Abstract
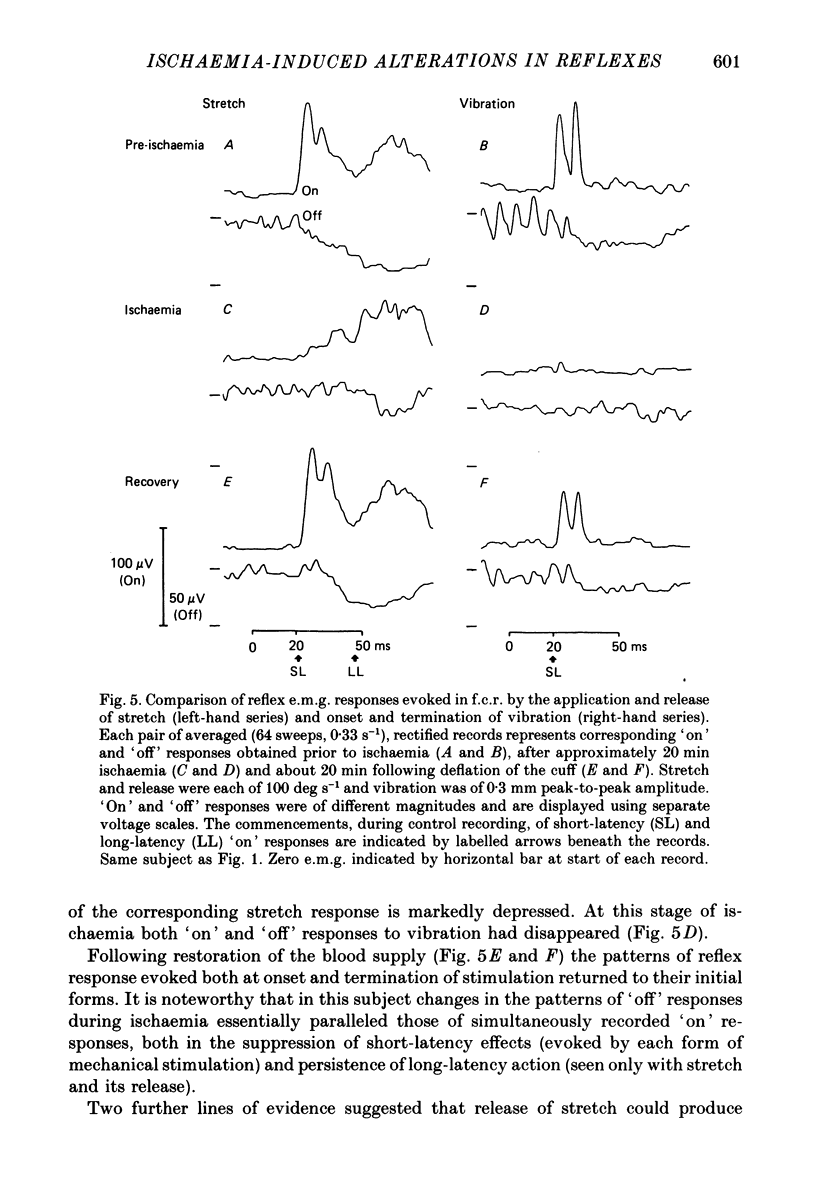
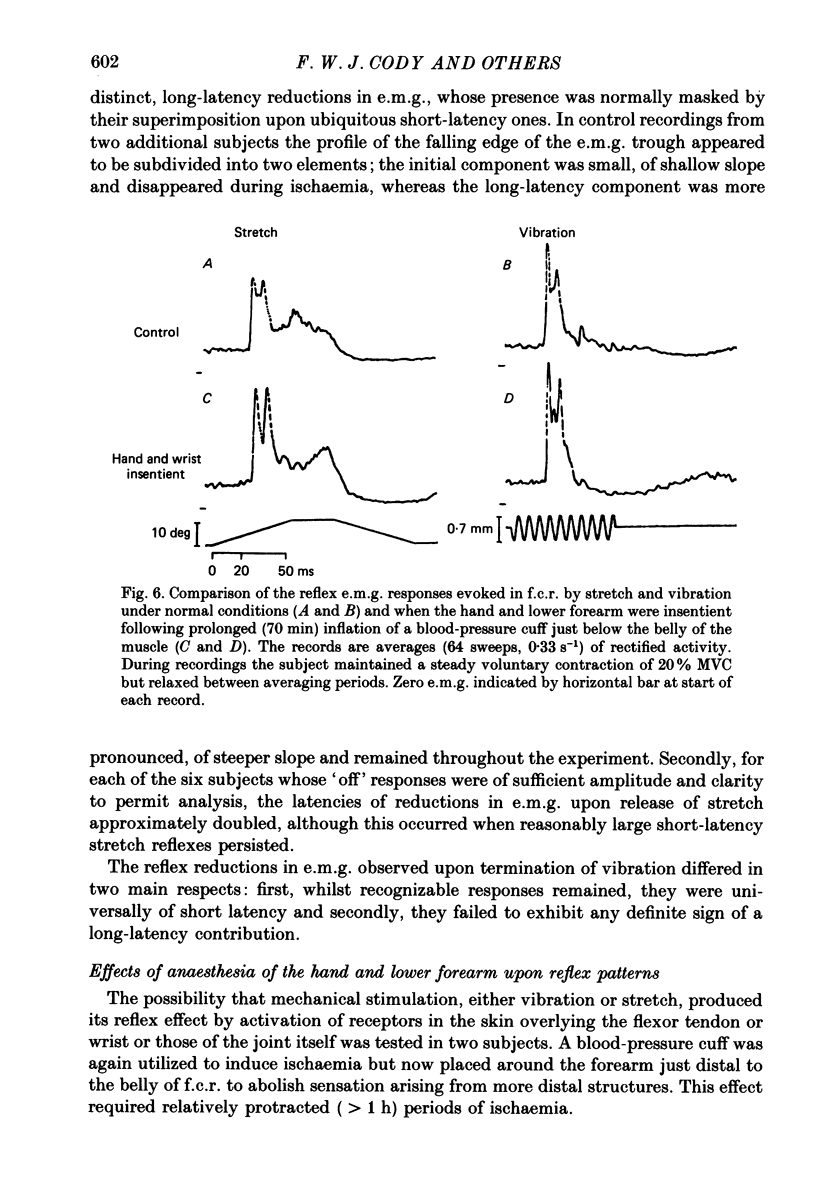
1. The reflex electromyographic responses evoked in a wrist flexor muscle, flexor carpi radialis (f.c.r.), by forcible extension of the wrist ('stretch') and by vibration of the flexor tendon have been studied in normal subjects. Reflexes were elicited during the maintenance of a low level of voluntary flexor contraction (5% maximum). Stretch regularly produced a relatively prolonged (ca. 100 ms duration) increase in e.m.g. activity which was usually divisible into short-latency (ca. 25 ms, M1) and long-latency (ca. 50 ms, M2) peaks. Vibration produced a single, phasic peak, at short latency, with no sign of an accompanying long-latency wave comparable to the M2 stretch response. 2. Ischaemia was induced by inflation of a blood-pressure cuff around the upper arm and its effects upon the reflex patterns were studied. During ischaemia M1 stretch responses showed a more rapid and pronounced decline than did M2 responses and were abolished before voluntary power was appreciably affected. Vibration-evoked short-latency peaks changed in an essentially parallel manner to M1 stretch reflexes. During recovery from ischaemia M2 reflexes were restored before short-latency responses. 3. The patterns of reflex reductions in e.m.g. upon withdrawal of stimulation were also studied. Such troughs in activity, under non-ischaemic conditions, regularly commenced at short latency and were of relatively small amplitude. The records of several of the subjects, and particularly ones obtained during ischaemia, suggested that release of stretch (with concomitant stretch of antagonists) could elicit an additive, long-latency decline in e.m.g. The existence of any such separate, delayed component was never observed upon termination of vibration. 4. Measurements of changes in the latencies and durations of reflex components, accompanying the progression of ischaemia, indicated that depression of early reflex activity resulted in part from increases in the latencies of these initial peaks but predominantly reflected simultaneous and separate reductions in their amplitudes. 5. The generation of short-latency reflexes by stretch and vibration, both of which stimuli powerfully excite muscle spindle primary endings, and the marked susceptibility of these responses to ischaemia supports their being mediated by group Ia afferents. The contrasting behaviour of M2 stretch responses, both regarding their absence with vibration and their resistance to ischaemia, suggests that they depend crucially upon a separate group of reflex afferents.