Abstract
1. The gated membrane currents (a tetrodotoxin-sensitive Na+ current and a tetraethylammonium- and 4-aminopyridine-sensitive K+ current) of the rapidly adapting stretch receptor neurone of lobster were investigated with respect to their kinetic properties using electrophysiological, pharmacological, and mathematical techniques. 2. The currents were found to be controlled by slow inactivations as well as by fast Hodgkin-Huxley (1952) gating processes. They could be described by kinetic expressions which differed from those inferred for the slowly adapting receptor (Gestrelius & Grampp, 1983a; Gestrelius, Grampp & Sjölin, 1983) only with respect to some of the parameter values. 3. With these expressions, and additional equations for the cell's pump and leak current components (Edman, Gestrelius & Grampp, 1986), a mathematical receptor model was formulated which accurately predicts the impulse activity of the living preparation in different functional circumstances and which, therefore, was adopted as an appropriate theory of firing regulation. 4. From a model analysis it appeared (a) that the 'rapid' adaptation of the receptor's impulse activity is mainly an effect of slow Na+ current inactivation starting a regenerative process of accommodation which, basically, is due to a small ratio of subthreshold Na+ to K+ currents; (b) that, because of the transmembrane Na+ influx being limited by accommodation, impulse firing is only little affected by a Na+-dependent pump current activation; and (c) that the phenomenon of increased firing frequency initially during prolonged stimulation ('negative adaptation') is an effect of the slow K+ current inactivation being faster than the slow Na+ current inactivation at comparable degrees of membrane polarization. 5. From further model studies it also appeared that, during depolarizations between successive action potentials evoked by constant stimulation, the membrane behaves like a high-resistance