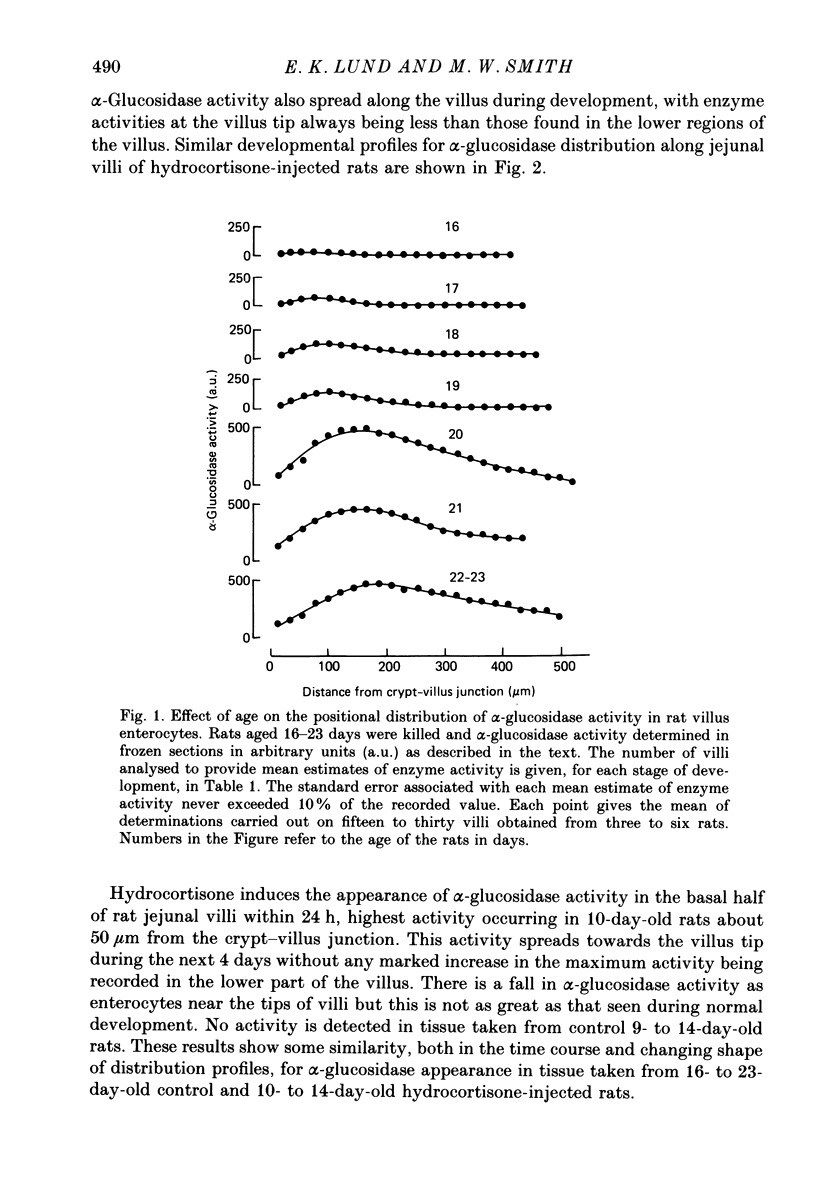
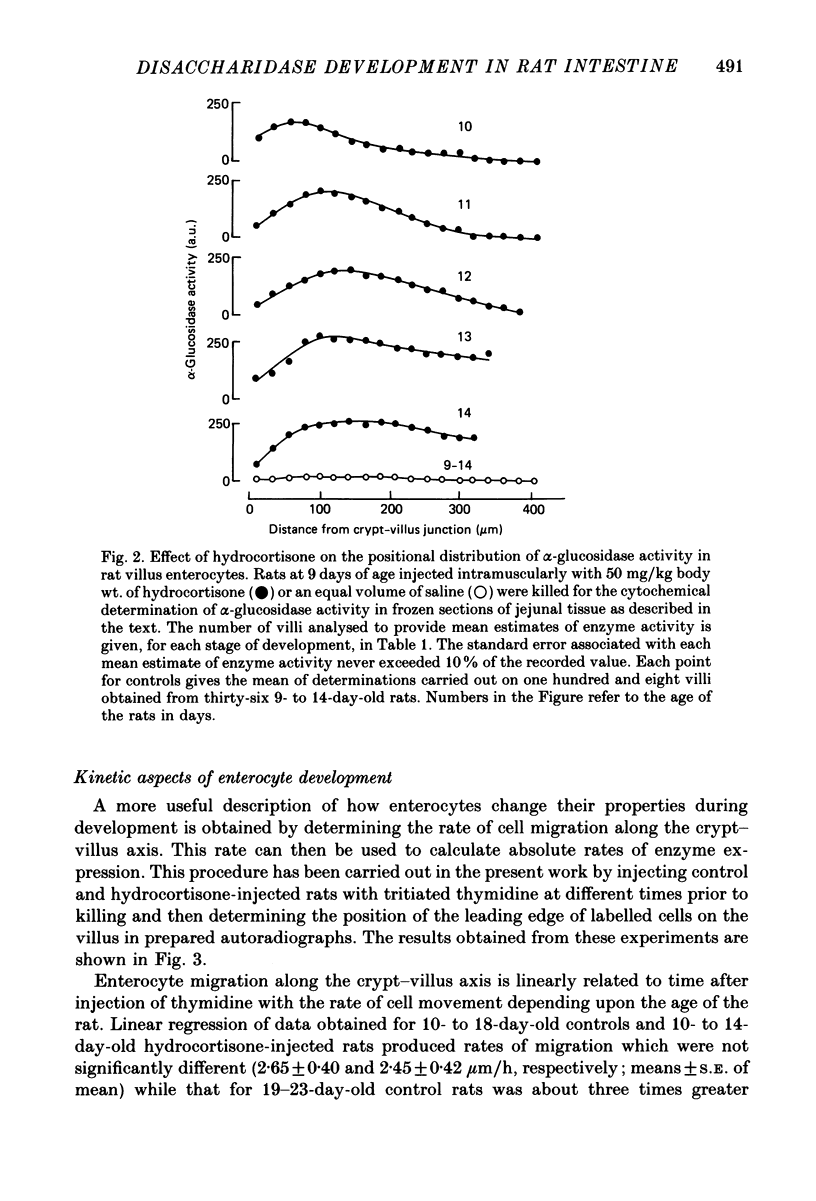
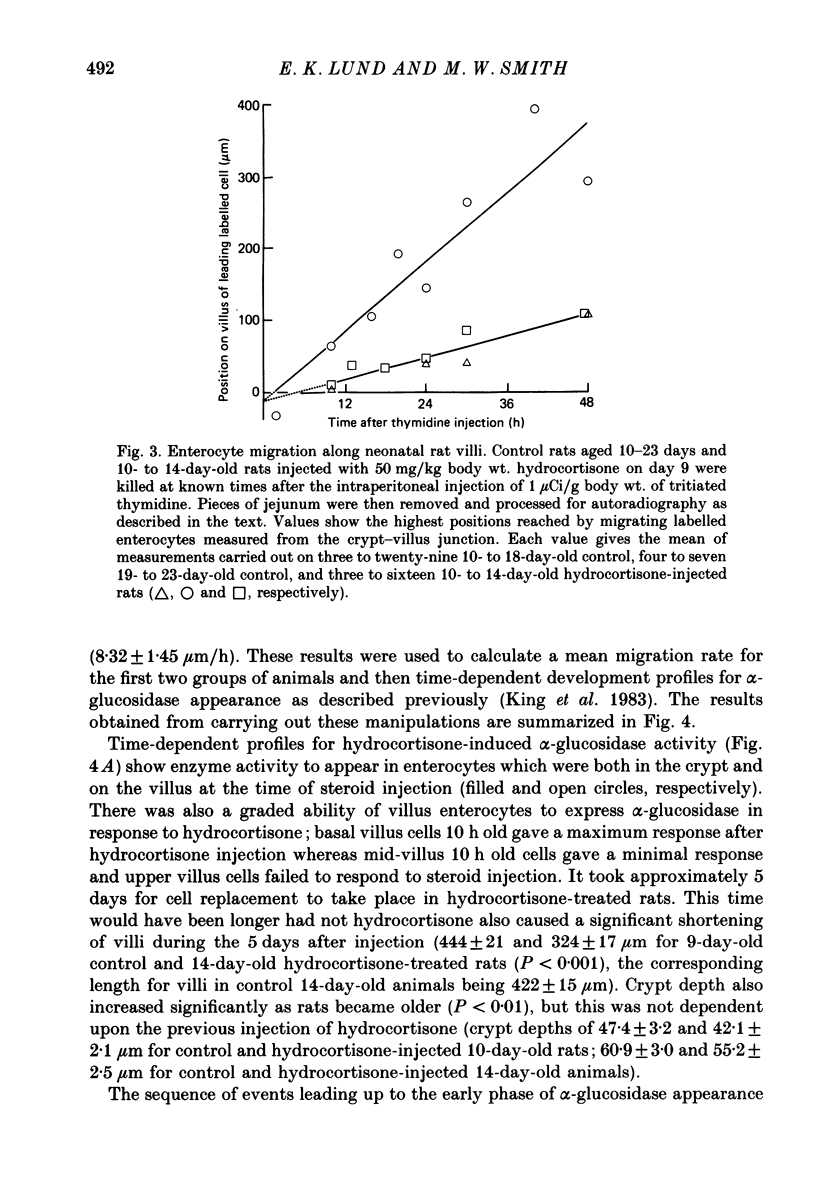
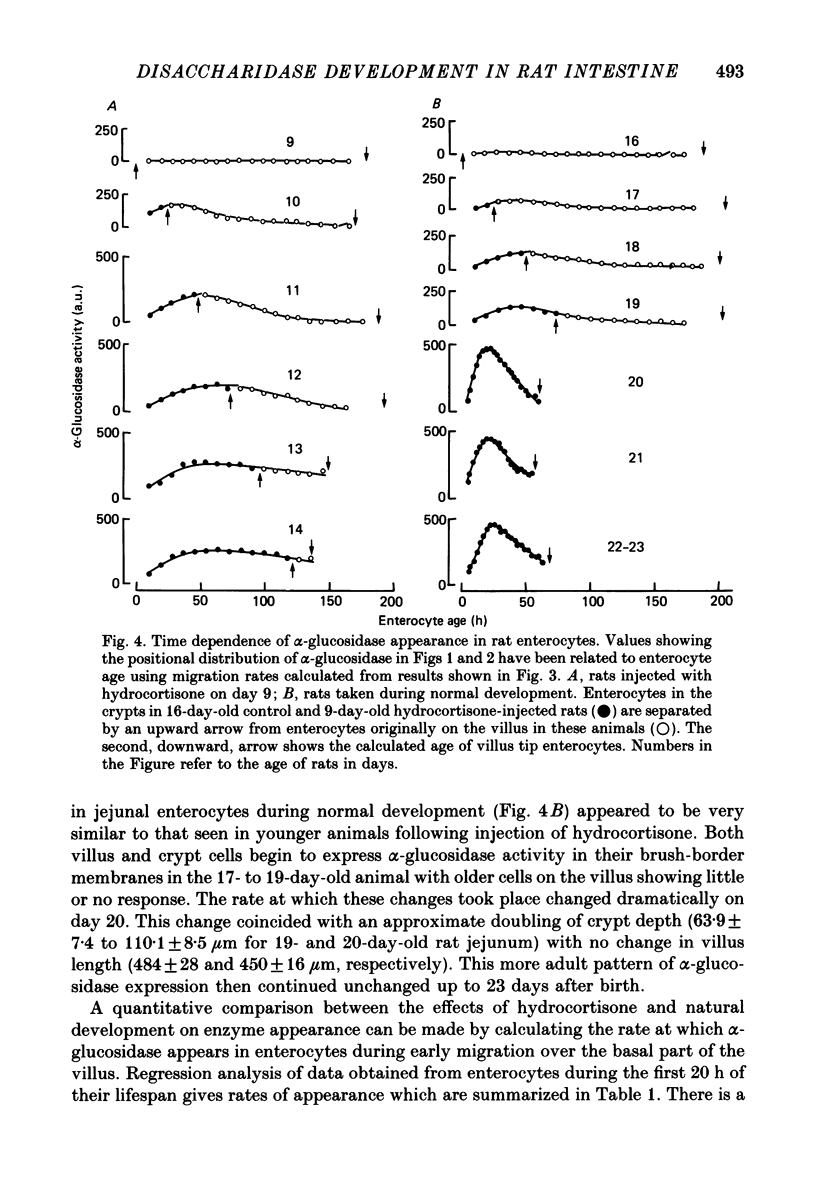
Abstract
1. Injection of hydrocortisone into 9-day-old rats induces the early appearance of sucrase in jejunal homogenates, the time course of the subsequent increase and magnitude of the final effect being similar to that seen to start on day 16 during normal development. 2. Cytochemical comparison of the effect of hydrocortisone and normal development on the appearance of a mixture of sucrase, maltase, isomaltase and trehalase disaccharidases (alpha-glucosidase activity) shows this enzyme to appear first in enterocytes at the base of the villus. Enzyme activity then increases and spreads along the whole villus during the next 96 h. 3. The rate at which enterocytes migrate along the villus after hydrocortisone injection is not significantly different from that measured during the early phase of normal development. The later phase of normal development is associated with a threefold increase in cell migration rate and a twofold increase in crypt depth. 4. The rate at which alpha-glucosidase activity increases in enterocytes at the base of the villus during early normal development is similar to that determined after hydrocortisone injection into younger animals. This rate of appearance increases eight to tenfold during normal development, shortly after the appearance of solid food in the stomach of normal control animals. 