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bawa P., McKenzie D. C. Contribution of joint and cutaneous afferents to longer-latency reflexes in man. Brain Res. 1981 Apr 27;211(1):185–189. doi: 10.1016/0006-8993(81)90081-0. [DOI] [PubMed] [Google Scholar]
- Bentley F. H., Schlapp W. Experiments on the blood supply of nerves. J Physiol. 1943 Jun 30;102(1):62–71. doi: 10.1113/jphysiol.1943.sp004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley F. H., Schlapp W. The effects of pressure on conduction in peripheral nerve. J Physiol. 1943 Jun 30;102(1):72–82. doi: 10.1113/jphysiol.1943.sp004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976 Oct;261(3):695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B., Bawa P. Voluntary and reflexive recruitment of flexor carpi radialis motor units in humans. J Neurophysiol. 1985 May;53(5):1194–1200. doi: 10.1152/jn.1985.53.5.1194. [DOI] [PubMed] [Google Scholar]
- Chan C. W., Jones G. M., Catchlove R. F. The 'late' electromyographic response to limb displacement in man. II. Sensory origin. Electroencephalogr Clin Neurophysiol. 1979 Feb;46(2):182–188. doi: 10.1016/0013-4694(79)90067-1. [DOI] [PubMed] [Google Scholar]
- Cody F. W., MacDermott N., Matthews P. B., Richardson H. C. Observations on the genesis of the stretch reflex in Parkinson's disease. Brain. 1986 Apr;109(Pt 2):229–249. doi: 10.1093/brain/109.2.229. [DOI] [PubMed] [Google Scholar]
- Darton K., Lippold O. C., Shahani M., Shahani U. Long-latency spinal reflexes in humans. J Neurophysiol. 1985 Jun;53(6):1604–1618. doi: 10.1152/jn.1985.53.6.1604. [DOI] [PubMed] [Google Scholar]
- Deuschl G., Schenck E., Lücking C. H. Long-latency responses in human thenar muscles mediated by fast conducting muscle and cutaneous afferents. Neurosci Lett. 1985 Apr 19;55(3):361–366. doi: 10.1016/0304-3940(85)90462-8. [DOI] [PubMed] [Google Scholar]
- Eklund G., Hagbarth K. E., Hägglund J. V., Wallin E. U. The 'late' reflex responses to muscle stretch: the 'resonance hypothesis' versus the 'long-loop hypothesis'. J Physiol. 1982 May;326:79–90. doi: 10.1113/jphysiol.1982.sp014178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles J. F. Responses in human pretibial muscles to sudden stretch and to nerve stimulation. Exp Brain Res. 1977 Dec 19;30(4):451–470. doi: 10.1007/BF00237637. [DOI] [PubMed] [Google Scholar]
