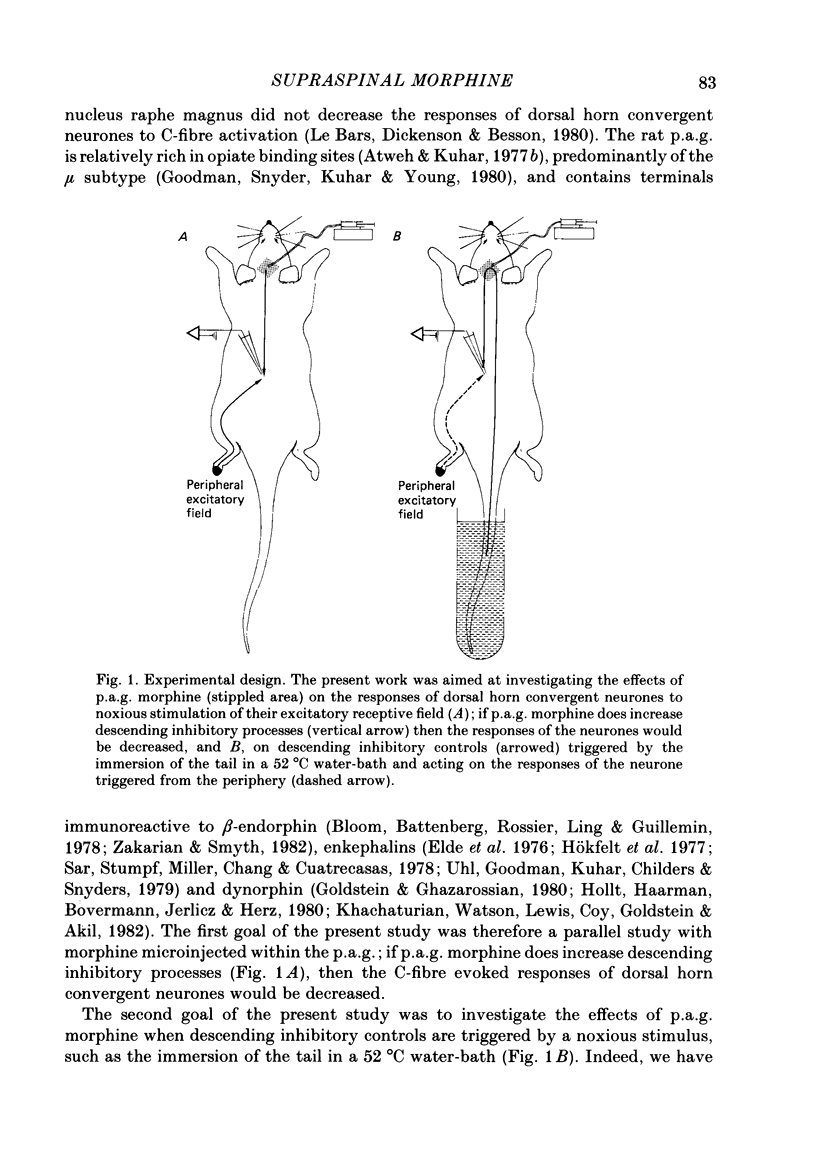
Abstract
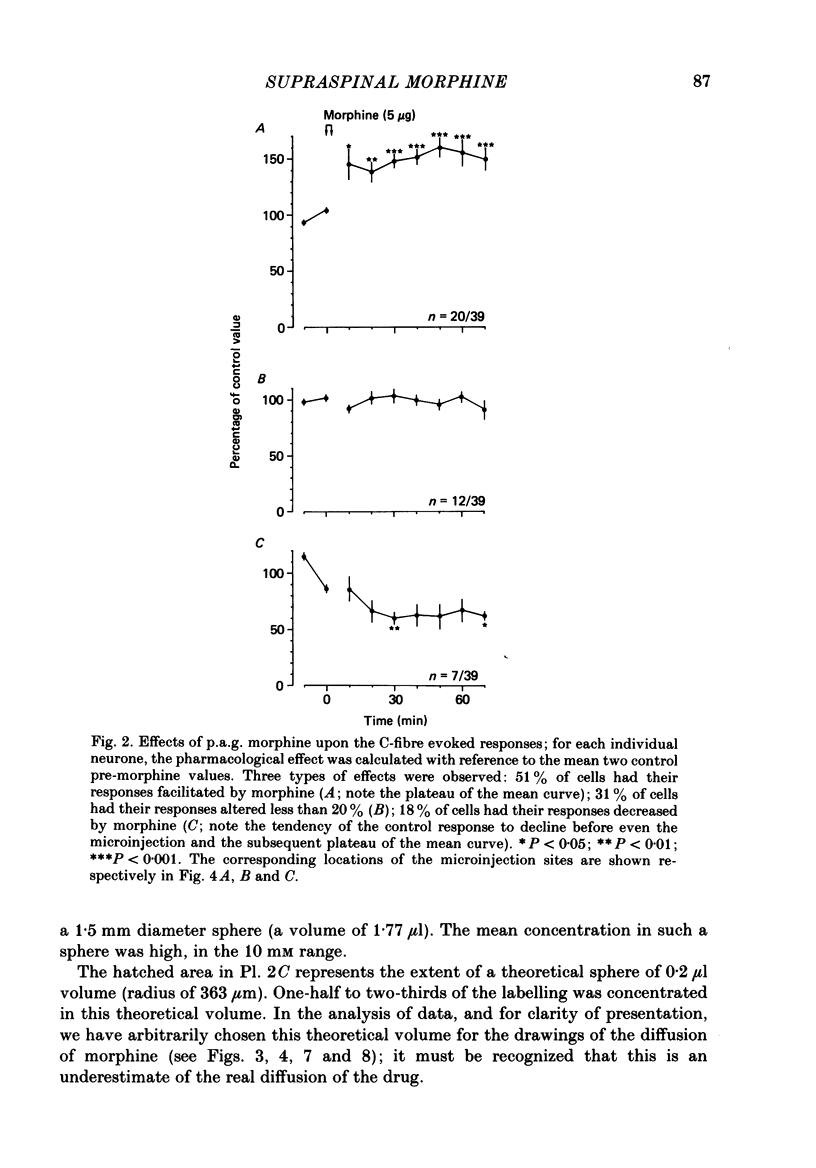
1. Recordings were made from thirty-nine convergent neurones in the lumbar enlargement of the rat spinal cord. These neurones were activated by both innocuous and noxious stimuli applied to their excitatory receptive fields located on the extremity of the ipsilateral hind paw. Transcutaneous application of suprathreshold 2 ms square-wave pulses to the centre of the receptive field resulted in responses to A- and C-fibre activation being observed; a mean of 18.8 +/- 1.8 C-fibre latency spikes was evoked per stimulus. This type of response was inhibited by applying noxious conditioning stimuli to heterotopic body areas; immersing the tail in a 52 degrees C water-bath caused a mean 54.5 +/- 2.3% inhibition of the C-fibre-evoked response; such inhibitory processes have been termed diffuse noxious inhibitory controls (d.n.i.c.). 2. The effects of microinjections of morphine (5 micrograms; 0.2 microliter) on both the unconditioned C-fibre-evoked response and inhibitory processes triggered from the tail were investigated in an attempt to answer two questions: (a) does morphine increase tonic descending inhibitory processes and (b) what are the effects of morphine on descending inhibitory processes triggered by noxious stimuli? 3. The predominant effect of periaqueductal grey matter (p.a.g.) morphine on the C-fibre-evoked responses was a facilitation: 51% of cells had their C-fibre-evoked responses increased by morphine (by roughly 50%); 31% of cells were not influenced while the remaining 18% of units were depressed; however the cells classified as depressed were only marginally so. No clear relationships were found either between the microinjection sites in the p.a.g. and their corresponding effects or between the number of C-fibre-spikes evoked in the control sequences and the subsequent effect of morphine. 4. While d.n.i.c. was not altered by morphine in 56% of cases, it was clearly reduced in the remaining cells. The effects were immediate but peaked at 40 min following the microinjection (a mean 77% reduction) and then returned towards control values. All but three of the corresponding microinjection sites were such as to include the medio-ventral p.a.g. including the nucleus raphé dorsalis. In contrast none of the cases where d.n.i.c. was unaltered included microinjection sites in this region. 5. No relationship was found between the changes in d.n.i.c. and the number of spikes evoked in the control sequences, or the changes in the C-fibre responses. 6. Autoradiographic controls using [3H]morphine showed a large diffusion of the drug within an area of about 0.75 mm around the tip of the cannula.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




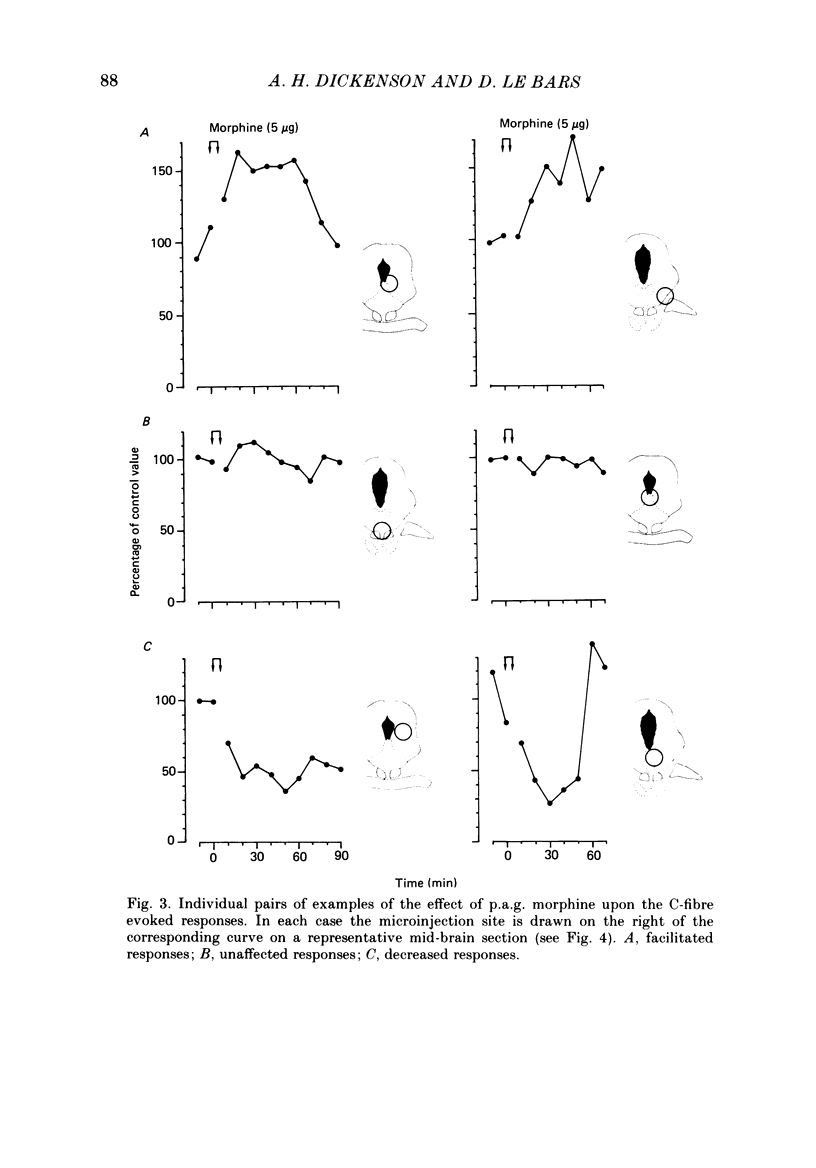

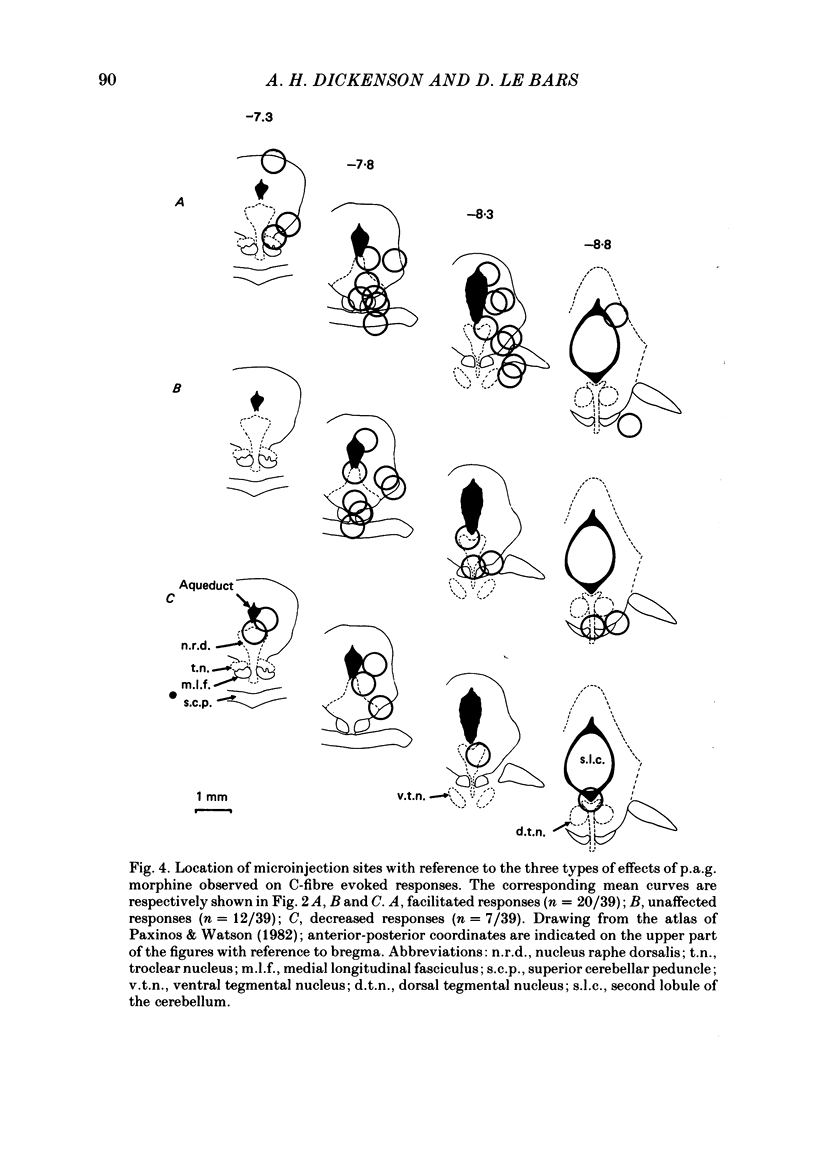
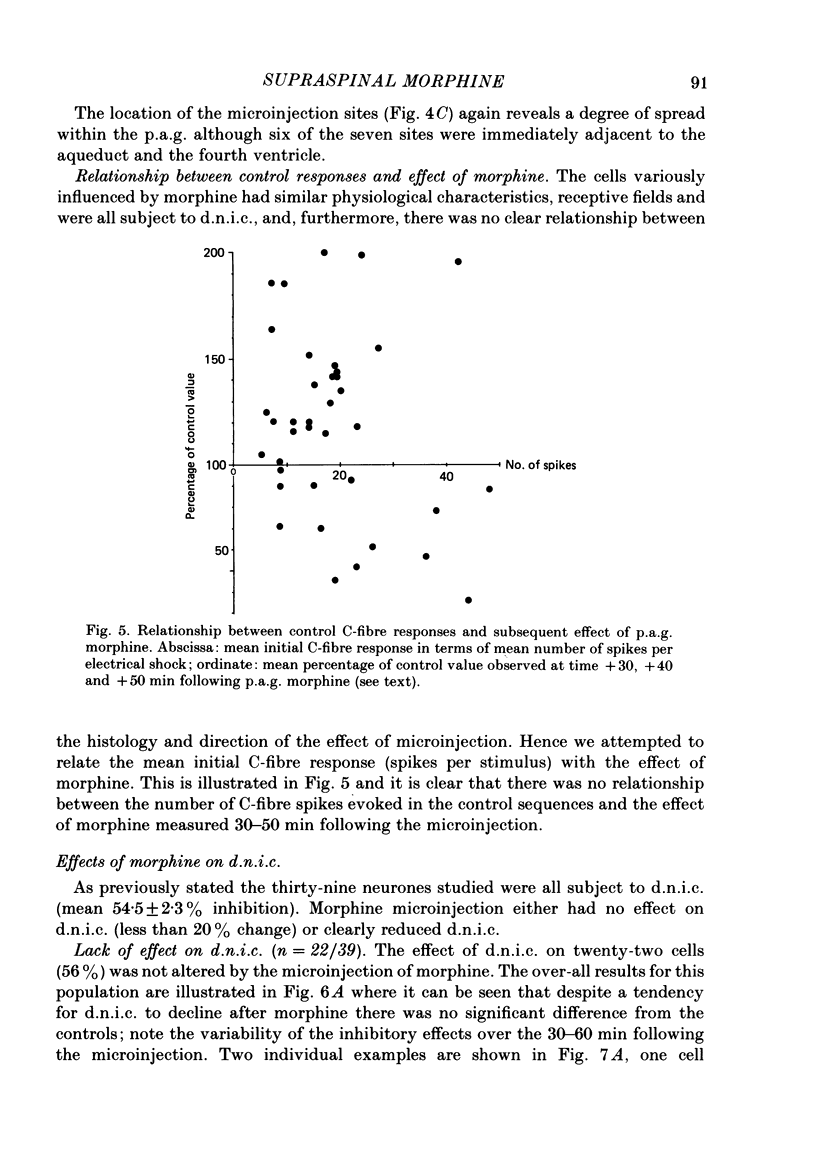
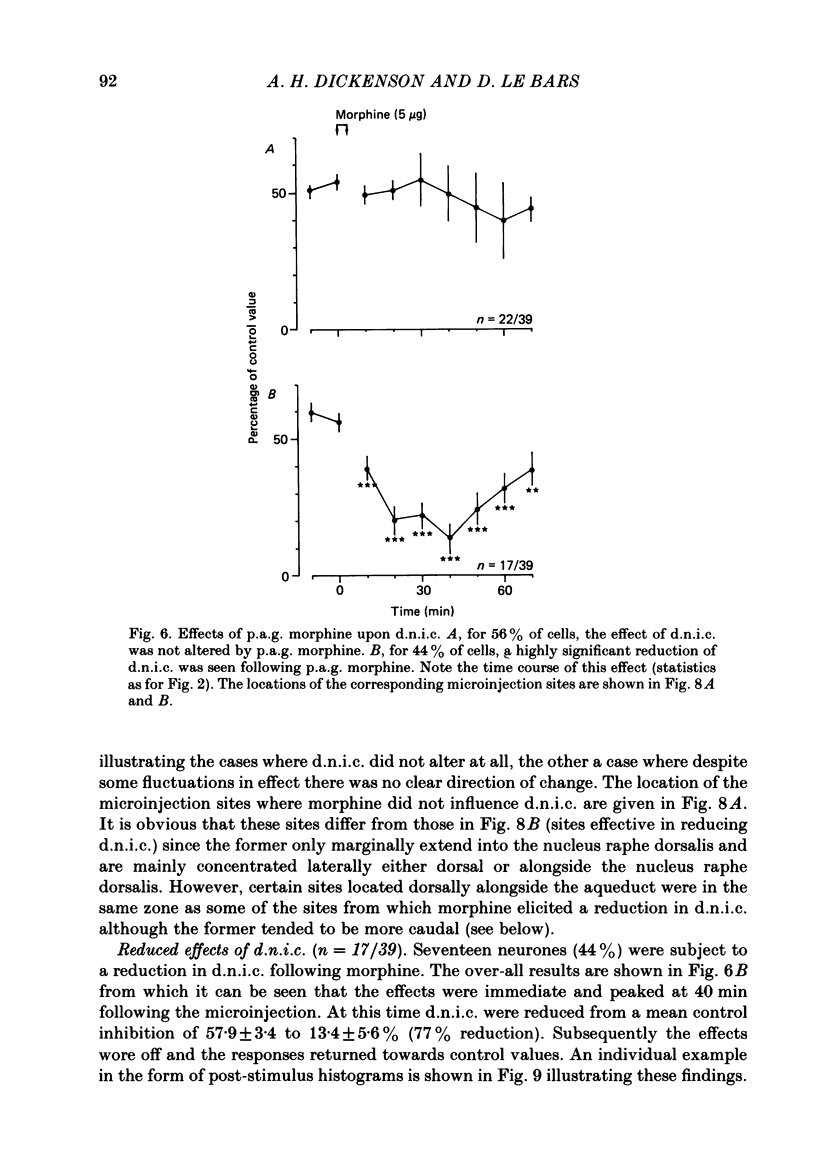
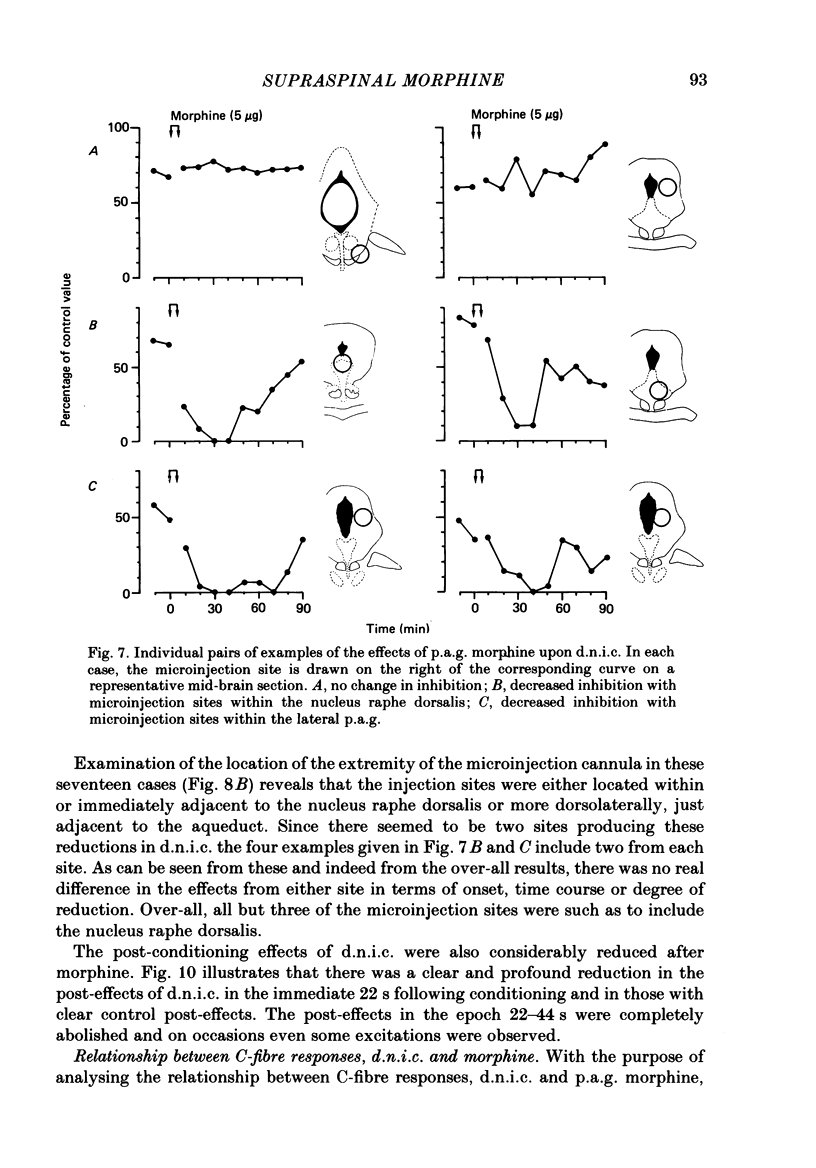
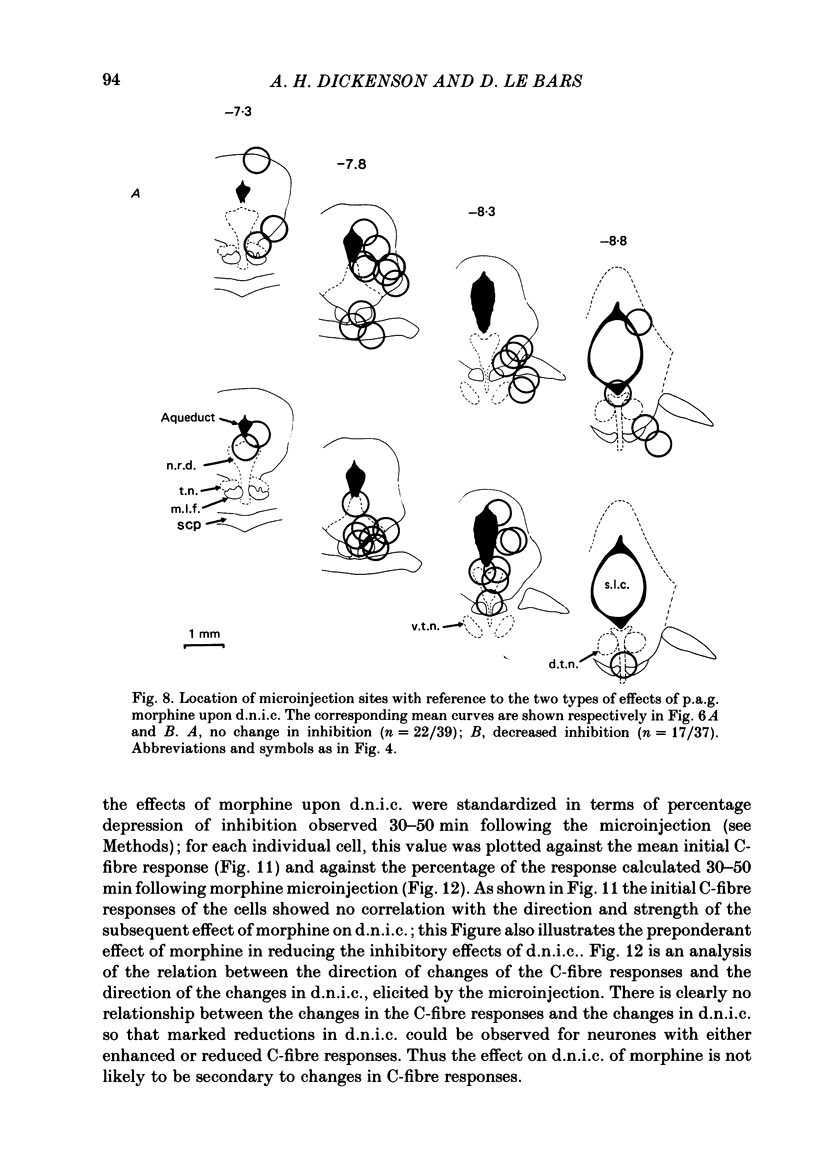
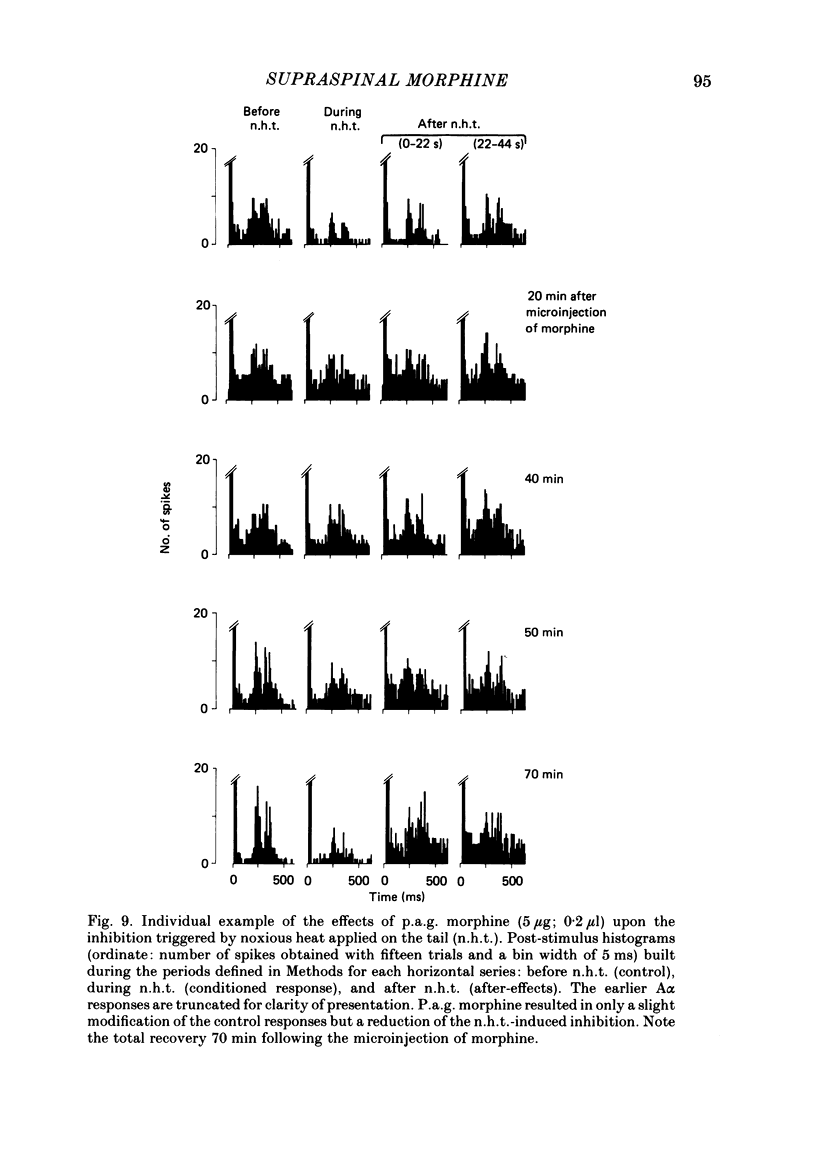