5. Injection of steroid hormones into young rats is generally supposed to mimic events taking place normally at weaning. Present results show alpha-glucosidase induction during normal development to be under more complicated control than had been previously suspected.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOELL R. G., KRETCHMER N. INTESTINAL INVERTASE: PRECOCIOUS DEVELOPMENT OF ACTIVITY AFTER INJECTION OF HYDROCORTISONE. Science. 1964 Jan 3;143(3601):42–44. doi: 10.1126/science.143.3601.42. [DOI] [PubMed] [Google Scholar]
- Doell R. G., Rosen G., Kretchmer N. Immunochemical studies of intestinal disaccharidases during normal and precocious development. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1268–1273. doi: 10.1073/pnas.54.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Gerskowitch V. P., Russell R. I. Pre- and postweaning disaccharidase patterns in isografts of fetal mouse intestine. Gastroenterology. 1973 Feb;64(2):292–297. [PubMed] [Google Scholar]
- Galand G., Forstner G. G. Isolation of microvillus plasma membranes from suckling-rat intestine. The influence of premature induction of digestive enzymes by injection of cortisol acetate. Biochem J. 1974 Nov;144(2):293–302. doi: 10.1042/bj1440293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschmidt S., Kaul W., Riecken E. O. A quantitative histochemical technique for the characterisation of alpha-glucosidases in the brush-border membrane of rat jejunum. Histochemistry. 1979 Sep;63(1):81–101. doi: 10.1007/BF00508014. [DOI] [PubMed] [Google Scholar]
