Abstract
1. Na+ and K+ flux measurements and membrane potential (Vm) determinations were performed on normal and denervated rat extensor digitorum longus (e.d.l.) muscles. 2. The mean Vm in normal muscle fibres was -74.6 mV. During the first week after denervation Vm fell about 20 mV following an S-shaped time course. 3. In that period the Na+ permeability (PNa) increased and the K+ permeability (PK) decreased, so that by the sixth day post-denervation, the PNa/PK ratio was increased by a factor of 2.7. 4. The decrease in PK preceded the increase in PNa. 5. No major contribution to the fall of Vm by a reduced activity of an electrogenic Na+ pump could be detected. 6. A good agreement was found between the experimental values of the depolarization and those calculated using the constant-field equation assuming Cl- is at equilibrium and no significant change of the intracellular K+ concentration ([K+]i) during the first week after denervation. 7. It is concluded that the depolarization promoted by denervation in e.d.l. rat muscle fibres can be fully explained in terms of changes in PNa and PK.
Full text
PDF




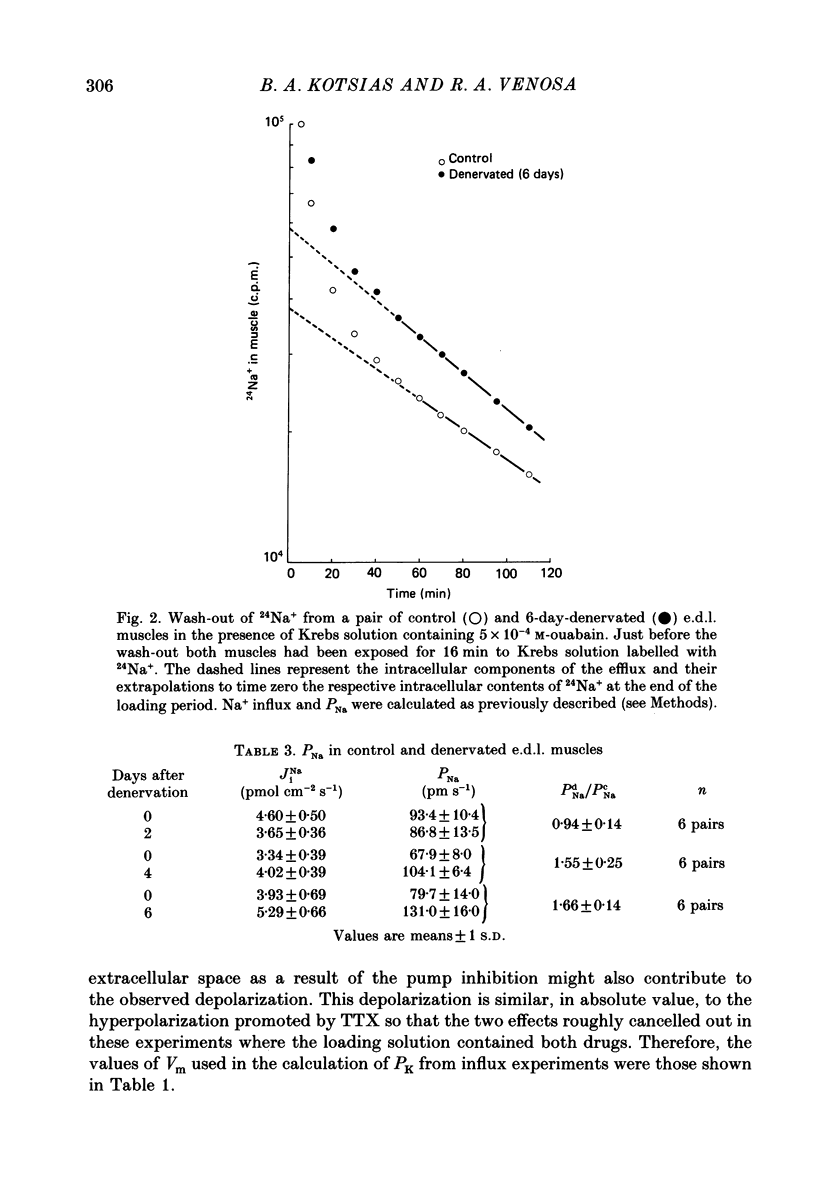
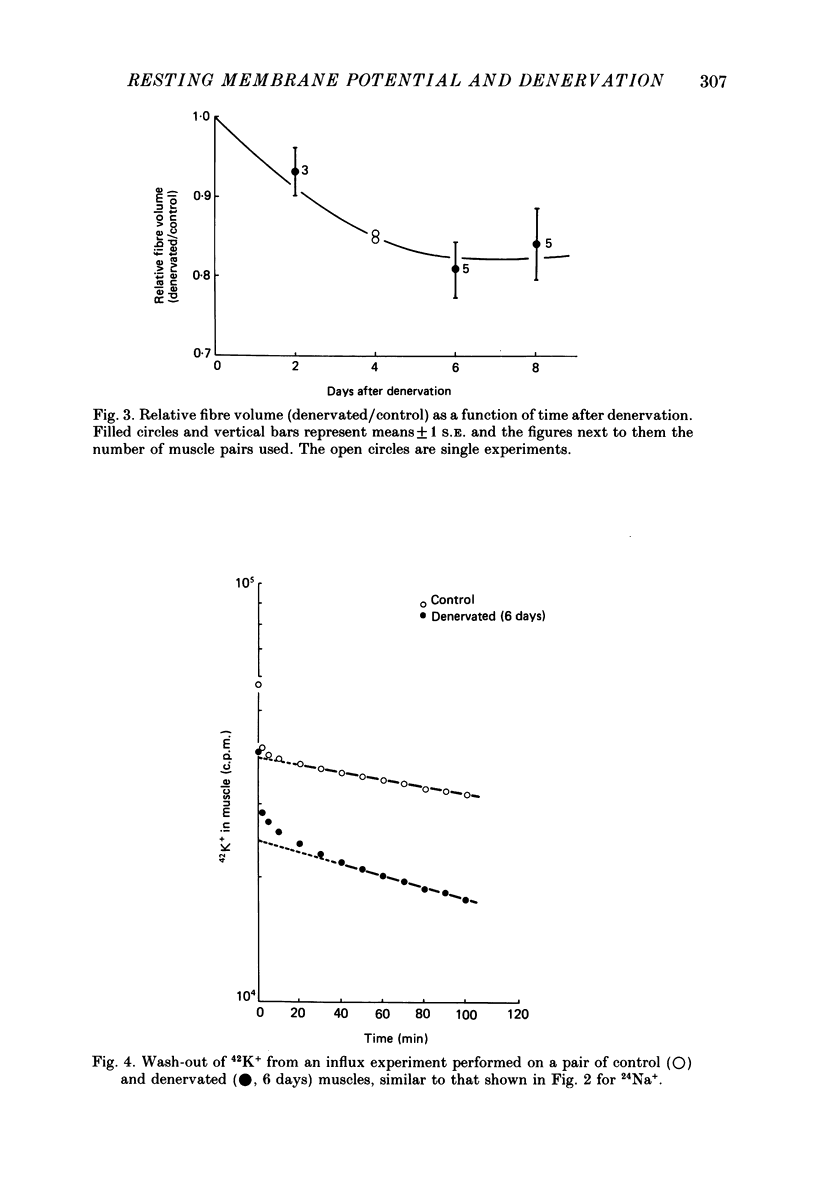

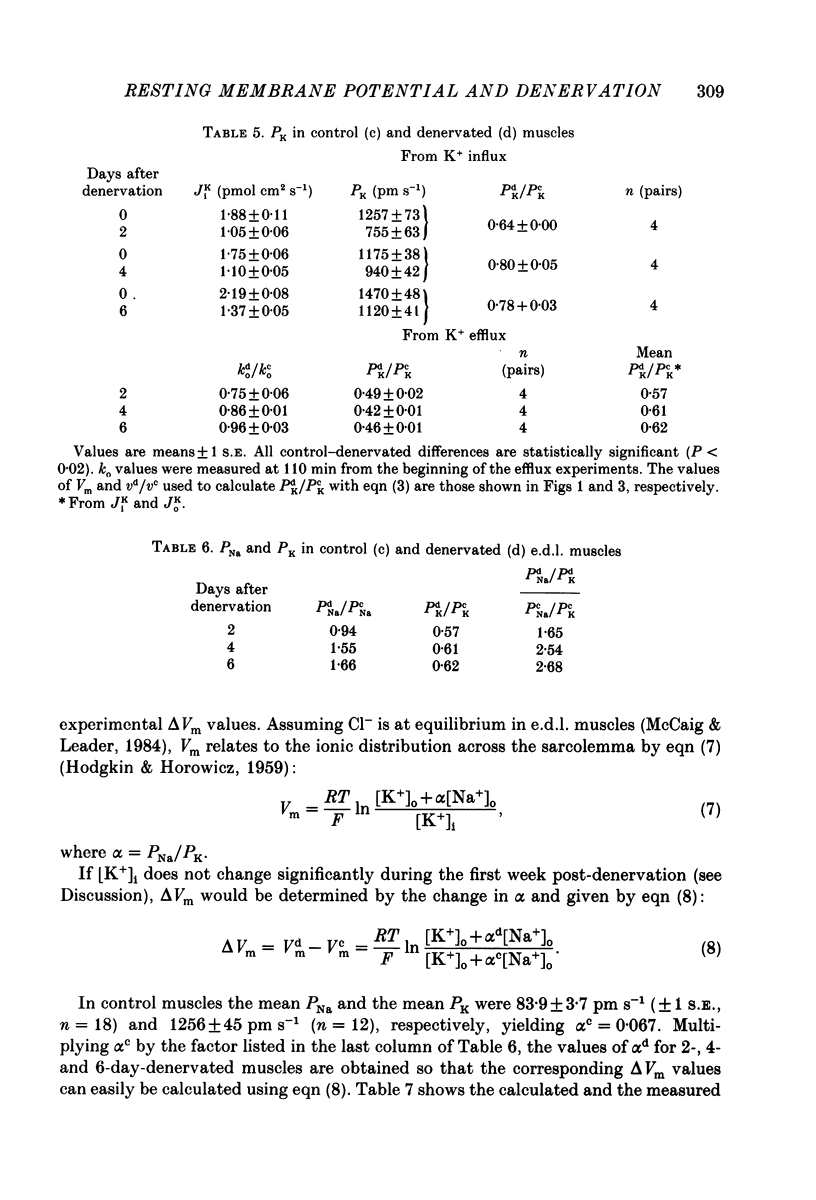
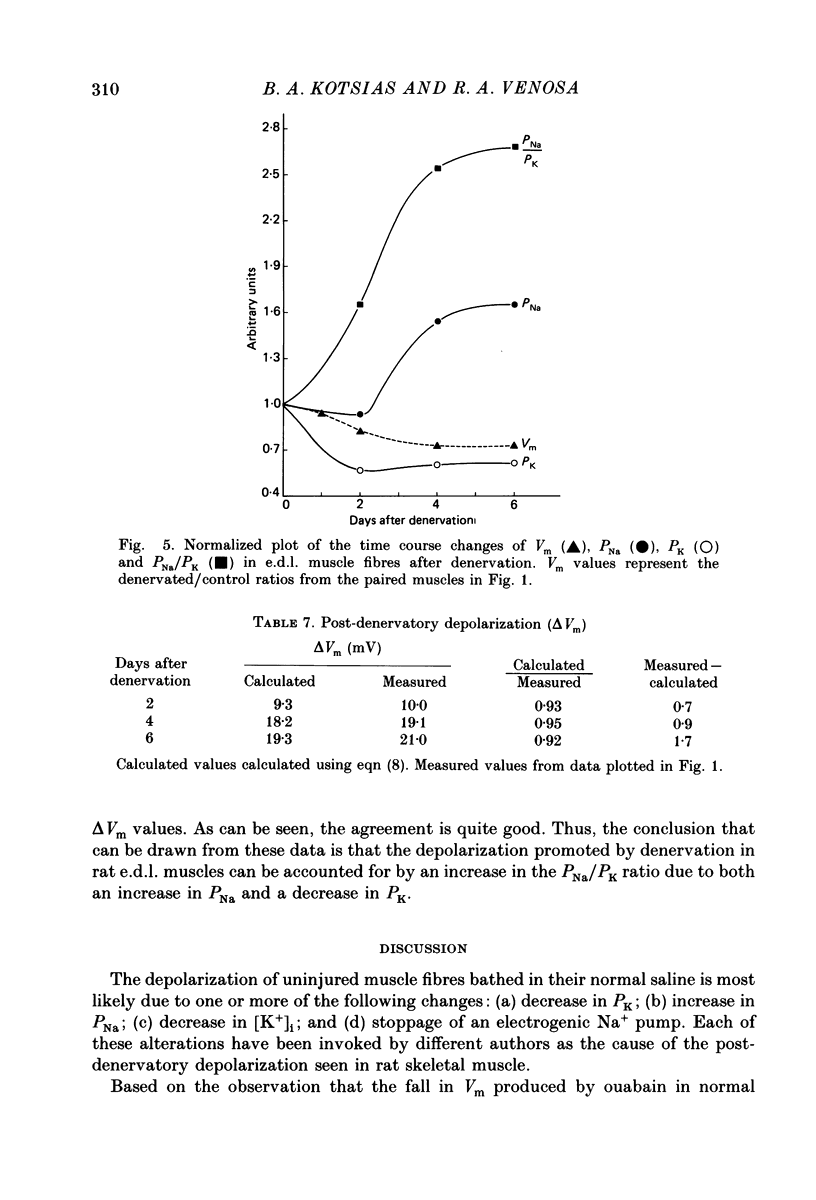