- Jaeger R. J., Gottlieb G. L., Agarwal G. C., Tahmoush A. J. Afferent contributions to stretch-evoked myoelectric responses. J Neurophysiol. 1982 Aug;48(2):403–418. doi: 10.1152/jn.1982.48.2.403. [DOI] [PubMed] [Google Scholar]
- Jenner J. R., Stephens J. A. Cutaneous reflex responses and their central nervous pathways studied in man. J Physiol. 1982 Dec;333:405–419. doi: 10.1113/jphysiol.1982.sp014461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson R. S., Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56(3):550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Loo C. K., McCloskey D. I. Effects of prior instruction and anaesthesia on long-latency responses to stretch in the long flexor of the human thumb. J Physiol. 1985 Aug;365:285–296. doi: 10.1113/jphysiol.1985.sp015772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in human voluntary movement. Nature. 1972 Jul 21;238(5360):140–143. doi: 10.1038/238140a0. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in the human thumb. J Physiol. 1976 May;257(1):1–44. doi: 10.1113/jphysiol.1976.sp011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. The sensory mechanism of servo action in human muscle. J Physiol. 1977 Feb;265(2):521–535. doi: 10.1113/jphysiol.1977.sp011728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. H. Nerve endings in mammalian muscle. J Physiol. 1933 Apr 13;78(1):1–53. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol. 1984 Mar;348:383–415. doi: 10.1113/jphysiol.1984.sp015116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Facilitation and depression of muscle stretch receptors by repetitive antidromic stimulation, adrenaline and asphyxia. J Physiol. 1959 Oct;148:252–266. doi: 10.1113/jphysiol.1959.sp006286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C. G. The Ferrier lecture, 1968. Motor apparatus of the baboon's hand. Proc R Soc Lond B Biol Sci. 1969 May 20;173(1031):141–174. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- Tatton W. G., Lee R. G. Evidence for abnormal long-loop reflexes in rigid Parkinsonian patients. Brain Res. 1975 Dec 26;100(3):671–676. doi: 10.1016/0006-8993(75)90167-5. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E., Hallin R. G. Perceptual changes accompanying controlled preferential blocking of A and C fibre responses in intact human skin nerves. Exp Brain Res. 1973 Jan 29;16(3):321–332. doi: 10.1007/BF00233334. [DOI] [PubMed] [Google Scholar]


