Abstract
1. The spatial and temporal characteristics of the visual information transmitted across the corpus callosum have been studied in normal cats by recording directly from the corpus callosum and in split-chiasm cats by means of visual evoked potentials (v.e.p.s) and single-unit recordings at the 17/18 border. 2. The modulation transfer functions (m.t.f.s) obtained by recording from the corpus callosum are comparable to the m.t.f.s evaluated by various techniques for the whole visual system of the cat. The spatial and temporal acuities, however, do not reach the values obtained behaviourally or estimated with cortical evoked potentials. 3. In split-chiasm cats, both v.e.p.s and single-unit recordings indicate that the contrast gain of the callosal pathway is considerably lower than the gain of the direct, geniculo-cortical system. Spatial and temporal acuities are lower for the callosal than for the direct system. 4. The same differences in contrast gain between the spatial m.t.f. obtained for the callosal and the direct system have been found in alert split-chiasm cats. 5. Our data suggest that the cross-talk between the hemispheres taking place across the corpus callosum is nearly abolished at low contrasts and high spatial and temporal frequencies.
Full text
PDF


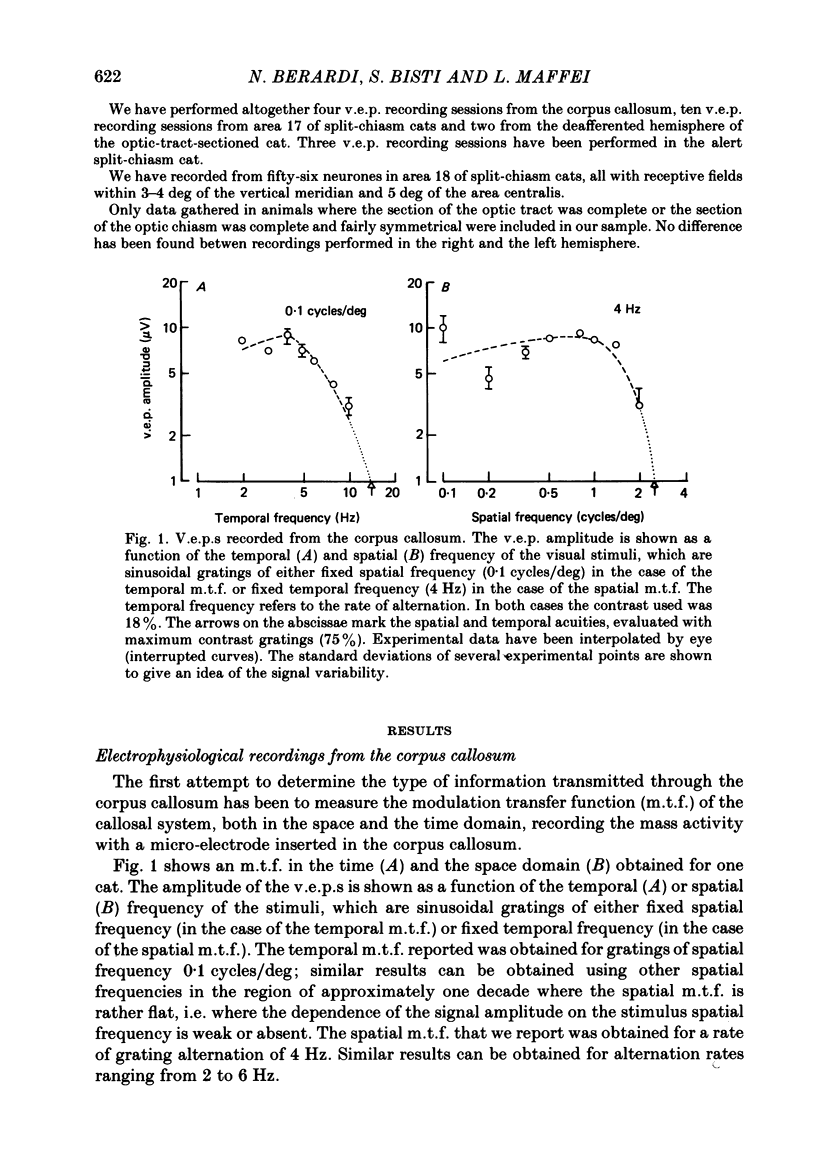
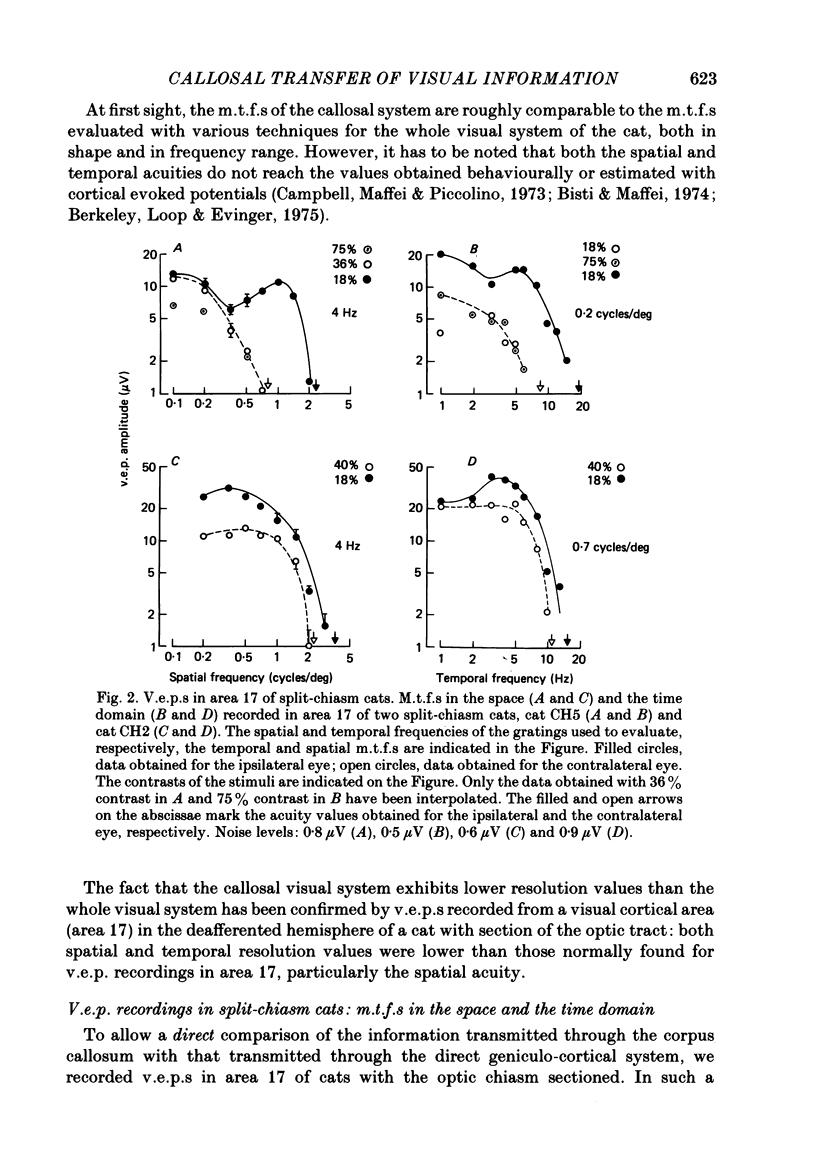
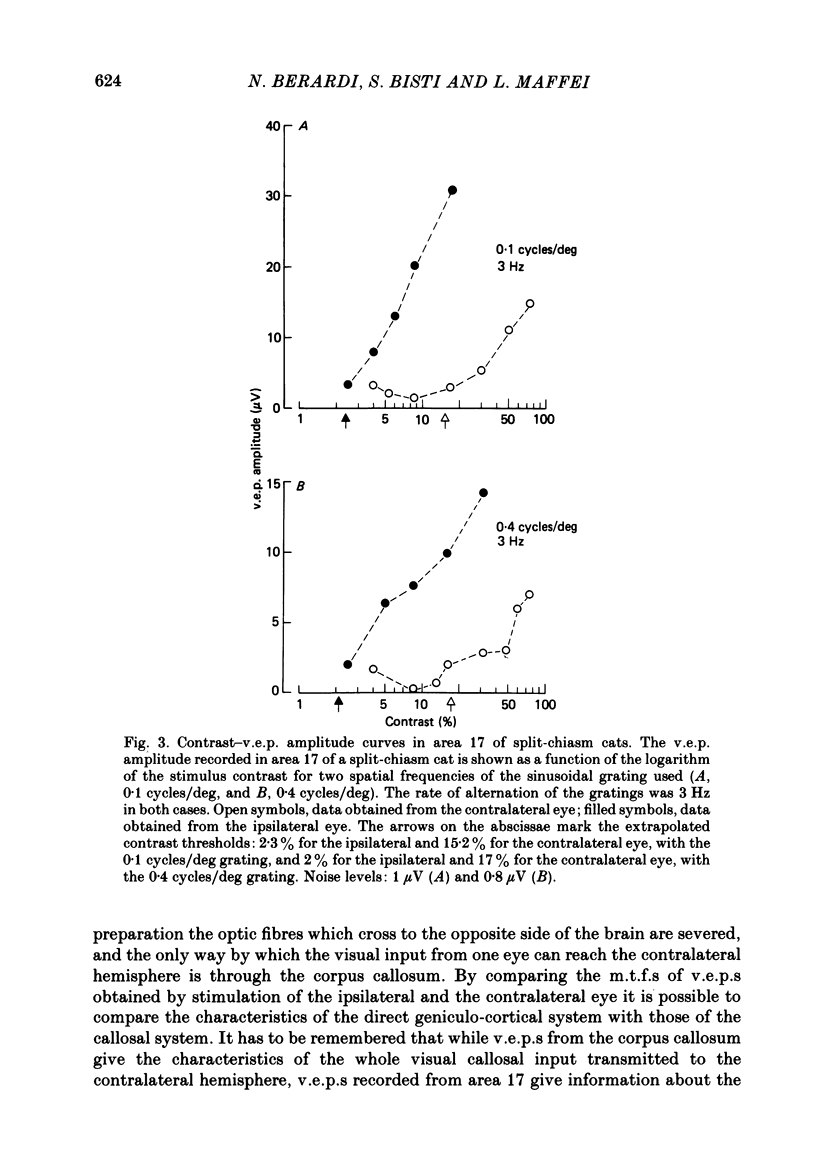
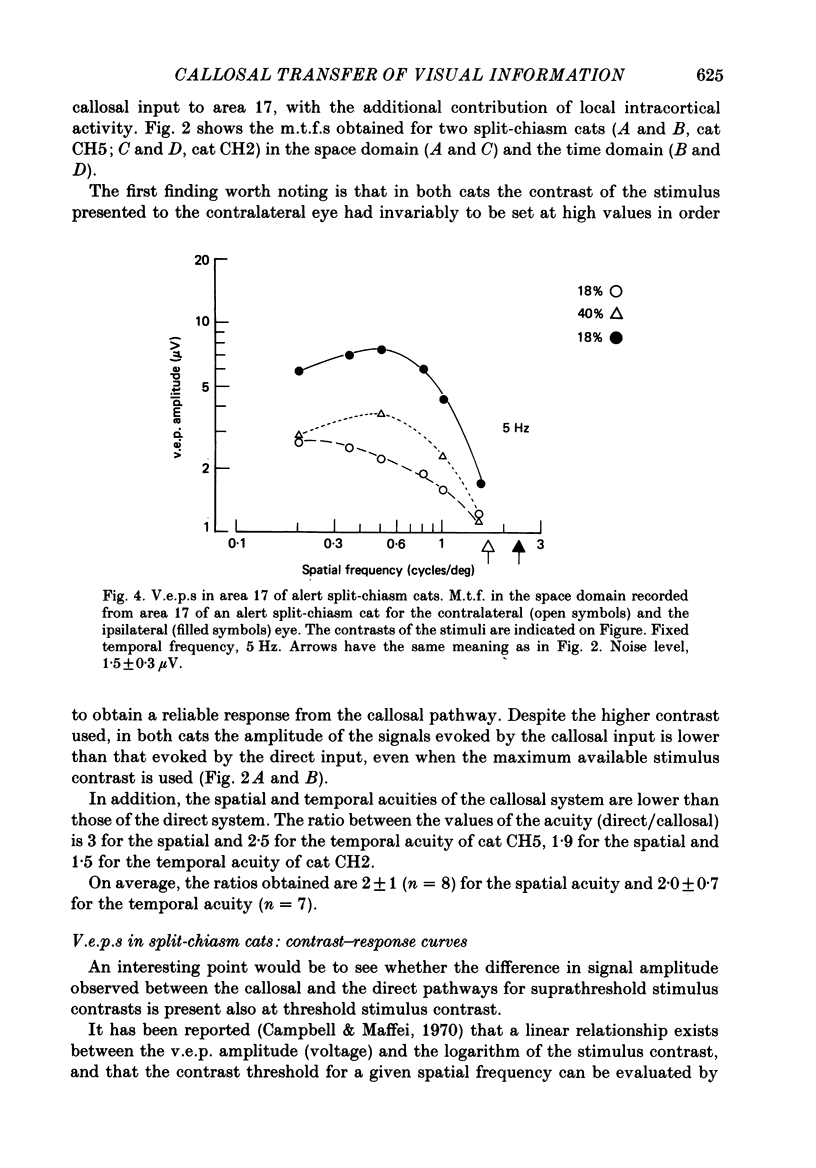