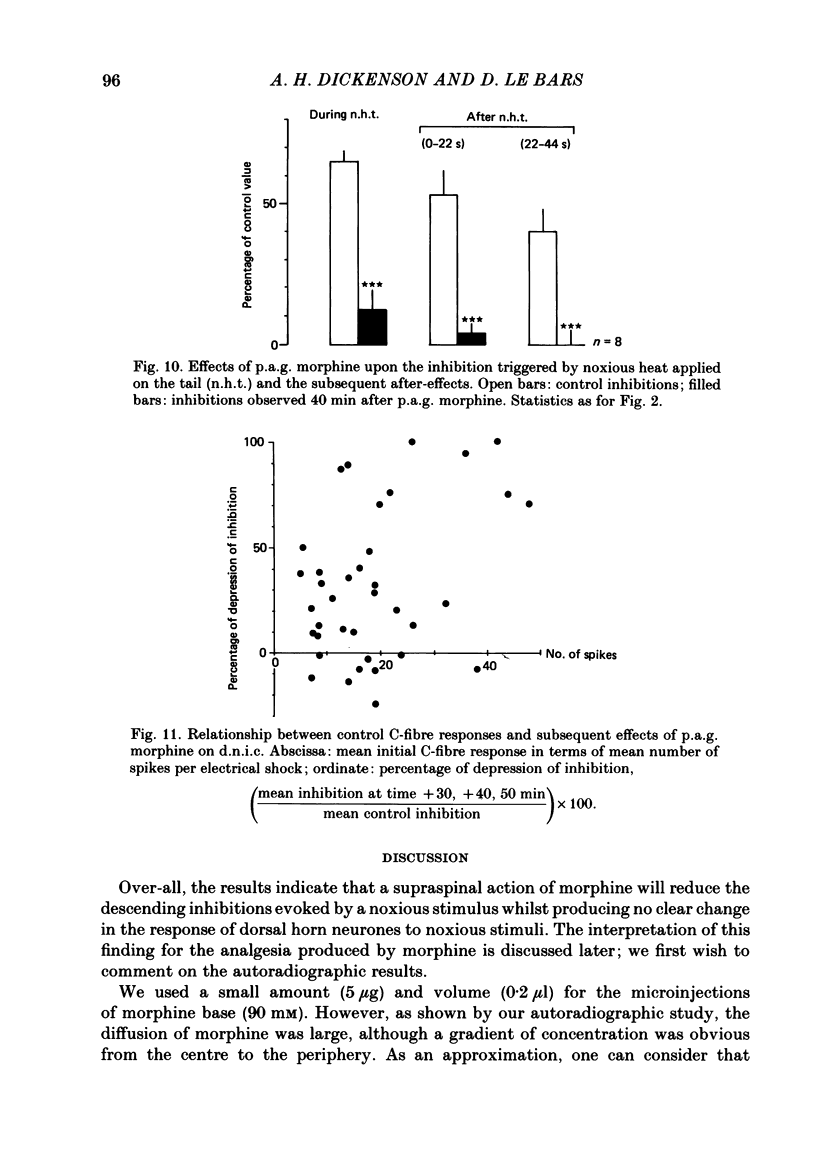
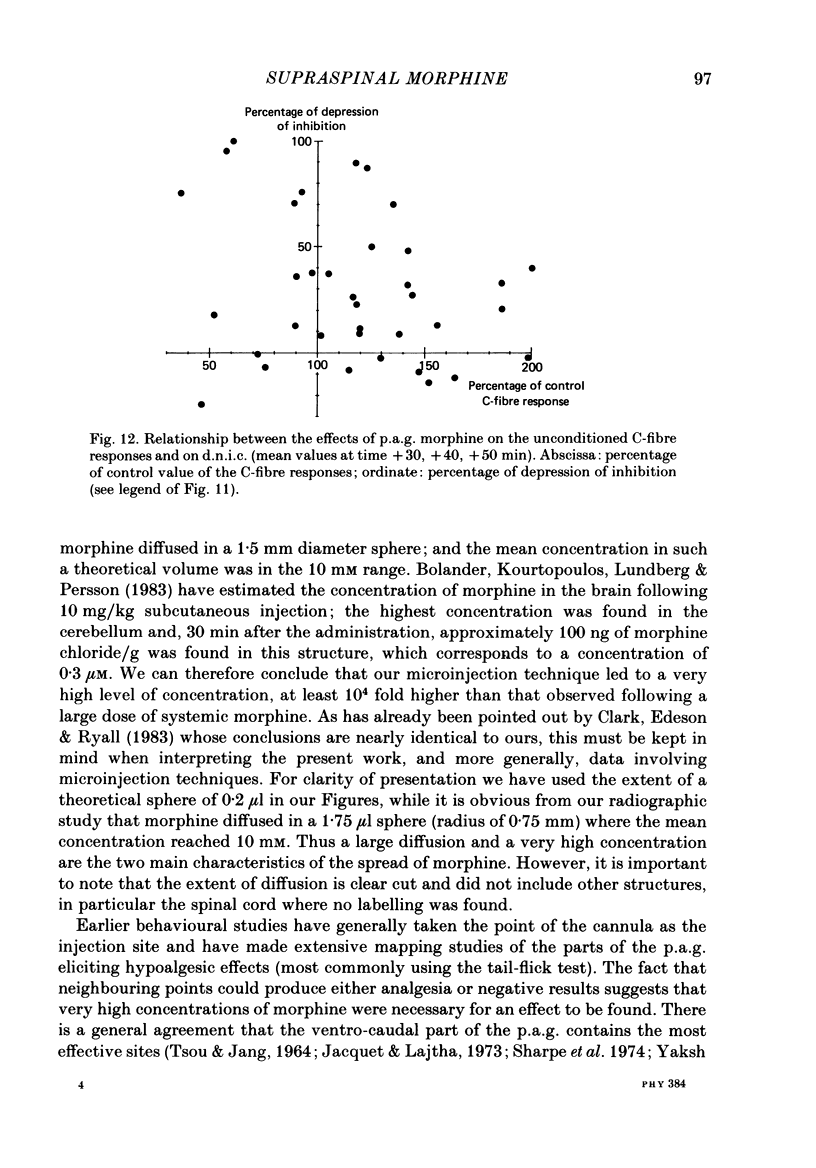









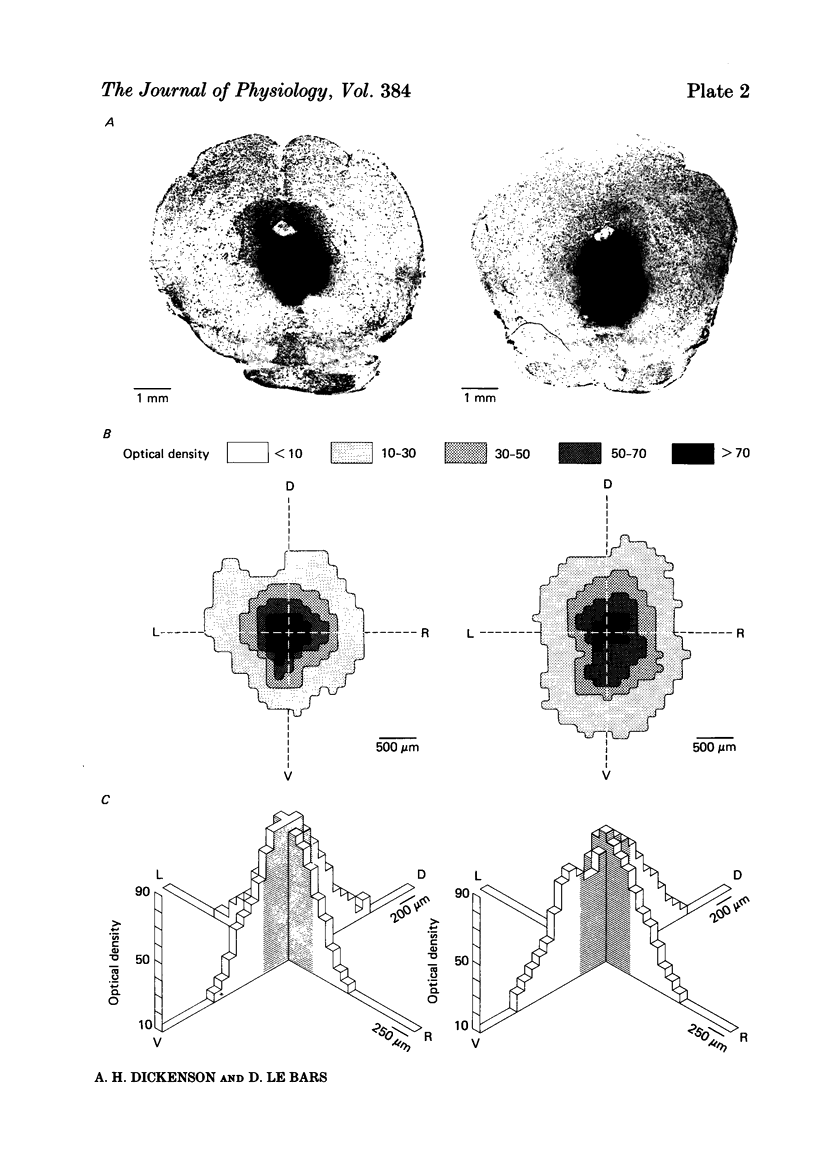

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike A., Shibata T., Satoh M., Takagi H. Analgesia induced by microinjection of morphine into, and electrical stimulation of, the nucleus reticularis paragigantocellularis of rat medulla oblongata. Neuropharmacology. 1978 Sep;17(9):775–778. doi: 10.1016/0028-3908(78)90093-x. [DOI] [PubMed] [Google Scholar]
- Akil H., Mayer D. J., Liebeskind J. C. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976 Mar 5;191(4230):961–962. doi: 10.1126/science.1251210. [DOI] [PubMed] [Google Scholar]
- Atweh S. F., Kuhar M. J. Autoradiographic localization of opiate receptors in rat brain. I. Spinal cord and lower medulla. Brain Res. 1977 Mar 18;124(1):53–67. doi: 10.1016/0006-8993(77)90863-0. [DOI] [PubMed] [Google Scholar]
- Atweh S. F., Kuhar M. J. Autoradiographic localization of opiate receptors in rat brain. II. The brain stem. Brain Res. 1977 Jun 24;129(1):1–12. doi: 10.1016/0006-8993(77)90965-9. [DOI] [PubMed] [Google Scholar]
- Azami J., Llewelyn M. B., Roberts M. H. The contribution of nucleus reticularis paragigantocellularis and nucleus raphe magnus to the analgesia produced by systemically administered morphine, investigated with the microinjection technique. Pain. 1982 Mar;12(3):229–246. doi: 10.1016/0304-3959(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Fields H. L. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978 Nov;4(5):451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- Bennett G. J., Mayer D. J. Inhibition of spinal cord interneurons by narcotic microinjection and focal electrical stimulation in the periaqueductal central gray matter. Brain Res. 1979 Aug 24;172(2):243–257. doi: 10.1016/0006-8993(79)90536-5. [DOI] [PubMed] [Google Scholar]
- Bloom F., Battenberg E., Rossier J., Ling N., Guillemin R. Neurons containing beta-endorphin in rat brain exist separately from those containing enkephalin: immunocytochemical studies. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1591–1595. doi: 10.1073/pnas.75.3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolander H., Kourtopoulos H., Lundberg S., Persson S. A. Morphine concentrations in serum, brain and cerebrospinal fluid in the rat after intravenous administration of a single dose. J Pharm Pharmacol. 1983 Oct;35(10):656–659. doi: 10.1111/j.2042-7158.1983.tb02860.x. [DOI] [PubMed] [Google Scholar]
- Botticelli L. J., Cox B. M., Goldstein A. Immunoreactive dynorphin in mammalian spinal cord and dorsal root ganglia. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7783–7786. doi: 10.1073/pnas.78.12.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadden S. W., Villanueva L., Chitour D., Le Bars D. Depression of activities of dorsal horn convergent neurones by propriospinal mechanisms triggered by noxious inputs; comparison with diffuse noxious inhibitory controls (DNIC). Brain Res. 1983 Sep 19;275(1):1–11. doi: 10.1016/0006-8993(83)90412-2. [DOI] [PubMed] [Google Scholar]