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., McIsaac R. J. Fast and slow mammalian muscles after denervation. Exp Neurol. 1970 Jan;26(1):183–202. doi: 10.1016/0014-4886(70)90099-3. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Schuh F. T., Kauffman F. C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflugers Arch. 1971;328(1):36–50. doi: 10.1007/BF00587359. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Warnick J. E. The pharmacology of batrachotoxin. IV. Interaction with tetrodotoxin on innervated and chronically denervated rat skeletal muscle. J Pharmacol Exp Ther. 1972 Mar;180(3):683–697. [PubMed] [Google Scholar]
- Arrizurieta de Muchnik E., Sosa M., Kotsias B. A., Muchnik S. Ionic changes following denervation and reinnervation in mammalian skeletal muscle. Medicina (B Aires) 1981;41(1):60–64. [PubMed] [Google Scholar]
- Bray J. J., Hawken M. J., Hubbard J. I., Pockett S., Wilson L. The membrane potential of rat diaphragm muscle fibres and the effect of denervation. J Physiol. 1976 Mar;255(3):651–667. doi: 10.1113/jphysiol.1976.sp011301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerino D., Bryant S. H. Effects of denervation and colchicine treatment on the chloride conductance of rat skeletal muscle fibers. J Neurobiol. 1976 May;7(3):221–228. doi: 10.1002/neu.480070305. [DOI] [PubMed] [Google Scholar]