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berardi N., Bisti S., Cattaneo A., Fiorentini A., Maffei L. Correlation between the preferred orientation and spatial frequency of neurones in visual areas 17 and 18 of the cat. J Physiol. 1982 Feb;323:603–618. doi: 10.1113/jphysiol.1982.sp014094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N., Fiorentini A. Interhemispheric transfer of visual information in humans: spatial characteristics. J Physiol. 1987 Mar;384:633–647. doi: 10.1113/jphysiol.1987.sp016474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley M. A., Loop M. S., Evinger C. Temporal modulation sensitivity of the cat. II. Evoked potential estimates. Vision Res. 1975 May;15(5):563–568. doi: 10.1016/0042-6989(75)90303-x. [DOI] [PubMed] [Google Scholar]
- Berlucchi G. Anatomical and physiological aspects of visual functions of corpus callosum. Brain Res. 1972 Feb 25;37(2):371–392. doi: 10.1016/0006-8993(72)90708-1. [DOI] [PubMed] [Google Scholar]
- Berlucchi G., Gazzaniga M. S., Rizzolatti G. Microelectrode analysis of transfer of visual information by the corpus callosum. Arch Ital Biol. 1967 Nov;105(4):583–596. [PubMed] [Google Scholar]
- Berlucchi G., Rizzolatti G. Binocularly driven neurons in visual cortex of split-chiasm cats. Science. 1968 Jan 19;159(3812):308–310. doi: 10.1126/science.159.3812.308. [DOI] [PubMed] [Google Scholar]
- Bisti S., Carmignoto G., Galli L., Maffei L. Spatial-frequency characteristics of neurones of area 18 in the cat: dependence on the velocity of the visual stimulus. J Physiol. 1985 Feb;359:259–268. doi: 10.1113/jphysiol.1985.sp015584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisti S., Carmignoto G. Monocular deprivation in kittens differently affects crossed and uncrossed visual pathways. Vision Res. 1986;26(6):875–884. doi: 10.1016/0042-6989(86)90145-8. [DOI] [PubMed] [Google Scholar]
