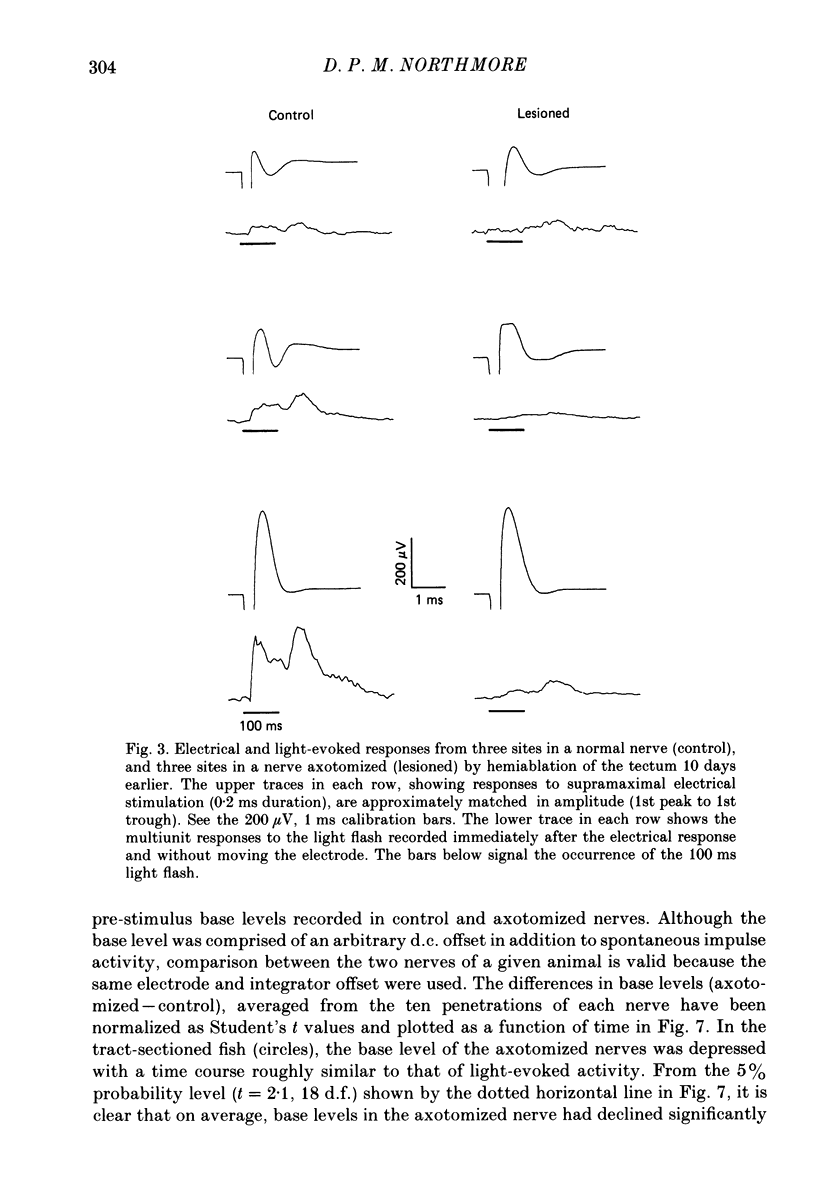
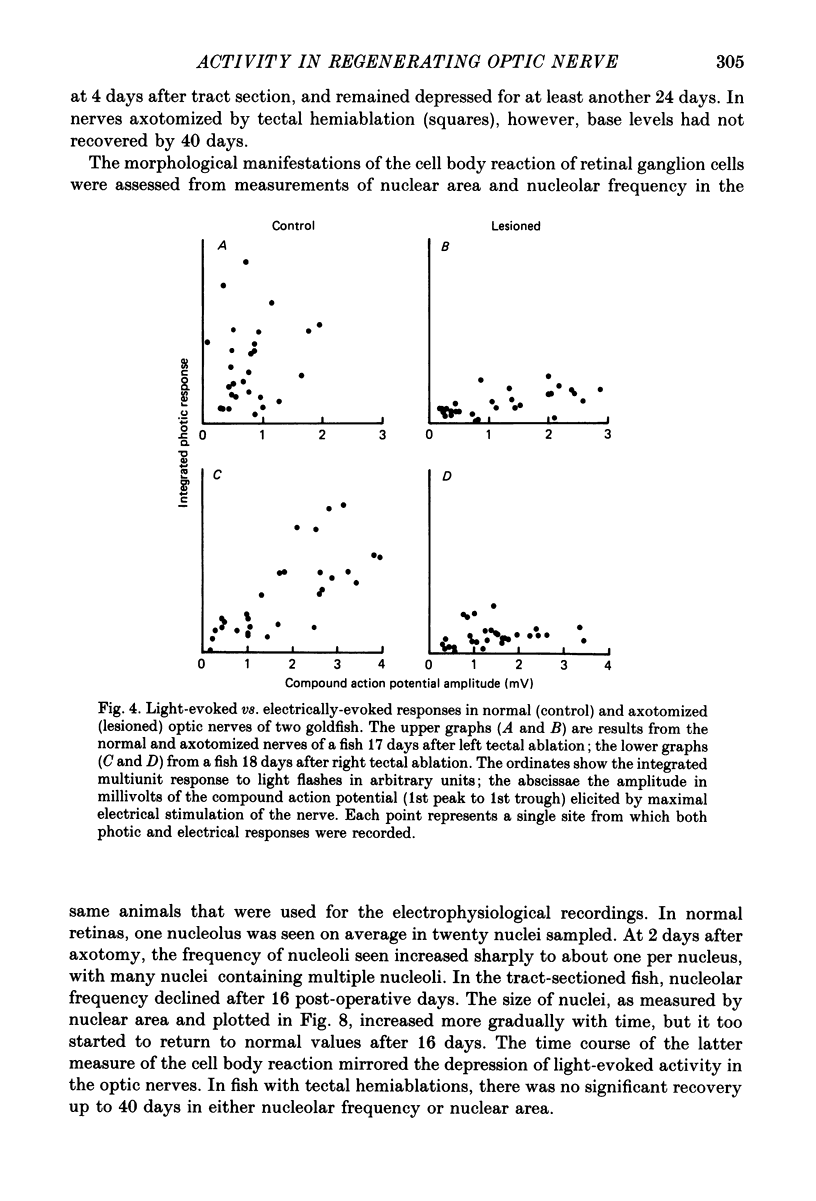
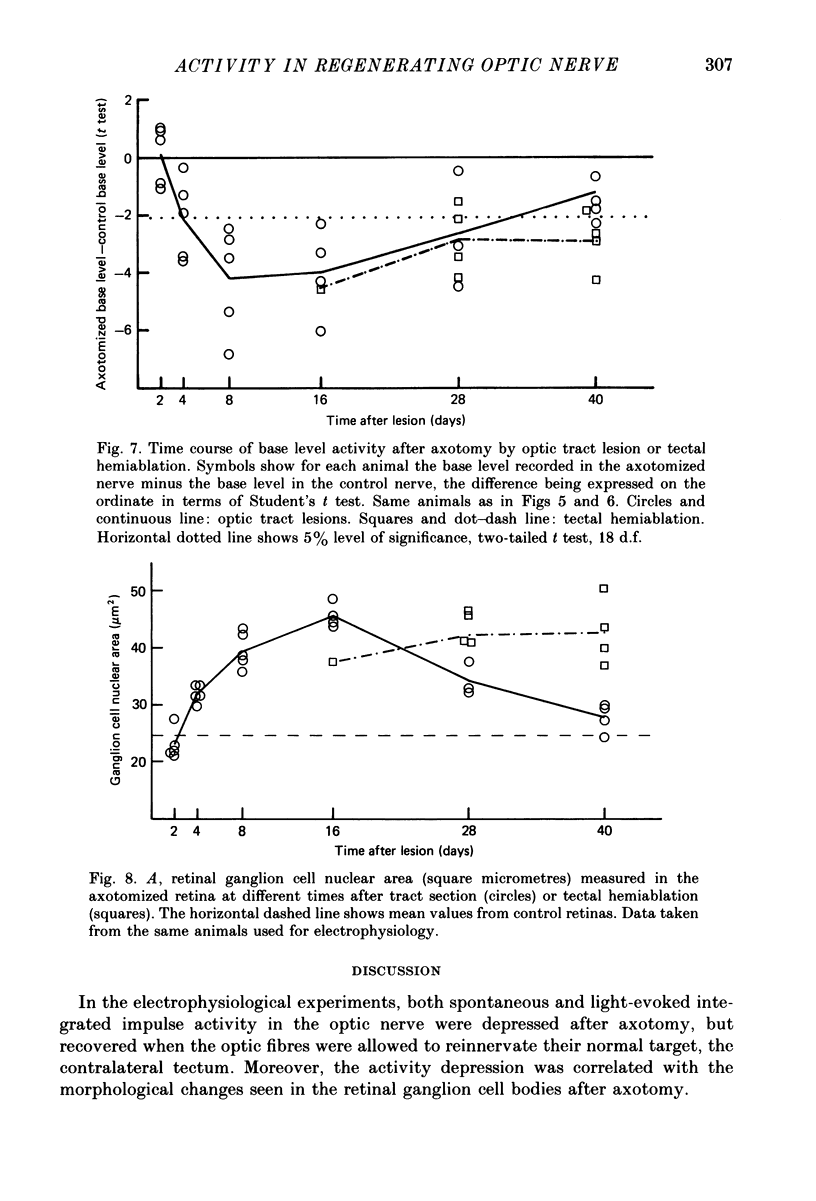





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blinzinger K., Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85(2):145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Brenner H. R., Martin A. R. Reduction in acetylcholine sensitivity of axotomized ciliary ganglion cells. J Physiol. 1976 Aug;260(1):159–175. doi: 10.1113/jphysiol.1976.sp011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister D. W., Grafstein B. Removal of optic tectum prolongs the cell body reaction to axotomy in goldfish retinal ganglion cells. Brain Res. 1985 Feb 18;327(1-2):45–51. doi: 10.1016/0006-8993(85)91497-0. [DOI] [PubMed] [Google Scholar]
- Cohan C. S., Kater S. B. Suppression of neurite elongation and growth cone motility by electrical activity. Science. 1986 Jun 27;232(4758):1638–1640. doi: 10.1126/science.3715470. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M., Law M. I. The development of maps and stripes in the brain. Sci Am. 1982 Dec;247(6):62–70. doi: 10.1038/scientificamerican1282-62. [DOI] [PubMed] [Google Scholar]
- Cook J. E., Rankin E. C. Impaired refinement of the regenerated retinotectal projection of the goldfish in stroboscopic light: a quantitative WGA-HRP study. Exp Brain Res. 1986;63(2):421–430. doi: 10.1007/BF00236861. [DOI] [PubMed] [Google Scholar]
- Davis R. E., Schlumpf B. E. Visual recovery in goldfish following unilateral optic tectum ablation: evidence of competition between optic axons for tectal targets. Behav Brain Res. 1984 Sep;13(3):287–291. doi: 10.1016/0166-4328(84)90172-4. [DOI] [PubMed] [Google Scholar]