- Creese R., el-Shafie A. L., Vrbová G. Sodium movements in denervated muscle and the effects of antimycin A. J Physiol. 1968 Jul;197(2):279–294. doi: 10.1113/jphysiol.1968.sp008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAHOTA Z. Electrolytes in denervated and reinnervated muscle. Proc Soc Exp Biol Med. 1960 Apr;103:849–851. doi: 10.3181/00379727-103-25693. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The resting exchange of radioactive potassium in crab nerve. J Physiol. 1951 Mar;113(1):73–98. doi: 10.1113/jphysiol.1951.sp004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The permeability of frog muscle fibres to lithium ions. J Physiol. 1959 Oct;147:626–638. doi: 10.1113/jphysiol.1959.sp006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsias B. A., Muchnik S., Arrizurieta E. E., Losavio A. S., Sosa M. Influence of trophic substances in the regulation of resting membrane potential and ionic concentration in skeletal muscle. Exp Neurol. 1985 Apr;88(1):56–67. doi: 10.1016/0014-4886(85)90113-x. [DOI] [PubMed] [Google Scholar]
- Kotsias B. A., Venosa R. A., Horowicz P. Denervated frog skeletal muscle. Some electrical and mechanical properties. Pflugers Arch. 1984 Mar;400(3):262–268. doi: 10.1007/BF00581557. [DOI] [PubMed] [Google Scholar]
- Kovács T., Vissy A., Went E. The effect of denervation on iontransport in tonic and tetanic skeletal muscles of the rat. Acta Physiol Acad Sci Hung. 1968;33(1):55–68. [PubMed] [Google Scholar]
