Abstract
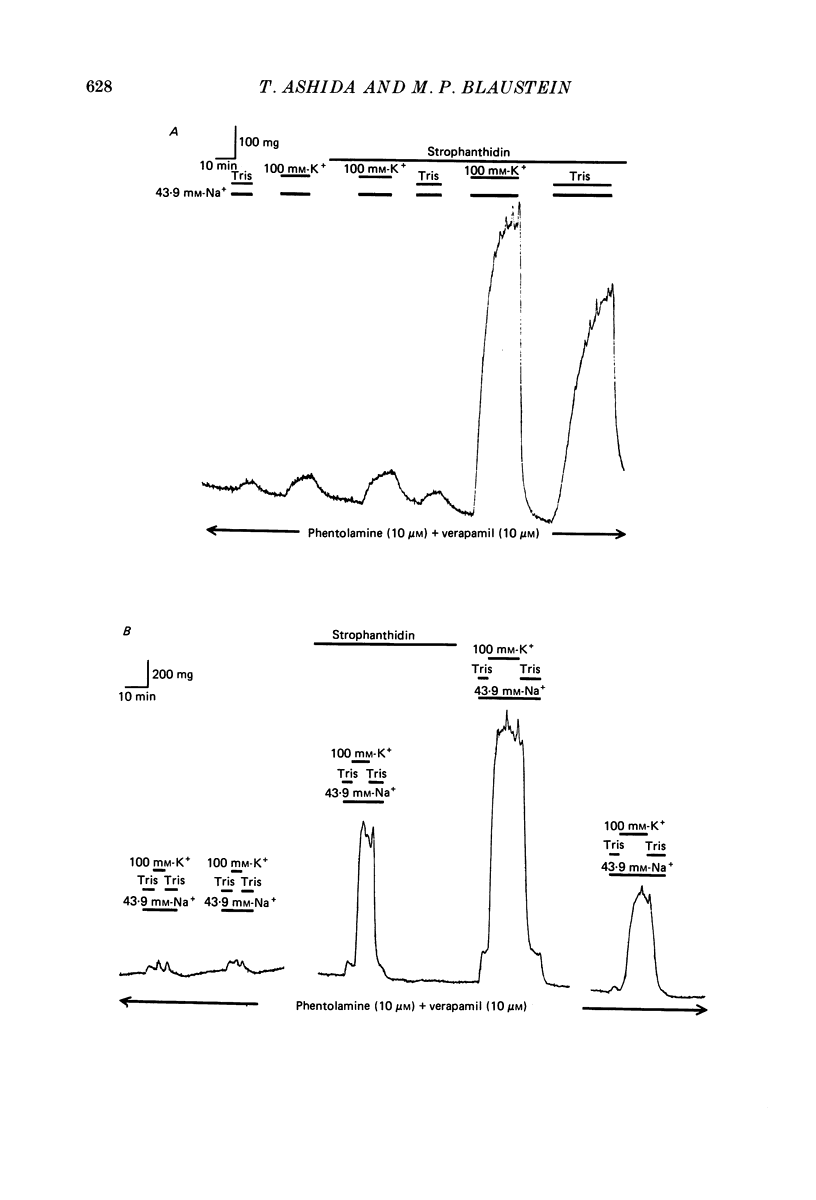
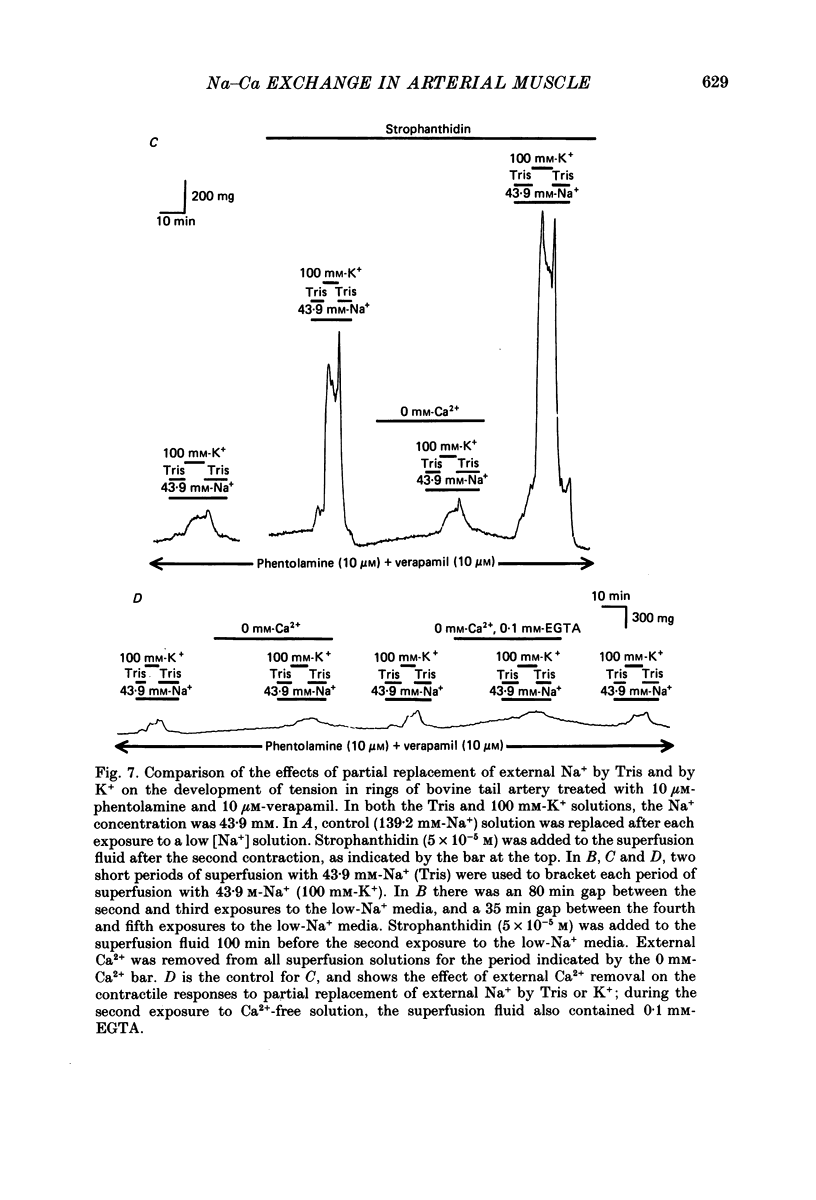
1. The contraction and relaxation of rings of rat thoracic aorta and bovine tail artery were examined as a function of changes in the Na+ electrochemical gradient in order to determine the role of Na-Ca exchange in the control of contractility. 2. Inhibition of the Na+ pump in rat aorta by K+-free media or a low concentration (5 x 10(-5) M) of strophanthidin reversibly increased the contractile responses to caffeine and noradrenaline. These effects were dependent upon external Ca2+ and were observed even in the presence of a Ca2+ channel blocker (10 microM-verapamil or 10 microM-diltiazem) and an alpha-receptor blocker (10 microM-phentolamine). 3. Reduction of external Na+ concentration, [Na+]o (replaced by N-methylglucamine, tetramethylammonium or Tris), also caused an external Ca2+-dependent increase in tonic tension and, in rat aorta, an increase in the response to caffeine. These effects were also observed in the presence of verapamil and phentolamine. 4. Caffeine relaxed the bovine tail artery, but increased the sensitivity of the rat aorta to reduced [Na+]o. The latter effect was presumably due to block of Ca2+ sequestration in the sarcoplasmic reticulum, so that entering Ca2+ was more effective in raising the intracellular free Ca2+ level, [Ca2+]i. 5. Relaxation from K+-free or low-Na+ contractions, in Ca2+-free media, depended upon [Na+]o. Reduction of [Na+]o to 1.2 or 7.5 mM slowed the relaxation of rat aorta (5 mM-caffeine present) 3- to 5-fold, and the relaxation of bovine tail artery (without caffeine) 5- to 10-fold. These effects were seen in the presence of verapamil and phentolamine. 