- Edwards D. L., Grafstein B. Intraocular injection of tetrodotoxin in goldfish decreases fast axonal transport of [3H]glucosamine-labeled materials in optic axons. Brain Res. 1984 May 7;299(1):190–194. doi: 10.1016/0006-8993(84)90807-2. [DOI] [PubMed] [Google Scholar]
- Edwards D. L., Grafstein B. Intraocular tetrodotoxin in goldfish hinders optic nerve regeneration. Brain Res. 1983 Jun 13;269(1):1–14. doi: 10.1016/0006-8993(83)90957-5. [DOI] [PubMed] [Google Scholar]
- Edwards D. L., Grafstein B. Intraocular tetrodotoxin reduces axonal transport and transcellular transfer of adenosine and other nucleosides in the visual system of goldfish. Brain Res. 1986 Feb 5;364(2):258–267. doi: 10.1016/0006-8993(86)90838-3. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Tani N., Watanabe K., Ibata Y. Branching of regenerating retinal axons and preferential selection of appropriate branches for specific neuronal connection in the newt. Dev Biol. 1982 Mar;90(1):43–57. doi: 10.1016/0012-1606(82)90210-x. [DOI] [PubMed] [Google Scholar]
- Harris W. A. Axonal pathfinding in the absence of normal pathways and impulse activity. J Neurosci. 1984 Apr;4(4):1153–1162. doi: 10.1523/JNEUROSCI.04-04-01153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. F., Easter S. S., Jr Retinal ganglion cells in goldfish: a qualitative classification into four morphological types, and a quantitative study of the development of one of them. J Neurosci. 1986 Apr;6(4):1037–1050. doi: 10.1523/JNEUROSCI.06-04-01037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock J. H., Reuter T. Retinal ganglion cells in the crucian carp (Carassius carassius). I. Size and number of somata in eyes of different size. J Comp Neurol. 1978 Jun 1;179(3):535–547. doi: 10.1002/cne.901790306. [DOI] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Differential reaction of fast and slow alpha-motoneurones to axotomy. J Physiol. 