- Carstens E., Bihl H., Irvine D. R., Zimmermann M. Descending inhibition from medial and lateral midbrain of spinal dorsal horn neuronal responses to noxious and nonnoxious cutaneous stimuli in the cat. J Neurophysiol. 1981 Jun;45(6):1029–1042. doi: 10.1152/jn.1981.45.6.1029. [DOI] [PubMed] [Google Scholar]
- Carstens E., Klumpp D., Zimmermann M. Differential inhibitory effects of medial and lateral midbrain stimulation on spinal neuronal discharges to noxious skin heating in the cat. J Neurophysiol. 1980 Feb;43(2):332–342. doi: 10.1152/jn.1980.43.2.332. [DOI] [PubMed] [Google Scholar]
- Carstens E., Klumpp D., Zimmermann M. The opiate antagonist, naloxone, does not affect descending inhibition from midbrain of nociceptive spinal neuronal discharges in the cat. Neurosci Lett. 1979 Mar;11(3):323–327. doi: 10.1016/0304-3940(79)90016-8. [DOI] [PubMed] [Google Scholar]
- Carstens E., Yokota T., Zimmermann M. Inhibition of spinal neuronal responses to noxious skin heating by stimulation of mesencephalic periaqueductal gray in the cat. J Neurophysiol. 1979 Mar;42(2):558–568. doi: 10.1152/jn.1979.42.2.558. [DOI] [PubMed] [Google Scholar]
- Chitour D., Dickenson A. H., Le Bars D. Pharmacological evidence for the involvement of serotonergic mechanisms in diffuse noxious inhibitory controls (DNIC). Brain Res. 1982 Mar 25;236(2):329–337. doi: 10.1016/0006-8993(82)90718-1. [DOI] [PubMed] [Google Scholar]
- Clark S. L., Edeson R. O., Ryall R. W. The relative significance of spinal and supraspinal actions in the antinociceptive effect of morphine in the dorsal horn: an evaluation of the microinjection technique. Br J Pharmacol. 1983 Jul;79(3):807–818. doi: 10.1111/j.1476-5381.1983.tb10019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. L., Ryall R. W. The antinociceptive action of etorphine in the dorsal horn is due to a direct spinal action and not to activation of descending inhibition. Br J Pharmacol. 1983 Feb;78(2):307–319. doi: 10.1111/j.1476-5381.1983.tb09396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. R., Beitz A. J., Fletcher T. F., Mullett M. A. Immunocytochemical localization of serotonin in the rat periaqueductal gray: a quantitative light and electron microscopic study. J Comp Neurol. 1985 Jun 1;236(1):60–70. doi: 10.1002/cne.902360106. [DOI] [PubMed] [Google Scholar]
- Descarries L., Watkins K. C., Garcia S., Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982 May 20;207(3):239–254. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Le Bars D., Besson J. M. Diffuse noxious inhibitory controls (DNIC). Effects on trigeminal nucleus caudalis neurones in the rat. Brain Res. 1980 Nov 3;200(2):293–305. doi: 10.1016/0006-8993(80)90921-x. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Le Bars D. Diffuse noxious inhibitory controls (DNIC) involve trigeminothalamic and spinothalamic neurones in the rat. Exp Brain Res. 1983;49(2):174–180. doi: 10.1007/BF00238577. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Le Bars D. Morphine microinjections into periaqueductal grey matter of the rat: effects on dorsal horn neuronal responses to C-fibre activity and diffuse noxious inhibitory controls. Life Sci. 1983;33 (Suppl 1):549–552. doi: 10.1016/0024-3205(83)90562-3. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Oliveras J. L., Besson J. M. Role of the nucleus raphe magnus in opiate analgesia as studied by the microinjection technique in the rat. Brain Res. 1979 Jul 6;170(1):95–111. doi: 10.1016/0006-8993(79)90943-0. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Rivot J. P., Chaouch A., Besson J. M., Le Bars D. Diffuse noxious inhibitory controls (DNIC) in the rat with or without pCPA pretreatment. Brain Res. 1981 Jul 20;216(2):313–321. doi: 10.1016/0006-8993(81)90133-5. [DOI] [PubMed] [Google Scholar]
- Du H. J., Kitahata L. M., Thalhammer J. G., Zimmermann M. Inhibition of nociceptive neuronal responses in the cat's spinal dorsal horn by electrical stimulation and morphine microinjection in nucleus raphe magnus. Pain. 1984 Jul;19(3):249–257. doi: 10.1016/0304-3959(84)90003-4. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Griersmith B. T. Inhibition of the spinal transmission of nociceptive information by supraspinal stimulation in the cat. Pain. 1979 Apr;6(2):149–161. doi: 10.1016/0304-3959(79)90122-2. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Griersmith B. T., North R. A. Morphine and supraspinal inhibition of spinal neurones: evidence that morphine decreases tonic descending inhibition in the anaesthetized cat. Br J Pharmacol. 1980 Jul;69(3):461–466. doi: 10.1111/j.1476-5381.1980.tb07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardin V., Oliveras J. L., Besson J. M. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. I. The production of behavioral side effects together with analgesia. Brain Res. 1984 Jul 23;306(1-2):105–123. doi: 10.1016/0006-8993(84)90360-3. [DOI] [PubMed] [Google Scholar]
- Fardin V., Oliveras J. L., Besson J. M. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. II. Differential characteristics of the analgesia induced by ventral and dorsal PAG stimulation. Brain Res. 1984 Jul 23;306(1-2):125–139. doi: 10.1016/0006-8993(84)90361-5. [DOI] [PubMed] [Google Scholar]
- Gebhart G. F., Sandkühler J., Thalhammer J. G., Zimmermann M. Inhibition in spinal cord of nociceptive information by electrical stimulation and morphine microinjection at identical sites in midbrain of the cat. J Neurophysiol. 1984 Jan;51(1):75–89. doi: 10.1152/jn.1984.51.1.75. [DOI] [PubMed] [Google Scholar]
- Gebhart G. F., Toleikis J. R. An evaluation of stimulation-produced analgesia in the cat. Exp Neurol. 1978 Dec;62(3):570–579. doi: 10.1016/0014-4886(78)90269-8. [DOI] [PubMed] [Google Scholar]
- Goldstein A., Ghazarossian V. E. Immunoreactive dynorphin in pituitary and brain. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6207–6210. doi: 10.1073/pnas.77.10.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. R., Snyder S. H., Kuhar M. J., Young W. S., 3rd Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbaud G., Besson J. M., Liebeskind J. C., Oliveras J. L. Analgésie induite par stimulation de la substance grise périaqueducale chez le chat: données comportementales et modifications de l'activité des interneurones de la corne dorsale de la moelle. C R Acad Sci Hebd Seances Acad Sci D. 1972 Sep 4;275(10):1055–1057. [PubMed] [Google Scholar]