- Bisti S., Maffei L. Behavioural contrast sensitivity of the cat in various visual meridians. J Physiol. 1974 Aug;241(1):201–210. doi: 10.1113/jphysiol.1974.sp010649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. Binocular depth discrimination and the nasotemporal division. J Physiol. 1969 Nov;205(2):471–497. doi: 10.1113/jphysiol.1969.sp008978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. Binocular depth perception and the optic chiasm. Vision Res. 1970 Jan;10(1):43–47. doi: 10.1016/0042-6989(70)90060-x. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Diao Y. C., Pu M. L., Wang Y. K., Xiao Y. M. Possible functions of the interhemispheric connexions between visual cortical areas in the cat. J Physiol. 1983 Apr;337:331–349. doi: 10.1113/jphysiol.1983.sp014627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOUDHURY B. P., WHITTERIDGE D., WILSON M. E. THE FUNCTION OF THE CALLOSAL CONNECTIONS OF THE VISUAL CORTEX. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:214–219. doi: 10.1113/expphysiol.1965.sp001783. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Maffei L. Electrophysiological evidence for the existence of orientation and size detectors in the human visual system. J Physiol. 1970 May;207(3):635–652. doi: 10.1113/jphysiol.1970.sp009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Maffei L., Piccolino M. The contrast sensitivity of the cat. J Physiol. 