constant-current generator feeding into a short-circuiting capacitor. In consequence, the cell's stimulus sensitivity (change in firing frequency with stimulation strength) is, at functionally relevant stimulation intensities, mainly determined by the membrane capacitance and by the amplitude of the interspike membrane depolarization while, at higher stimulation intensities and firing frequencies, it becomes more and more a function of the spike duration itself.
Full text
PDF


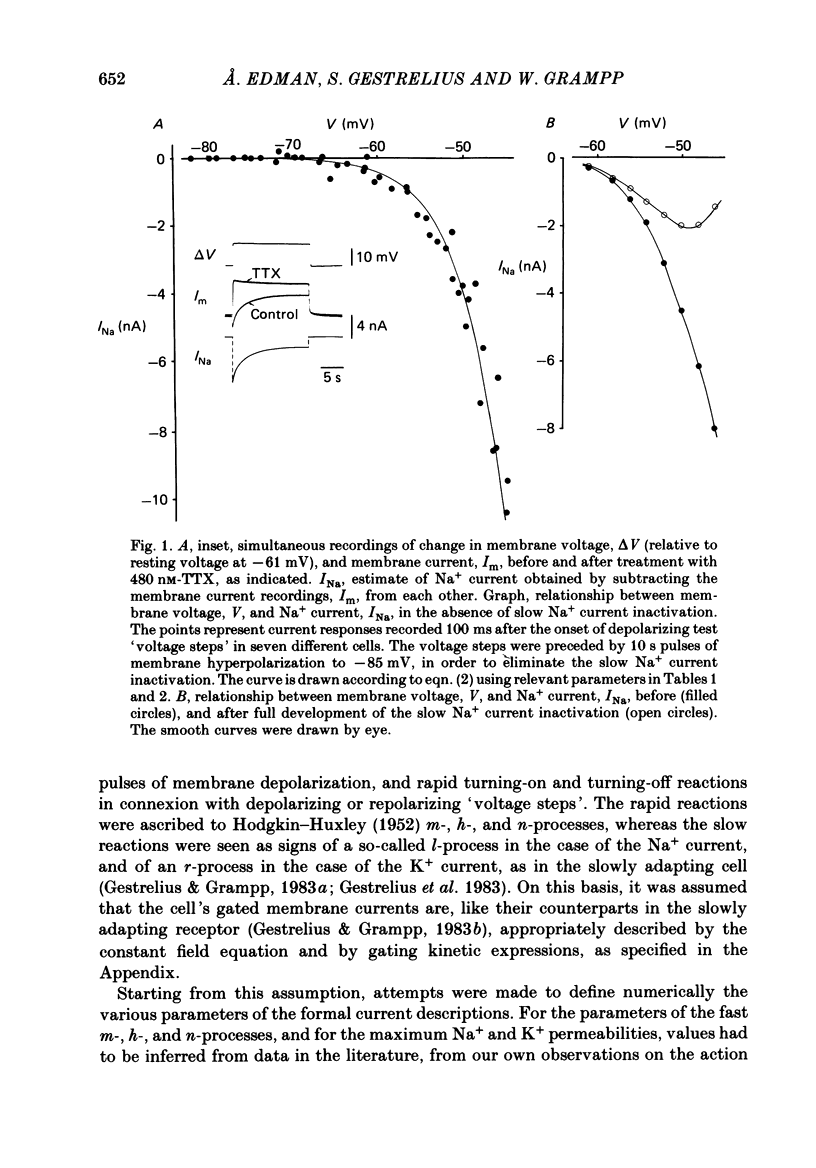
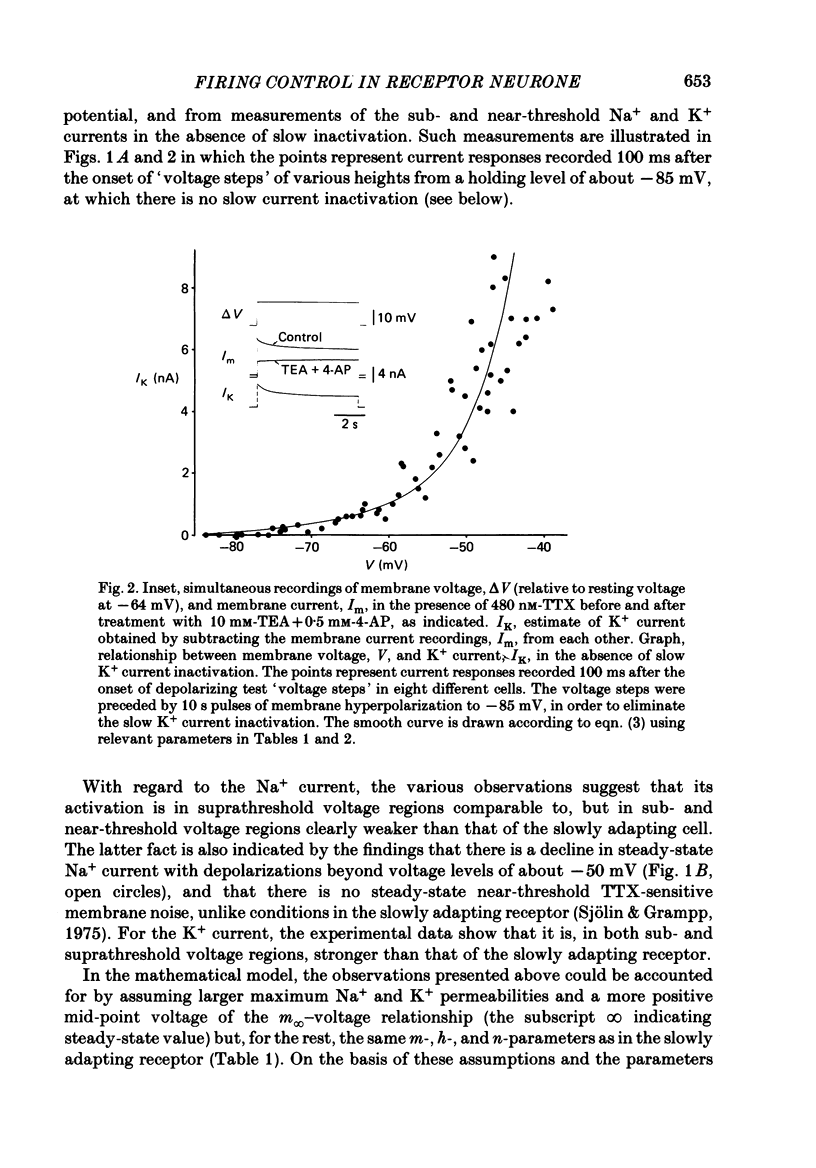

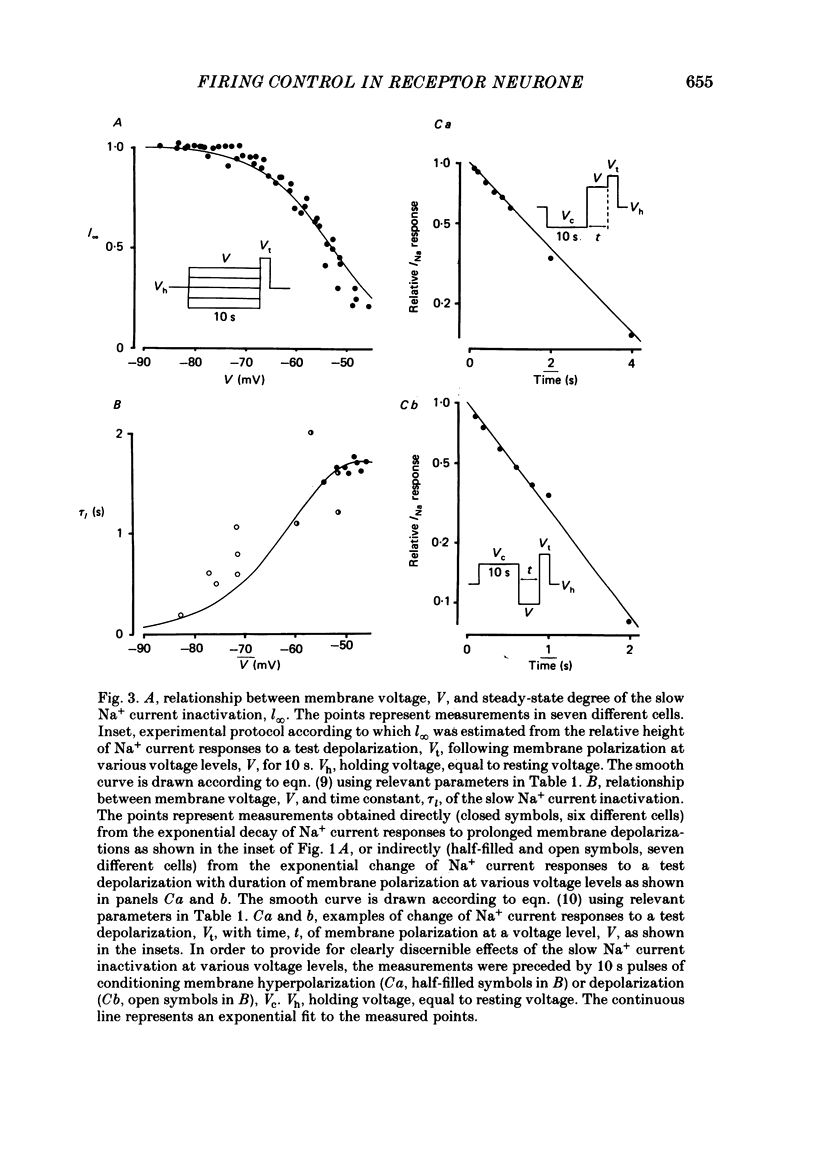
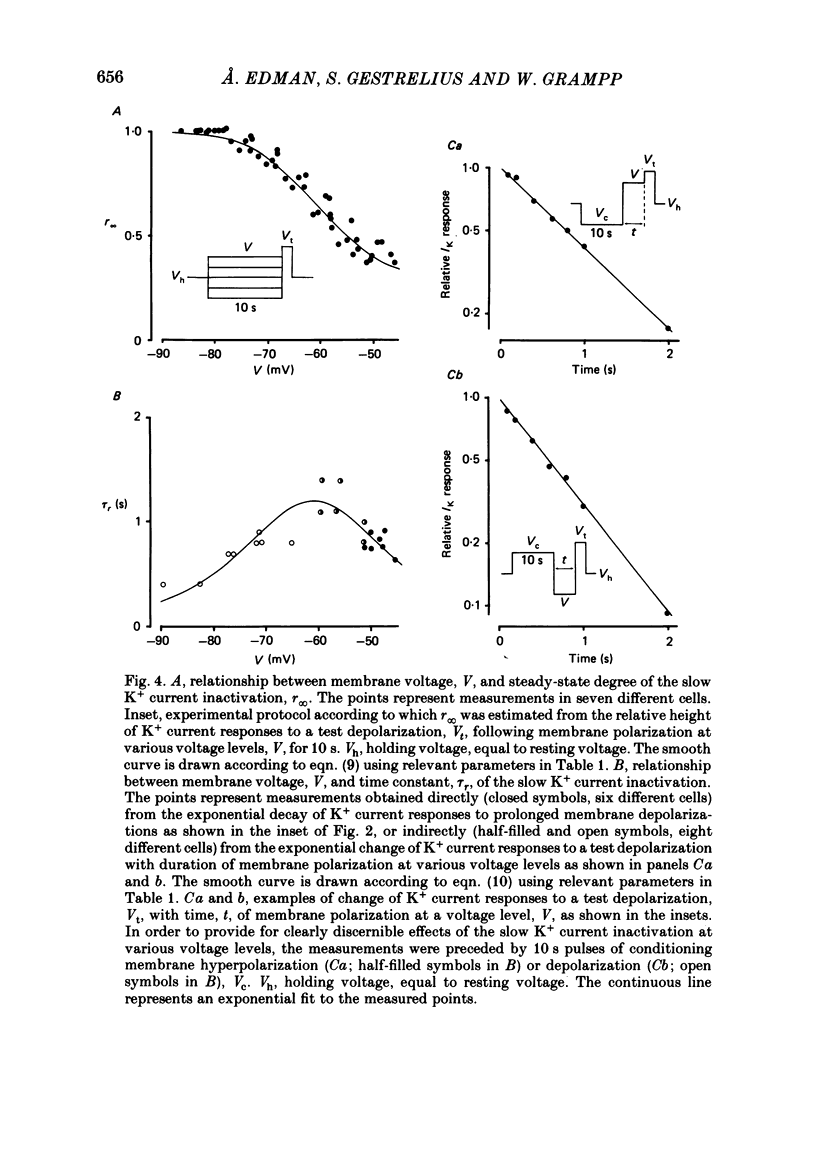

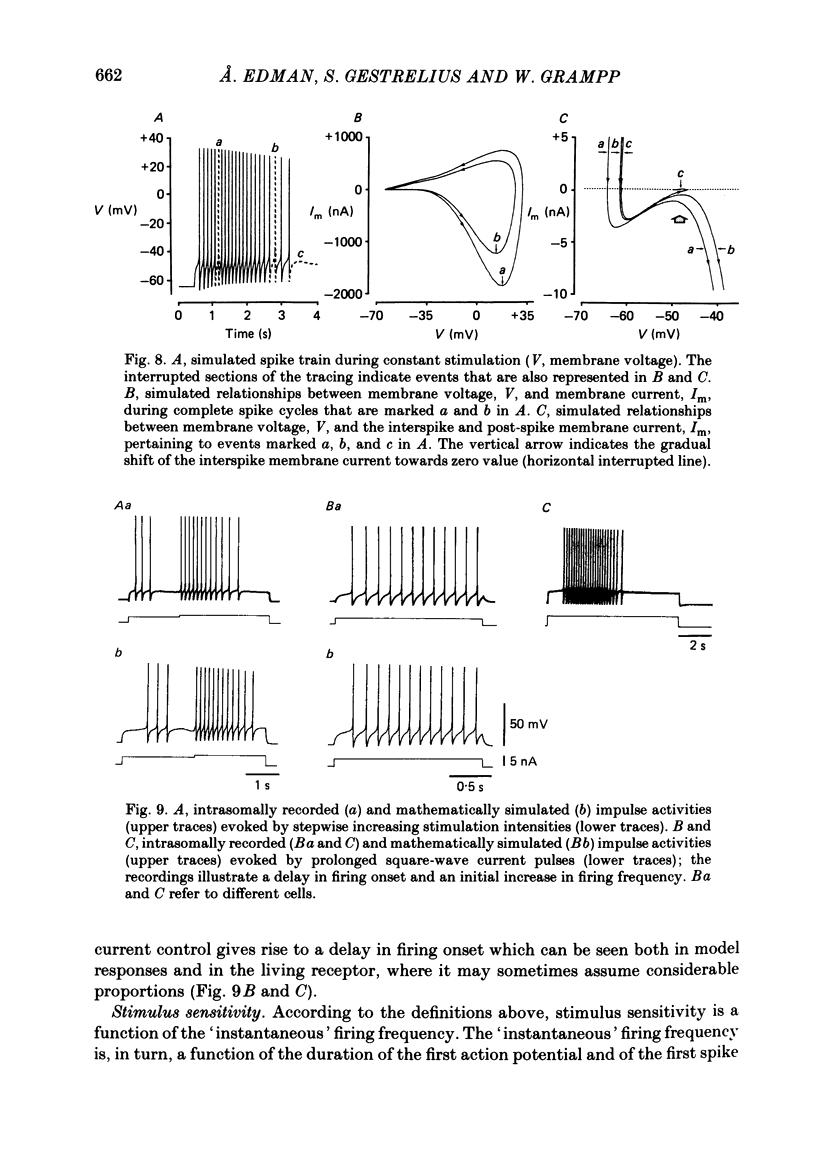
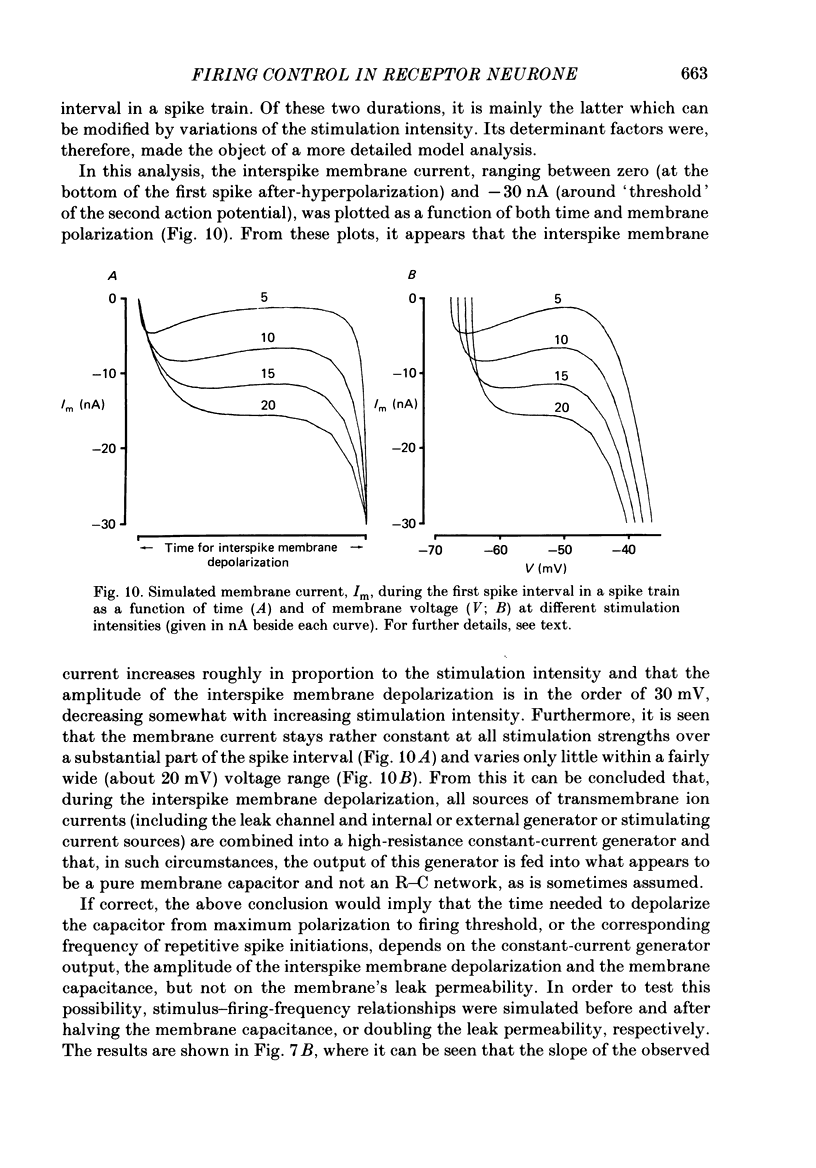






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clementz B., Grampp W. A method for rapid bevelling of micropipette electrodes. Acta Physiol Scand. 1976 Feb;96(2):286–288. doi: 10.1111/j.1748-1716.1976.tb10199.x. [DOI] [PubMed] [Google Scholar]