- Hayes R. L., Price D. D., Ruda M., Dubner R. Suppression of nociceptive responses in the primate by electrical stimulation of the brain or morphine administration: behavioral and electrophysiological comparisons. Brain Res. 1979 May 11;167(2):417–421. doi: 10.1016/0006-8993(79)90838-2. [DOI] [PubMed] [Google Scholar]
- Herz A., Albus K., Metys J., Schubert P., Teschemacher H. On the central sites for the antinociceptive action of morphine and fentanyl. Neuropharmacology. 1970 Nov;9(6):539–551. doi: 10.1016/0028-3908(70)90004-3. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A., Terenius L., Elde R., Nilsson G. Immunohistochemical analysis of peptide pathways possibly related to pain and analgesia: enkephalin and substance P. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3081–3085. doi: 10.1073/pnas.74.7.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höllt V., Haarmann I., Bovermann K., Jerlicz M., Herz A. Dynorphin-related immunoreactive peptides in rat brain and pituitary. Neurosci Lett. 1980 Jun;18(2):149–153. doi: 10.1016/0304-3940(80)90318-3. [DOI] [PubMed] [Google Scholar]
- Iwamoto E. T., Harris R. A., Loh H. H., Way E. L. Antinociceptive responses after microinjection of morphine or lanthanum in discrete rat brain sites. J Pharmacol Exp Ther. 1978 Jul;206(1):46–55. [PubMed] [Google Scholar]
- Jacquet Y. F., Lajtha A. Morphine action at central nervous system sites in rat: analgesia or hyperalgesia depending on site and dose. Science. 1973 Nov 2;182(4111):490–492. doi: 10.1126/science.182.4111.490. [DOI] [PubMed] [Google Scholar]
- Jacquet Y. F., Lajtha A. Paradoxical effects after microinjection of morphine in the periaqueductal gray matter in the rat. Science. 1974 Sep 20;185(4156):1055–1057. doi: 10.1126/science.185.4156.1055. [DOI] [PubMed] [Google Scholar]
- Khachaturian H., Watson S. J., Lewis M. E., Coy D., Goldstein A., Akil H. Dynorphin immunocytochemistry in the rat central nervous system. Peptides. 1982 Nov-Dec;3(6):941–954. doi: 10.1016/0196-9781(82)90063-8. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Chitour D. Do convergent neurones in the spinal dorsal horn discriminate nociceptive from non-nociceptive information? Pain. 1983 Sep;17(1):1–19. doi: 10.1016/0304-3959(83)90123-9. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Chitour D., Kraus E., Clot A. M., Dickenson A. H., Besson J. M. The effect of systemic morphine upon diffuse noxious inhibitory controls (DNIC) in the rat: evidence for a lifting of certain descending inhibitory controls of dorsal horn convergent neurones. Brain Res. 1981 Jun 29;215(1-2):257–274. doi: 10.1016/0006-8993(81)90506-0. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Chitour D., Kraus E., Dickenson A. H., Besson J. M. Effect of naloxone upon diffuse noxious inhibitory controls (DNIC) in the rat. Brain Res. 1981 Jan 12;204(2):387–402. doi: 10.1016/0006-8993(81)90597-7. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Dickenson A. H., Besson J. M. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979 Jun;6(3):283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Dickenson A. H., Besson J. M. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979 Jun;6(3):305–327. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Dickenson A. H., Besson J. M. Microinjection of morphine within nucleus raphe magnus and dorsal horn neurone activities related to nociception in the rat. Brain Res. 1980 May 12;189(2):467–481. doi: 10.1016/0006-8993(80)90106-7. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Guilbaud G., Chitour D., Besson J. M. Does systemic morphine increase descending inhibitory controls of dorsal horn neurones involved in nociception? Brain Res. 1980 Nov 24;202(1):223–228. doi: 10.1016/0006-8993(80)90659-9. [DOI] [PubMed] [Google Scholar]
- Lewis V. A., Gebhart G. F. Evaluation of the periaqueductal central gray (PAG) as a morphine-specific locus of action and examination of morphine-induced and stimulation-produced analgesia at coincident PAG loci. Brain Res. 1977 Mar 25;124(2):283–303. doi: 10.1016/0006-8993(77)90886-1. [DOI] [PubMed] [Google Scholar]
- Lewis V. A., Gebhart G. F. Morphine-induced and stimulation-produced analgesias at coincident periaqueductal central gray loci: evaluation of analgesic congruence, tolerance, and cross-tolerance. Exp Neurol. 1977 Dec;57(3):934–955. doi: 10.1016/0014-4886(77)90119-4. [DOI] [PubMed] [Google Scholar]
- Liebeskind J. C., Guilbaud G., Besson J. M., Oliveras J. L. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: behavioral observations and inhibitory effects on spinal cord interneurons. Brain Res. 1973 Feb 28;50(2):441–446. doi: 10.1016/0006-8993(73)90748-8. [DOI] [PubMed] [Google Scholar]
- Mayer D. J., Price D. D. Central nervous system mechanisms of analgesia. Pain. 1976 Dec;2(4):379–404. doi: 10.1016/0304-3959(76)90080-4. [DOI] [PubMed] [Google Scholar]
- Menétrey D., Giesler G. J., Jr, Besson J. M. An analysis of response properties of spinal cord dorsal horn neurones to nonnoxious and noxious stimuli in the spinal rat. Exp Brain Res. 1977 Jan 18;27(1):15–33. doi: 10.1007/BF00234822. [DOI] [PubMed] [Google Scholar]
- Misra A. L., Pontani R. B., Vadlamani N. L., Mulé S. J. Physiological disposition and biotransformation of [allyl-1', 3' - 14C naloxone in the rat and some comparative observations on nalorphine. J Pharmacol Exp Ther. 1976 Feb;196(2):257–268. [PubMed] [Google Scholar]
- Moskowitz A. S., Goodman R. R. Light microscopic autoradiographic localization of mu and delta opioid binding sites in the mouse central nervous system. J Neurosci. 1984 May;4(5):1331–1342. doi: 10.1523/JNEUROSCI.04-05-01331.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveras J. L., Besson J. M., Guilbaud G., Liebeskind J. C. Behavioral and electrophysiological evidence of pain inhibition from midbrain stimulation in the cat. Exp Brain Res. 1974 Apr 30;20(1):32–44. doi: 10.1007/BF00239016. [DOI] [PubMed] [Google Scholar]