6. These observations are all consistent with an Na-Ca exchange transport system that can move Ca2+ either into or out of the arterial smooth muscle cells. Ca2+ entry is enhanced by raising [Na+]i (by Na+ pump inhibition) and/or lowering [Na+]o. Ca2+ extrusion from the contracted muscles is largely dependent upon external Na+. The latter observation implies that, when [Ca2+] exceeds the contraction threshold, Ca2+ efflux is mediated primarily by the Na-Ca exchanger, rather than by the sarcolemmal ATP-driven Ca2+ pump. 7. When bovine tail artery was treated with verapamil and phentolamine, and [Na+]o was reduced from 139.2 to 43.9 mM, substitution of K+ for Na+ induced a larger external Ca2+-dependent contraction than did substitution of Tris for Na+. The amplitudes of these contractions were greatly increased when the Na+ pump was inhibited by 5 x 10(-5) M-strophanthidin, presumably because of the rise in [Na+]i.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Mulvany M. J. Effect of ouabain on tone, membrane potential and sodium efflux compared with [3H]ouabain binding in rat resistance vessels. J Physiol. 1985 May;362:215–231. doi: 10.1113/jphysiol.1985.sp015672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F., Burdyga T. V. Evidence for sodium-calcium exchange in the guinea-pig ureter. J Physiol. 1984 Feb;347:411–430. doi: 10.1113/jphysiol.1984.sp015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biamino G., Johansson B. Effects of calcium and sodium on contracture tension in the smooth muscle of the rat portal vein. Pflugers Arch. 1970;321(2):143–158. doi: 10.1007/BF00586369. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Ashida T., Goldman W. F., Wier W. G., Hamlyn J. M. Sodium/calcium exchange in vascular smooth muscle: a link between sodium metabolism and hypertension. Ann N Y Acad Sci. 1986;488:199–216. doi: 10.1111/j.1749-6632.1986.tb46559.x. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol. 1977 May;232(5):C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. The energetics and kinetics of sodium-calcium exchange in barnacle muscles, squid axons, and mammalian heart: the role of ATP. Soc Gen Physiol Ser. 1984;38:129–147. [PubMed] [Google Scholar]