1973 Mar;229(3):719–731. doi: 10.1113/jphysiol.1973.sp010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald R., Chase R. An improved method for plotting retinal landmarks and focusing the eyes. Vision Res. 1971 Jan;11(1):95–96. doi: 10.1016/0042-6989(71)90207-0. [DOI] [PubMed] [Google Scholar]
- Fiorentini A., Berardi N. Right-hemisphere superiority in the discrimination of spatial phase. Perception. 1984;13(6):695–708. doi: 10.1068/p130695. [DOI] [PubMed] [Google Scholar]
- Harvey A. R. A physiological analysis of subcortical and commissural projections of areas 17 and 18 of the cat. J Physiol. 1980 May;302:507–534. doi: 10.1113/jphysiol.1980.sp013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Cortical and callosal connections concerned with the vertical meridian of visual fields in the cat. J Neurophysiol. 1967 Nov;30(6):1561–1573. doi: 10.1152/jn.1967.30.6.1561. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M. The primary visual pathway through the corpus callosum: morphological and functional aspects in the cat. Arch Ital Biol. 1980 May;118(2):124–188. [PubMed] [Google Scholar]
- Maffei L., Berardi N., Bisti S. Interocular transfer of adaptation after effect in neurons of area 17 and 18 of split chiasm cats. J Neurophysiol. 1986 May;55(5):966–976. doi: 10.1152/jn.1986.55.5.966. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Blakemore C. Binocular depth perception and the corpus callosum. Vision Res. 1970 Jan;10(1):49–54. doi: 10.1016/0042-6989(70)90061-1. [DOI] [PubMed] [Google Scholar]