- LULLMANN H. Uber die Konstanz des Membranpotentials bei spontanen Anderungen der Ionengradienten am normalen und denervierten Rattenzwerchfell. Pflugers Arch. 1958;267(2):188–189. doi: 10.1007/BF00362985. [DOI] [PubMed] [Google Scholar]
- Leader J. P., Bray J. J., Macknight A. D., Mason D. R., McCaig D., Mills R. G. Cellular ions in intact and denervated muscles of the rat. J Membr Biol. 1984;81(1):19–27. doi: 10.1007/BF01868806. [DOI] [PubMed] [Google Scholar]
- Locke S., Solomon H. C. Relation of resting potential of rat gastrocnemius and soleus muscles to innervation, activity, and the Na-K pump. J Exp Zool. 1967 Dec;166(3):377–386. doi: 10.1002/jez.1401660310. [DOI] [PubMed] [Google Scholar]
- Lorković H., Tomanek R. J. Potassium and chloride conductances in normal and denervated rat muscles. Am J Physiol. 1977 Mar;232(3):C109–C114. doi: 10.1152/ajpcell.1977.232.3.C109. [DOI] [PubMed] [Google Scholar]
- McArdle J. J., Albuquerque E. X. Effects of ouabain on denervated and dystrophic muscles of the mouse. Exp Neurol. 1975 May;47(2):353–356. doi: 10.1016/0014-4886(75)90263-0. [DOI] [PubMed] [Google Scholar]
- McCaig D., Leader J. P. Intracellular chloride activity in the extensor digitorum longus (EDL) muscle of the rat. J Membr Biol. 1984;81(1):9–17. doi: 10.1007/BF01868805. [DOI] [PubMed] [Google Scholar]