1974 Aug;240(3):725–739. doi: 10.1113/jphysiol.1974.sp010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. L., Jacobson M. Discontinuous mapping of retina onto tectum innervated by both eyes. Brain Res. 1975 Nov 7;98(1):172–176. doi: 10.1016/0006-8993(75)90517-x. [DOI] [PubMed] [Google Scholar]
- Lo R. Y., Levine R. L. Time course and pattern of optic fiber regeneration following tectal lobe removal in the goldfish. J Comp Neurol. 1980 May 15;191(2):295–314. doi: 10.1002/cne.901910210. [DOI] [PubMed] [Google Scholar]
- McQuarrie I. G., Grafstein B. Protein synthesis and fast axonal transport in regenerating goldfish retinal ganglion cells. Brain Res. 1982 Mar 11;235(2):213–223. doi: 10.1016/0006-8993(82)91001-0. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Munson J. B., Scott J. G. Alterations of synapses on axotomized motoneurones. J Physiol. 1976 Feb;255(1):67–79. doi: 10.1113/jphysiol.1976.sp011270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. L. Tetrodotoxin inhibits the formation of refined retinotopography in goldfish. Brain Res. 1983 Feb;282(3):293–298. doi: 10.1016/0165-3806(83)90068-8. [DOI] [PubMed] [Google Scholar]
- Murray M. A quantitative study of regenerative sprouting by optic axons in goldfish. J Comp Neurol. 1982 Aug 20;209(4):352–362. doi: 10.1002/cne.902090405. [DOI] [PubMed] [Google Scholar]
- Murray M., Grafstein B. Changes in the morphology and amino acid incorporation of regenerating goldfish optic neurons. Exp Neurol. 1969 Apr;23(4):544–560. doi: 10.1016/0014-4886(69)90124-1. [DOI] [PubMed] [Google Scholar]
- Murray M. Regeneration of retinal axons into the goldfish optic tectum. J Comp Neurol. 1976 Jul 15;168(2):175–195. doi: 10.1002/cne.901680202. [DOI] [PubMed] [Google Scholar]