- Payne B. R., Pearson H. E., Berman N. Role of corpus callosum in functional organization of cat striate cortex. J Neurophysiol. 1984 Sep;52(3):570–594. doi: 10.1152/jn.1984.52.3.570. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. Anatomy of interhemispheric connections in the visual system of Boston Siamese and ordinary cats. J Comp Neurol. 1977 Jun 1;173(3):497–518. doi: 10.1002/cne.901730307. [DOI] [PubMed] [Google Scholar]
- Shatz C. Abnormal interhemispheric connections in the visual system of Boston Siamese cats: a physiological study. J Comp Neurol. 1977 Jan 15;171(2):229–245. doi: 10.1002/cne.901710207. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F., Cynader M. Organization of cat striate cortex: a correlation of receptive-field properties with afferent and efferent connections. J Neurophysiol. 1975 Sep;38(5):1080–1098. doi: 10.1152/jn.1975.38.5.1080. [DOI] [PubMed] [Google Scholar]
- Stone J. A quantitative analysis of the distribution of ganglion cells in the cat's retina. J Comp Neurol. 1965 Jun;124(3):337–352. doi: 10.1002/cne.901240305. [DOI] [PubMed] [Google Scholar]
- Toyama K., Matsunami K., Ono T., Tokashiki S. An intracellular study of neuronal organization in the visual cortex. Exp Brain Res. 1974;21(1):45–66. doi: 10.1007/BF00234257. [DOI] [PubMed] [Google Scholar]
- Toyama K., Tokashiki S., Matsunami K. Synaptic action of commissural impulses upon association efferent cells in cat visual cortex. Brain Res. 1969 Jul;14(2):518–520. doi: 10.1016/0006-8993(69)90128-0. [DOI] [PubMed] [Google Scholar]


