Abstract
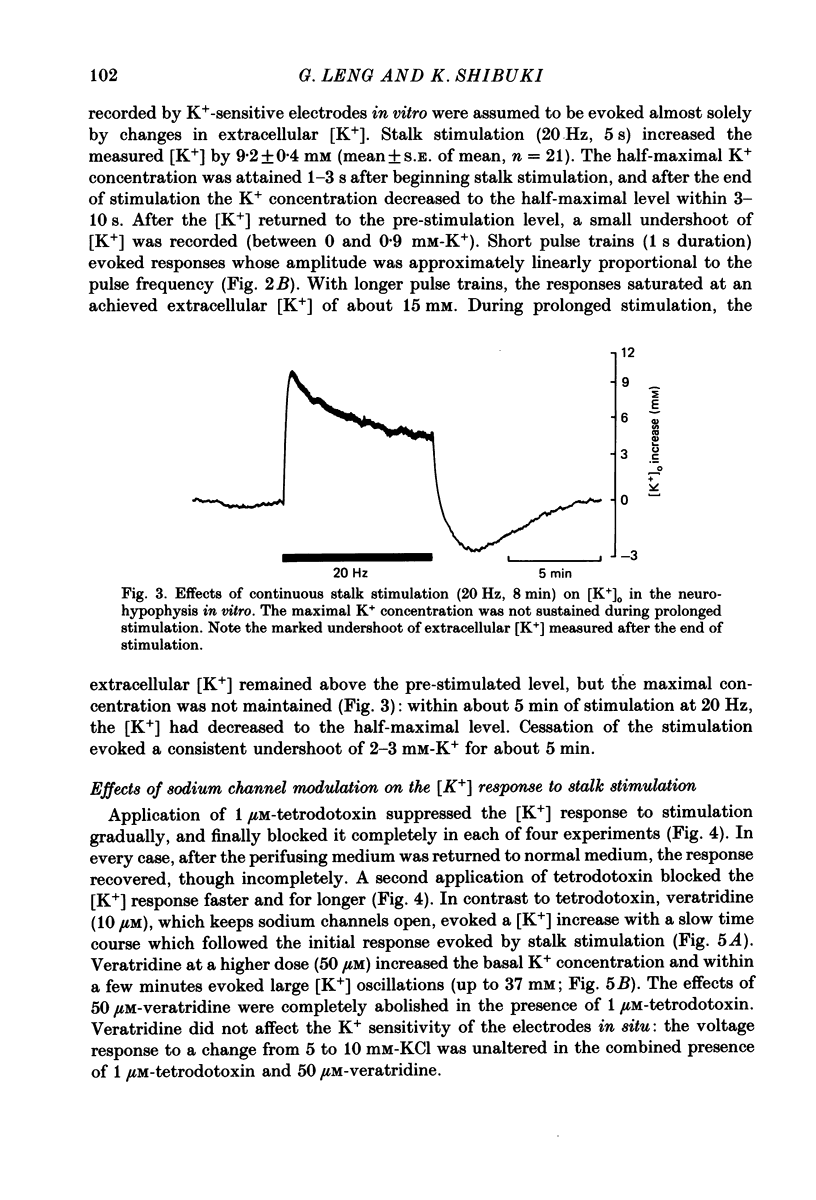
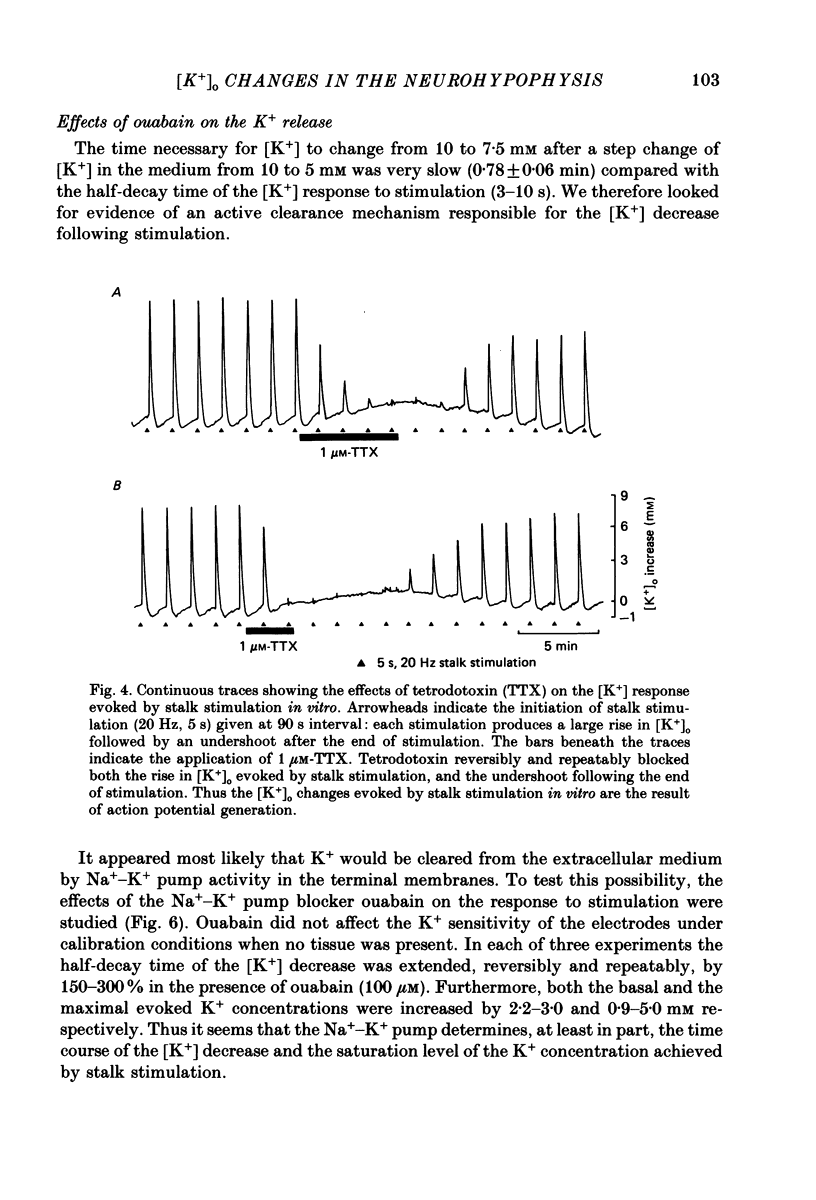
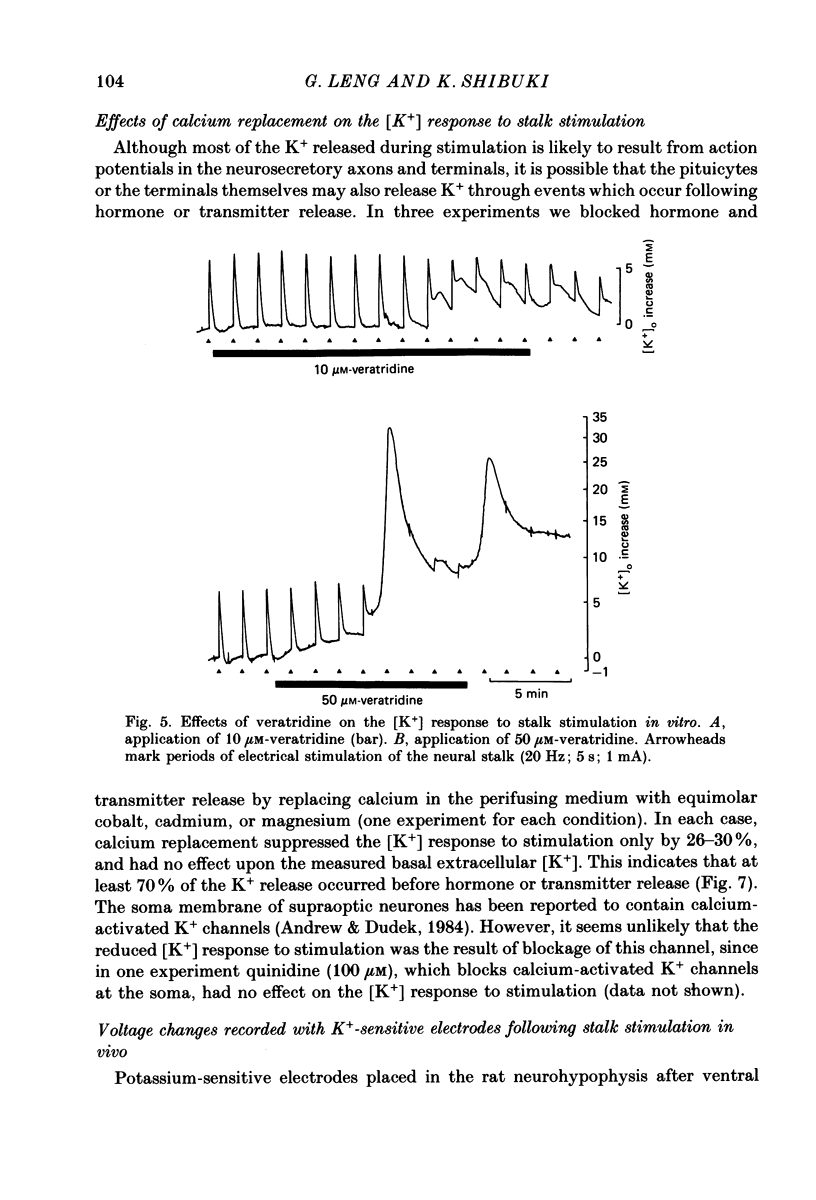
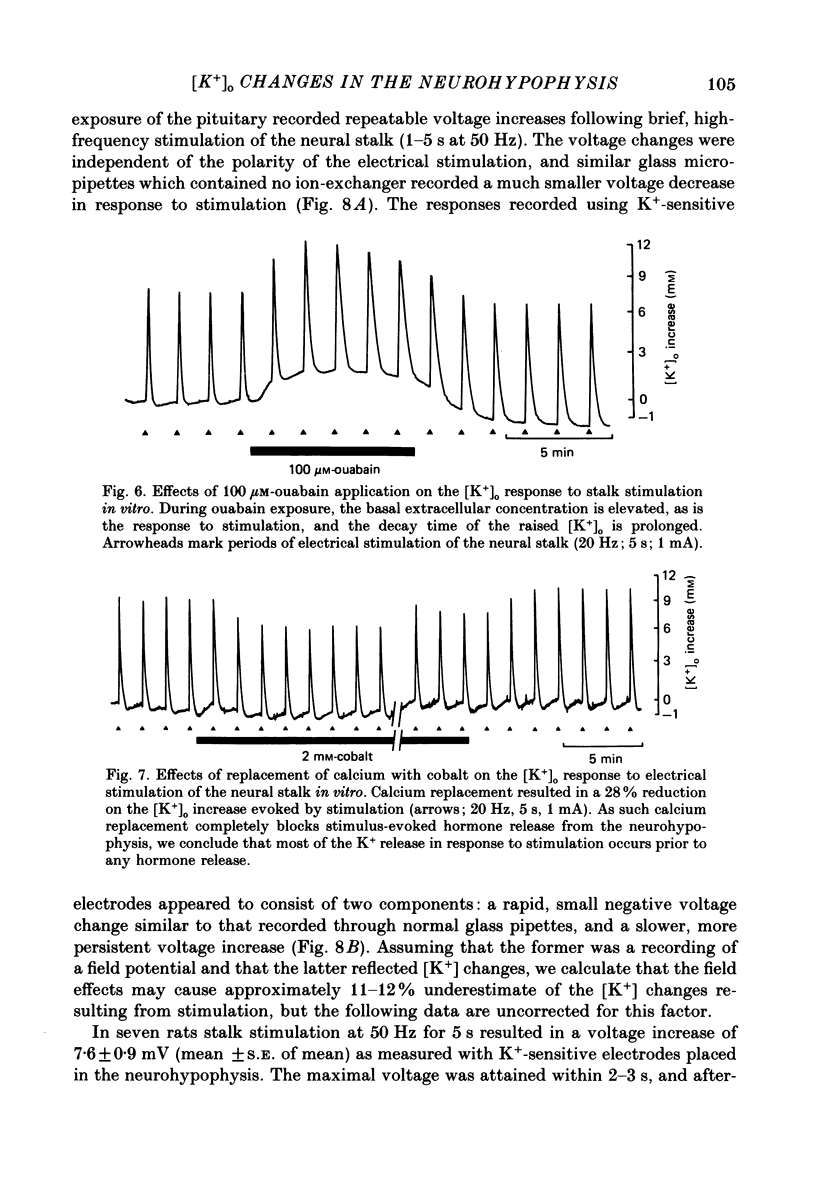
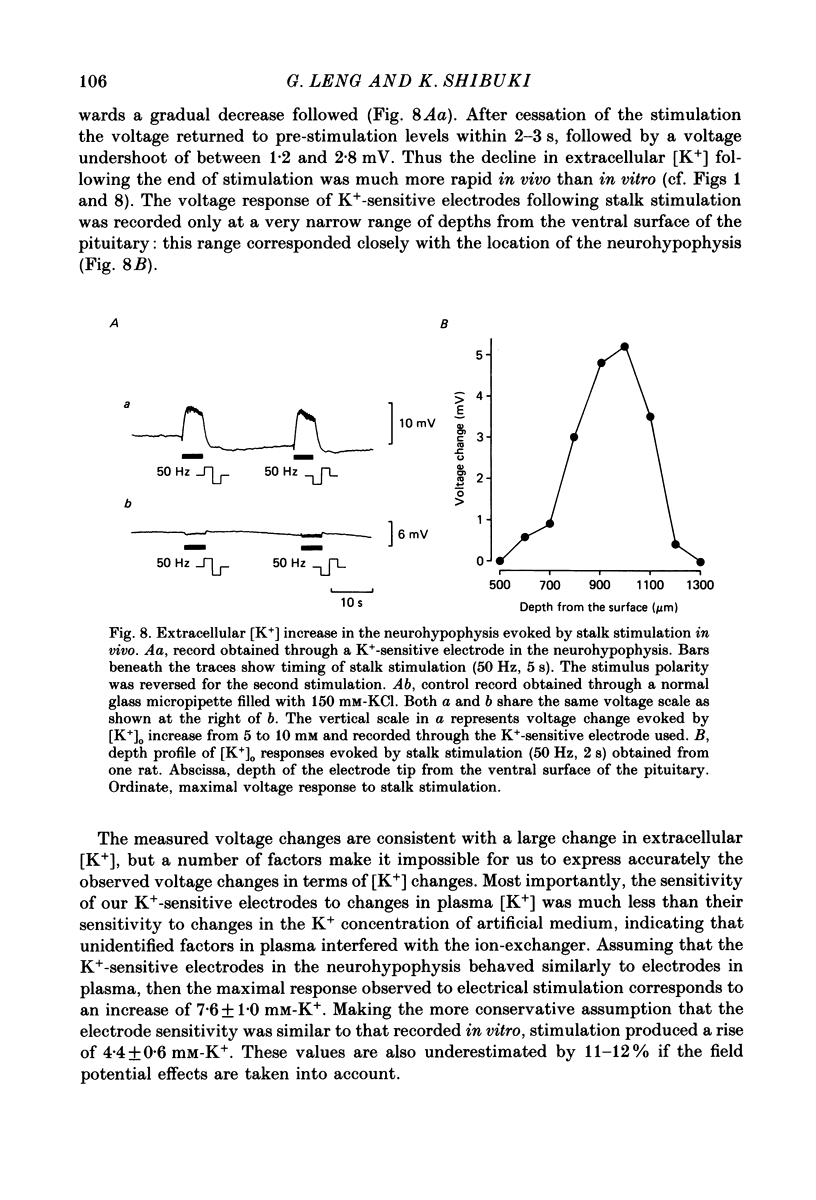
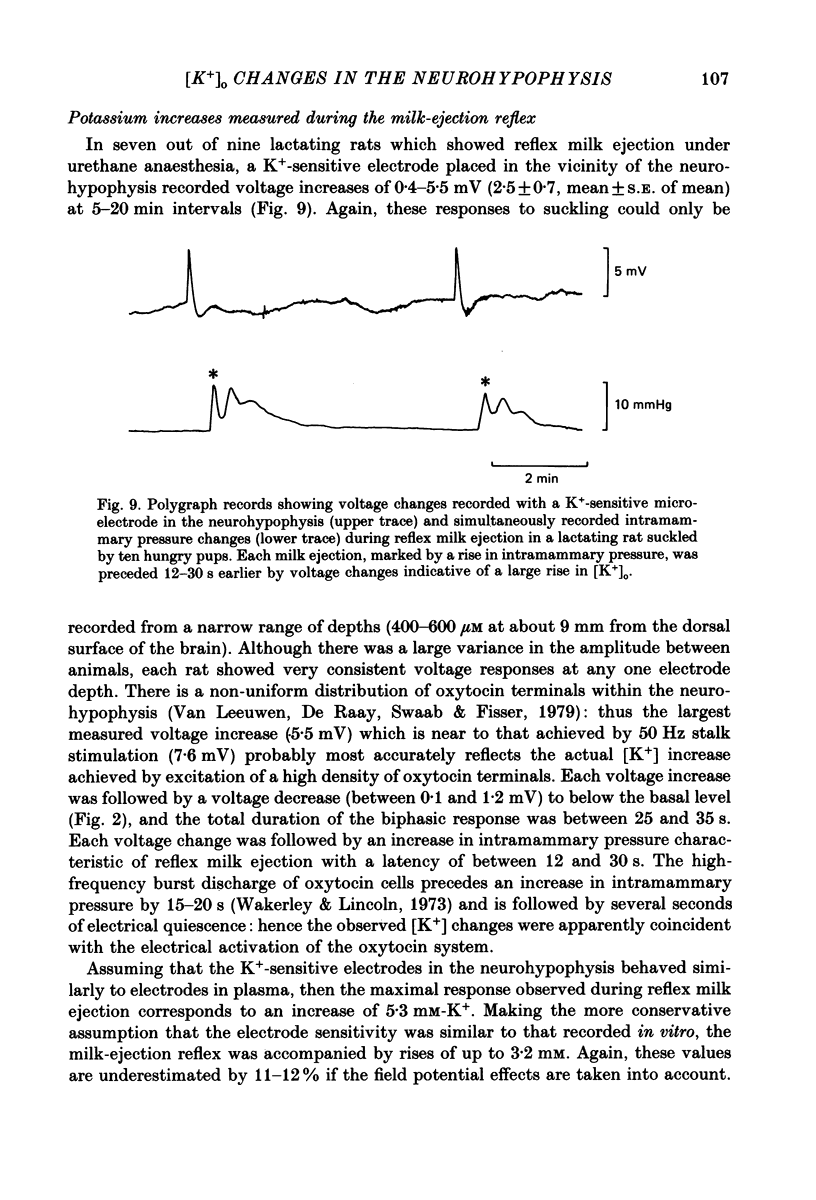
1. Potassium-sensitive microelectrodes were used to measure extracellular [K+] in the isolated rat neurohypophysis maintained in vitro. Electrical stimulation of the neurohypophysial stalk (20 Hz 5 s) increased the inferred extracellular [K+] by 9.2 +/- 0.4 mM (mean +/- S.E. of mean; n = 21). 2. Veratridine (10 microM) enhanced the response to stalk stimulation, and at a higher concentration (50 microM) increased extracellular [K+] in the absence of stimulation. By contrast, tetrodotoxin (1 microM) blocked the [K+] increase completely and reversibly in each of five experiments, indicating that the increase was a consequence of action potential generation. 3. At the end of brief periods of stimulation, the raised extracellular [K+] returned to pre-stimulation levels within 30 s. In the presence of ouabain (100 microM), the recovery was slower: the half-decay time was extended by 150-300% in each of three experiments. 