- Robbins N. Cation movements in normal and short-term denervated rat fast twitch muscle. J Physiol. 1977 Oct;271(3):605–624. doi: 10.1113/jphysiol.1977.sp012017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabunova I., Vyskocil F. Postdenervation changes of intracellular potassium and sodium measured by ion selective microelectrodes in rat soleus and extensor digitorum longus muscle fibres. Pflugers Arch. 1982 Aug;394(2):161–164. doi: 10.1007/BF00582919. [DOI] [PubMed] [Google Scholar]
- Venosa R. A., Horowicz P. Effects on sodium efflux of treating frog sartorius muscles with hypertonic glycerol solutions. J Membr Biol. 1973 Dec 6;14(1):33–56. doi: 10.1007/BF01868067. [DOI] [PubMed] [Google Scholar]
- Venosa R. A. Inward movement of sodium ions in resting and stimulated frog's sartorius muscle. J Physiol. 1974 Aug;241(1):155–173. doi: 10.1113/jphysiol.1974.sp010646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARE F., Jr, BENNETT A. L., McINTYRE A. R. Membrane resting potential of denervated mammalian skeletal muscle measured in vivo. Am J Physiol. 1954 Apr;177(1):115–118. doi: 10.1152/ajplegacy.1954.177.1.115. [DOI] [PubMed] [Google Scholar]
- Wareham A. C. Effect of denervation and ouabain on the response of the resting membrane potential of rat skeletal muscle to potassium. Pflugers Arch. 1978 Mar 20;373(3):225–228. doi: 10.1007/BF00580828. [DOI] [PubMed] [Google Scholar]