- Northmore D. P., Masino T. Recovery of vision in fish after optic nerve crush: a behavioral and electrophysiological study. Exp Neurol. 1984 Apr;84(1):109–125. doi: 10.1016/0014-4886(84)90009-8. [DOI] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh T. A., Constantine-Paton M. Eye-specific segregation requires neural activity in three-eyed Rana pipiens. J Neurosci. 1985 May;5(5):1132–1143. doi: 10.1523/JNEUROSCI.05-05-01132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. A., Joh T. H., Reis D. J. Reversible changes in the accumulation and activities of tyrosine hydroxylase and dopamine-beta-hydroxylase in neurons of nucleus locus coeruleus during the retrograde reaction. Brain Res. 1975 Jul 4;92(1):57–72. doi: 10.1016/0006-8993(75)90527-2. [DOI] [PubMed] [Google Scholar]
- Rotter A., Birdsall N. J., Burgen A. S., Field P. M., Smolen A., Raisman G. Muscarinic receptors in the central nervous system of the rat. IV. A comparison of the effects of axotomy and deafferentation on the binding of [3H]propylbenzilylcholine mustard and associated synaptic changes in the hypoglossal and pontine nuclei. Brain Res. 1979;180(2):207–224. doi: 10.1016/0165-0173(79)90005-5. [DOI] [PubMed] [Google Scholar]
- Schmidt J. T., Edwards D. L. Activity sharpens the map during the regeneration of the retinotectal projection in goldfish. Brain Res. 1983 Jun 13;269(1):29–39. doi: 10.1016/0006-8993(83)90959-9. [DOI] [PubMed] [Google Scholar]
- Schmidt J. T., Edwards D. L., Stuermer C. The re-establishment of synaptic transmission by regenerating optic axons in goldfish: time course and effects of blocking activity by intraocular injection of tetrodotoxin. Brain Res. 1983 Jun 13;269(1):15–27. doi: 10.1016/0006-8993(83)90958-7. [DOI] [PubMed] [Google Scholar]