4. Replacement of calcium in the medium with cobalt, cadmium or magnesium reduced the amplitude of the [K+] increase by 26-30%, indicating that the [K+] increase was largely independent of events subsequent to evoked release of hormone and/or transmitters. 5. Potassium-sensitive microelectrodes were placed in the neurohypophysis of rats anaesthetized with urethane. Electrical stimulation of the pituitary stalk (50 Hz, 5 s) produced transient voltage increases of 7.6 +/- 0.9 mV (mean +/- S.E. of mean of seven experiments). These voltage increases were similar in magnitude to the response of the electrodes to the addition of 7.6 +/- 1.0 mM-K+ to rat plasma. 6. In seven lactating rats, the suckling of a litter of hungry pups evoked periodic reflex milk ejections, as detected by increases in intramammary pressure. Potassium-sensitive microelectrodes in the neurohypophysis recorded transient voltage increases prior to each milk ejection (0.4-5.5 mV). Each increase preceded an increase in intramammary pressure by 12-30 s. 7. Thus synchronized high-frequency activation of magnocellular neurones can produce large changes in extracellular [K+]. The implications of these findings for stimulus-secretion coupling in the neurohypophysis are discussed in the light of previous reports that hormone release from the neurohypophysis is highly dependent on the frequency and pattern of electrical stimulation.