- ECKERT R. O. Reflex relationships of the abdominal stretch receptors of the crayfish. I. Feedback inhibition of the receptors. J Cell Comp Physiol. 1961 Jun;57:149–162. doi: 10.1002/jcp.1030570303. [DOI] [PubMed] [Google Scholar]
- EYZAGUIRRE C., KUFFLER S. W. Processes of excitation in the dendrites and in the soma of single isolated sensory nerve cells of the lobster and crayfish. J Gen Physiol. 1955 Sep 20;39(1):87–119. doi: 10.1085/jgp.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman A., Gestrelius S., Grampp W. Intracellular ion control in lobster stretch receptor neurone. Acta Physiol Scand. 1983 Jul;118(3):241–252. doi: 10.1111/j.1748-1716.1983.tb07268.x. [DOI] [PubMed] [Google Scholar]
- Edman A., Gestrelius S., Grampp W. Transmembrane ion balance in slowly and rapidly adapting lobster stretch receptor neurones. J Physiol. 1986 Aug;377:171–191. doi: 10.1113/jphysiol.1986.sp016180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestrelius S., Grampp W. Impulse firing in the slowly adapting stretch receptor neurone of lobster and its numerical simulation. Acta Physiol Scand. 1983 Jul;118(3):253–261. doi: 10.1111/j.1748-1716.1983.tb07269.x. [DOI] [PubMed] [Google Scholar]
- Gestrelius S., Grampp W. Kinetics of the TEA and 4-AP sensitive K+ current in the slowly adapting lobster stretch receptor neurone. Acta Physiol Scand. 1983 Jun;118(2):125–134. doi: 10.1111/j.1748-1716.1983.tb07252.x. [DOI] [PubMed] [Google Scholar]
- Gestrelius S., Grampp W., Sjölin L. Kinetics of the TTX sensitive Na+ current in the slowly adapting lobster stretch receptor neurone. Acta Physiol Scand. 1983 Jun;118(2):135–140. doi: 10.1111/j.1748-1716.1983.tb07253.x. [DOI] [PubMed] [Google Scholar]
- Gestrelius S., Grampp W., Sjölin L. Subthreshold and near-threshold membrane currents in lobster stretch receptor neurones. J Physiol. 1981 Jan;310:191–203. doi: 10.1113/jphysiol.1981.sp013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grampp W. The impulse activity in different parts of the slowly adapting stretch receptor neuron of the lobster. Acta Physiol Scand Suppl. 1966;262:1–36. [PubMed] [Google Scholar]
- Gustafsson B., Linström S., Zangger P. Firing behaviour of dorsal spinocerebellar tract neurones. J Physiol. 1978 Feb;275:321–343. doi: 10.1113/jphysiol.1978.sp012192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of 4-aminopyridine on potassium currents in a molluscan neuron. J Gen Physiol. 1981 Jul;78(1):63–86. doi: 10.1085/jgp.78.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Onodera K. Adaptation of the generator potential in the crayfish stretch receptors under constant length and constant tension. J Physiol. 1969 Jan;200(1):187–204. doi: 10.1113/jphysiol.1969.sp008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölin L., Grampp W. Membrane noise in slowly adapting stretch receptor neurone of lobster. Nature. 1975 Oct 23;257(5528):696–697. doi: 10.1038/257696a0. [DOI] [PubMed] [Google Scholar]