- Oliveras J. L., Hosobuchi Y., Redjemi F., Guilbaud G., Besson J. M. Opiate antagonist, naloxone, strongly reduces analgesia induced by stimulation of a raphe nucleus (centralis inferior). Brain Res. 1977 Jan 21;120(2):221–229. doi: 10.1016/0006-8993(77)90902-7. [DOI] [PubMed] [Google Scholar]
- Ossipov M. H., Goldstein F. J., Malseed R. T. Feline analgesia following central administration of opioids. Neuropharmacology. 1984 Aug;23(8):925–929. doi: 10.1016/0028-3908(84)90006-6. [DOI] [PubMed] [Google Scholar]
- Pert A., Walter M. Comparison between naloxone reversal of morphine and electrical stimulation induced analgesia in the rat mesencephalon. Life Sci. 1976 Oct 1;19(7):1023–1032. doi: 10.1016/0024-3205(76)90294-0. [DOI] [PubMed] [Google Scholar]
- Rivot J. P., Chaouch A., Besson J. M. The influence of naloxone on the C fiber response of dorsal horn neurons and their inhibitory control by raphe magnus stimulation. Brain Res. 1979 Nov 2;176(2):355–364. doi: 10.1016/0006-8993(79)90989-2. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J. P., Stocco S. Differential effects of systemic versus intracranial injection of opiates on central, orofacial and lower body nociception: somatotypy in bulbar analgesia systems. Pain. 1980 Dec;9(3):307–318. doi: 10.1016/0304-3959(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Sar M., Stumpf W. E., Miller R. J., Chang K. J., Cuatrecasas P. Immunohistochemical localization of enkephalin in rat brain and spinal cord. J Comp Neurol. 1978 Nov 1;182(1):17–37. doi: 10.1002/cne.901820103. [DOI] [PubMed] [Google Scholar]
- Schouenborg J., Dickenson A. Effects of a distant noxious stimulation on A and C fibre-evoked flexion reflexes and neuronal activity in the dorsal horn of the rat. Brain Res. 1985 Feb 25;328(1):23–32. doi: 10.1016/0006-8993(85)91318-6. [DOI] [PubMed] [Google Scholar]
- Sharpe L. G., Garnett J. E., Cicero T. J. Analgesia and hyperreactivity produced by intracranial microinjections of morphine into the periaqueductal gray matter of the rat. Behav Biol. 1974 Jul;11(3):303–313. doi: 10.1016/s0091-6773(74)90548-3. [DOI] [PubMed] [Google Scholar]
- Skagerberg G., Björklund A. Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. Neuroscience. 1985 Jun;15(2):445–480. doi: 10.1016/0306-4522(85)90225-8. [DOI] [PubMed] [Google Scholar]
- Soja P. J., Sinclair J. G. Spinal vs supraspinal actions of morphine on cat spinal cord multireceptive neurons. Brain Res. 1983 Aug 22;273(1):1–7. doi: 10.1016/0006-8993(83)91087-9. [DOI] [PubMed] [Google Scholar]
- Steinbusch H. W. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6(4):557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- TSOU K., JANG C. S. STUDIES ON THE SITE OF ANALGESIC ACTION OF MORPHINE BY INTRACEREBRAL MICRO-INJECTION. Sci Sin. 1964 Jul;13:1099–1109. [PubMed] [Google Scholar]
- Takagi H., Satoh M., Akaike A., Shibata T., Kuraishi Y. The nucleus reticularis gigantocellularis of the medulla oblongata is a highly sensitive site in the production of morphine analgesia in the rat. Eur J Pharmacol. 1977 Sep 1;45(1):91–92. doi: 10.1016/0014-2999(77)90064-4. [DOI] [PubMed] [Google Scholar]