- Schmidt J. T., Eisele L. E. Stroboscopic illumination and dark rearing block the sharpening of the regenerated retinotectal map in goldfish. Neuroscience. 1985 Feb;14(2):535–546. doi: 10.1016/0306-4522(85)90308-2. [DOI] [PubMed] [Google Scholar]
- Schmidt J. T. Selective stabilization of retinotectal synapses by an activity-dependent mechanism. Fed Proc. 1985 Sep;44(12):2767–2772. [PubMed] [Google Scholar]
- Sharma S. C. Anomalous retinal projection after removal of contralateral optic tectum in adult goldfish. Exp Neurol. 1973 Dec;41(3):661–669. doi: 10.1016/0014-4886(73)90058-7. [DOI] [PubMed] [Google Scholar]
- Springer A. D., Cohen S. M. Optic fiber segregation in goldfish with two eyes innervating one tectal lobe. Brain Res. 1981 Nov 23;225(1):23–36. doi: 10.1016/0006-8993(81)90315-2. [DOI] [PubMed] [Google Scholar]
- Springer A. D., Gaffney J. S. Retinal projections in the goldfish: a study using cobaltous-lysine. J Comp Neurol. 1981 Dec 10;203(3):401–424. doi: 10.1002/cne.902030306. [DOI] [PubMed] [Google Scholar]
- Stuermer C. A., Easter S. S., Jr Rules of order in the retinotectal fascicles of goldfish. J Neurosci. 1984 Apr;4(4):1045–1051. doi: 10.1523/JNEUROSCI.04-04-01045.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner B. E. A quantitative study of subsurface cisterns and their relationships in normal and axotomized hypoglossal neurones. Exp Brain Res. 1975;22(2):175–183. doi: 10.1007/BF00237687. [DOI] [PubMed] [Google Scholar]
- Sumner B. E., Watson W. E. Retraction and expansion of the dendritic tree of motor neurones of adult rats induced in vivo. Nature. 1971 Sep 24;233(5317):273–275. doi: 10.1038/233273a0. [DOI] [PubMed] [Google Scholar]
- Yip H. K., Grafstein B. Effect of nerve growth factor on regeneration of goldfish optic axons. Brain Res. 1982 Apr 29;238(2):329–339. doi: 10.1016/0006-8993(82)90108-1. [DOI] [PubMed] [Google Scholar]