- Henning S. J., Helman T. A., Kretchmer N. Studies on normal and precocious appearance of jejunal sucrase in suckling rats. Biol Neonate. 1975;26(3-4):249–262. doi: 10.1159/000240736. [DOI] [PubMed] [Google Scholar]
- Henning S. J. Ontogeny of enzymes in the small intestine. Annu Rev Physiol. 1985;47:231–245. doi: 10.1146/annurev.ph.47.030185.001311. [DOI] [PubMed] [Google Scholar]
- Henning S. J. Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol. 1981 Sep;241(3):G199–G214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- Herbst J. J., Koldovský O. Cell migration and cortisone induction of sucrase activity in jejunum and ileum. Biochem J. 1972 Feb;126(3):471–476. doi: 10.1042/bj1260471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J. E., Smith M. W. Thyroid hormone effects on lactase expression by rat enterocytes. J Physiol. 1986 Jul;376:253–265. doi: 10.1113/jphysiol.1986.sp016152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall K., Jumawan J., Koldovský O. Development of jejunoileal differences of activity of lactase, sucrase and acid beta-galactosidase in isografts of fetal rat intestine. Biol Neonate. 1979;36(3-4):206–214. doi: 10.1159/000241229. [DOI] [PubMed] [Google Scholar]
- King I. S., Paterson J. Y., Peacock M. A., Smith M. W., Syme G. Effect of diet upon enterocyte differentiation in the rat jejunum. J Physiol. 1983 Nov;344:465–481. doi: 10.1113/jphysiol.1983.sp014952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E. K., Bruce M. G., Smith M. W., Ferguson A. Selective effects of graft-versus-host reaction on disaccharidase expression by mouse jejunal enterocytes. Clin Sci (Lond) 1986 Aug;71(2):189–198. doi: 10.1042/cs0710189. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Montgomery R. K., Sybicki M. A., Grand R. J. Autonomous biochemical and morphological differentiation in fetal rat intestine transplanted at 17 and 20 days of gestation. Dev Biol. 1981 Oct 15;87(1):76–84. doi: 10.1016/0012-1606(81)90062-2. [DOI] [PubMed] [Google Scholar]
- RUBINO A., ZIMBALATTI F., AURICCHIO S. INTESTINAL DISACCHARIDASE ACTIVITIES IN ADULT AND SUCKLING RATS. Biochim Biophys Acta. 1964 Nov 22;92:305–311. doi: 10.1016/0926-6569(64)90187-7. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Wostmann B. S. Intestinal disaccharidase activities in the growing germfree and conventional rats. Arch Biochem Biophys. 1966 Mar;113(3):609–616. doi: 10.1016/0003-9861(66)90238-4. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Bruns M. E., Lawson E. D. Identification of intestinal cells responsive to calcitriol (1,25-dihydroxycholecalciferol). Biochem J. 1985 Jan 1;225(1):127–133. doi: 10.1042/bj2250127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Jarvis L. G., King I. S. Cell proliferation in follicle-associated epithelium of mouse Peyer's patch. Am J Anat. 1980 Oct;159(2):157–166. doi: 10.1002/aja.1001590204. [DOI] [PubMed] [Google Scholar]
- Yeh K. Y., Holt P. R. Ontogenic timing mechanism initiates the expression of rat intestinal sucrase activity. Gastroenterology. 1986 Mar;90(3):520–526. doi: 10.1016/0016-5085(86)91103-0. [DOI] [PubMed] [Google Scholar]