- Uhl G. R., Goodman R. R., Kuhar M. J., Childers S. R., Snyder S. H. Immunohistochemical mapping of enkephalin containing cell bodies, fibers and nerve terminals in the brain stem of the rat. Brain Res. 1979 Apr 20;166(1):75–94. doi: 10.1016/0006-8993(79)90651-6. [DOI] [PubMed] [Google Scholar]
- Villablanca J. R., Harris C. M., Burgess J. W., de Andres I. Reassessing morphine effects in cats: I. Specific behavioral responses in intact and unilaterally brain-lesioned animals. Pharmacol Biochem Behav. 1984 Dec;21(6):913–921. doi: 10.1016/s0091-3057(84)80073-8. [DOI] [PubMed] [Google Scholar]
- Weil-Fugazza J., Godefroy F., Le Bars D. Increase in 5-HT synthesis in the dorsal part of the spinal cord, induced by a nociceptive stimulus: blockade by morphine. Brain Res. 1984 Apr 16;297(2):247–264. doi: 10.1016/0006-8993(84)90566-3. [DOI] [PubMed] [Google Scholar]
- Willer J. C., Roby A., Le Bars D. Psychophysical and electrophysiological approaches to the pain-relieving effects of heterotopic nociceptive stimuli. Brain. 1984 Dec;107(Pt 4):1095–1112. doi: 10.1093/brain/107.4.1095. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L. Spinal opiate analgesia: characteristics and principles of action. Pain. 1981 Dec;11(3):293–346. doi: 10.1016/0304-3959(81)90633-3. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Yeung J. C., Rudy T. A. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976 Sep 10;114(1):83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]
- Yeung J. C., Rudy T. A. Multiplicative interaction between narcotic agonisms expressed at spinal and supraspinal sites of antinociceptive action as revealed by concurrent intrathecal and intracerebroventricular injections of morphine. J Pharmacol Exp Ther. 1980 Dec;215(3):633–642. [PubMed] [Google Scholar]
- Yezierski R. P., Wilcox T. K., Willis W. D. The effects of serotonin antagonists on the inhibition of primate spinothalamic tract cells produced by stimulation in nucleus raphe magnus or periaqueductal gray. J Pharmacol Exp Ther. 1982 Feb;220(2):266–277. [PubMed] [Google Scholar]
- Young W. S., 3rd, Kuhar M. J. A new method for receptor autoradiography: [3H]opioid receptors in rat brain. Brain Res. 1979 Dec 28;179(2):255–270. doi: 10.1016/0006-8993(79)90442-6. [DOI] [PubMed] [Google Scholar]
- Zakarian S., Smyth D. G. beta-Endorphin is processed differently in specific regions of rat pituitary and brain. Nature. 1982 Mar 18;296(5854):250–252. doi: 10.1038/296250a0. [DOI] [PubMed] [Google Scholar]
- Zorman G., Belcher G., Adams J. E., Fields H. L. Lumbar intrathecal naloxone blocks analgesia produced by microstimulation of the ventromedial medulla in the rat. Brain Res. 1982 Mar 18;236(1):77–84. doi: 10.1016/0006-8993(82)90035-x. [DOI] [PubMed] [Google Scholar]
- Zorman G., Hentall I. D., Adams J. E., Fields H. L. Naloxone-reversible analgesia produced by microstimulation in the rat medulla. Brain Res. 1981 Aug 24;219(1):137–148. doi: 10.1016/0006-8993(81)90273-0. [DOI] [PubMed] [Google Scholar]