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew R. D., Dudek F. E. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol. 1984 Mar;51(3):552–566. doi: 10.1152/jn.1984.51.3.552. [DOI] [PubMed] [Google Scholar]
- Belin V., Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol. 1986 Aug;377:369–390. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R. J., Brown D., Chapman C., Hancock P. D., Leng G. Reversible fatigue of stimulus-secretion coupling in the rat neurohypophysis. J Physiol. 1984 Mar;348:601–613. doi: 10.1113/jphysiol.1984.sp015128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R. J., Leng G. Relative efficiency of neural firing patterns for vasopressin release in vitro. Neuroendocrinology. 1981 Nov;33(5):295–299. doi: 10.1159/000123248. [DOI] [PubMed] [Google Scholar]
- Broadwell R. D., Brightman M. W. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol. 1976 Apr 1;166(3):257–283. doi: 10.1002/cne.901660302. [DOI] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985 Dec;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A., Orkand R. K. Modification of potassium movement through the retina of the drone (Apis mellifera male) by glial uptake. J Physiol. 1983 Jul;340:157–174. doi: 10.1113/jphysiol.1983.sp014756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A., Tsacopoulos M. Potassium activity in photoreceptors, glial cells and extracellular space in the drone retina: changes during photostimulation. J Physiol. 1979 May;290(2):525–549. doi: 10.1113/jphysiol.1979.sp012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Ransom B. R. Chloride conductance and extracellular potassium concentration interact to modify the excitability of rat optic nerve fibres. J Physiol. 1984 Oct;355:619–633. doi: 10.1113/jphysiol.1984.sp015442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. CALCIUM MOVEMENT IN THE NEUROHYPOPHYSIS OF THE RAT AND ITS RELATION TO THE RELEASE OF VASOPRESSIN. J Physiol. 1964 Jul;172:19–30. doi: 10.1113/jphysiol.1964.sp007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kalnins I., Kelly J. S., Ruf K. B. Action potentials and release of neurohypophysial hormones in vitro. J Physiol. 1971 Jul;215(3):805–817. doi: 10.1113/jphysiol.1971.sp009499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A., Dyball R. E. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979 May;290(2):433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Weight F. F. Extracellular potassium and trasmitter release at the giant synapse of squid. J Physiol. 1977 Apr;266(2):209–218. doi: 10.1113/jphysiol.1977.sp011764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Medwin A. R. A study of the mechanisms by which potassium moves through brain tissue in the rat. J Physiol. 1983 Feb;335:353–374. doi: 10.1113/jphysiol.1983.sp014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Medwin A. R., Nicholson C. Changes of extracellular potassium activity induced by electric current through brain tissue in the rat. J Physiol. 1983 Feb;335:375–392. doi: 10.1113/jphysiol.1983.sp014540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton G. I., Perlmutter L. S., Salm A. K., Tweedle C. D. Dynamic neuronal-glial interactions in hypothalamus and pituitary: implications for control of hormone synthesis and release. Peptides. 1984;5 (Suppl 1):121–138. doi: 10.1016/0196-9781(84)90271-7. [DOI] [PubMed] [Google Scholar]
- Ishida A. The oxytocin release and the compound action potential evoked by electrical stimulation on the isolated neurohypophysis of the rat. Jpn J Physiol. 1970 Feb 15;20(1):84–96. doi: 10.2170/jjphysiol.20.84. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Stuenkel E. L. Electrical properties of axons and neurohypophysial nerve terminals and their relationship to secretion in the rat. J Physiol. 1986 Nov;380:521–539. doi: 10.1113/jphysiol.1986.sp016300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Parsons R. L., Hofmann W. W., Feigen G. A. Presynaptic effects of potassium ion on the mammalian neuromuscular junction. Nature. 1965 Nov 6;208(5010):590–591. doi: 10.1038/208590a0. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Shaw F. D., Bicknell R. J., Dyball R. E. Facilitation of vasopressin release from the neurohypophysis by application of electrical stimuli in bursts. Relevant stimulation parameters. Neuroendocrinology. 1984 Oct;39(4):371–376. doi: 10.1159/000124007. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Extracellular potassium in the mammalian central nervous system. Annu Rev Physiol. 1979;41:159–177. doi: 10.1146/annurev.ph.41.030179.001111. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Changes in potassium concentration around motor nerve terminals, produced by current flow, and their effects on neuromuscular transmission. J Physiol. 1961 Jan;155:46–58. doi: 10.1113/jphysiol.1961.sp006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E., Clarke G., Dreifuss J. J., Lincoln D. W. The role of central adrenergic receptors in the reflex release of oxytocin. Brain Res. 1978 Feb 17;142(1):69–84. doi: 10.1016/0006-8993(78)90177-4. [DOI] [PubMed] [Google Scholar]
- Tweedle C. D., Hatton G. I. Evidence for dynamic interactions between pituicytes and neurosecretory axons in the rat. Neuroscience. 1980;5(3):661–671. doi: 10.1016/0306-4522(80)90063-9. [DOI] [PubMed] [Google Scholar]
- Tweedle C. D., Hatton G. I. Magnocellular neuropeptidergic terminals in neurohypophysis: rapid glial release of enclosed axons during parturition. Brain Res Bull. 1982 Feb;8(2):205–209. doi: 10.1016/0361-9230(82)90047-8. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J Endocrinol. 1973 Jun;57(3):477–493. doi: 10.1677/joe.0.0570477. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F. W., de Raay C., Swaab D. F., Fisser B. The localization of oxytocin, vasopressin, somatostatin and luteinizing hormone releasing hormone in the rat neurohypophysis. Cell Tissue Res. 1979 Nov;202(2):189–201. doi: 10.1007/BF00232234. [DOI] [PubMed] [Google Scholar]