- Bohlen H. G. Localization of vascular resistance changes during hypertension. Hypertension. 1986 Mar;8(3):181–183. doi: 10.1161/01.hyp.8.3.181. [DOI] [PubMed] [Google Scholar]
- Bohr D. F., Seidel C., Sobieski J. Possible role of sodium-calcium pumps in tension development of vascular smooth muscle. Microvasc Res. 1969 Oct;1(4):335–343. doi: 10.1016/0026-2862(69)90012-0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Calcium metabolism in vascular smooth muscle. Br Med Bull. 1986 Oct;42(4):421–429. doi: 10.1093/oxfordjournals.bmb.a072161. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Burnett M., Sneddon P. The effect of sodium removal on the contractile response of the guinea-pig taenia coli to carbachol. J Physiol. 1980 Sep;306:411–429. doi: 10.1113/jphysiol.1980.sp013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Lategan T. W. Na-Ca exchange in vascular smooth muscle. J Hypertens. 1985 Apr;3(2):109–116. doi: 10.1097/00004872-198504000-00002. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Plasma membrane Ca2+ transport, and Ca2+ handling by intracellular stores: an integrated picture with emphasis on regulation. Kroc Found Ser. 1984;17:121–134. [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L., Droogmans G., Wuytack F. Na+-K+ ATPase, Na-Ca exchange, and excitation-contraction coupling in smooth muscle. J Cardiovasc Pharmacol. 1985;7 (Suppl 3):S103–S110. [PubMed] [Google Scholar]
- Daniel E. E., Grover A. K., Kwan C. Y. Isolation and properties of plasma membrane from smooth muscle. Fed Proc. 1982 Oct;41(12):2898–2904. [PubMed] [Google Scholar]
- Daniel E. E. The use of subcellular membrane fractions in analysis of control of smooth muscle function. Experientia. 1985 Jul 15;41(7):905–913. doi: 10.1007/BF01970009. [DOI] [PubMed] [Google Scholar]
- DeFeo T. T., Morgan K. G. Calcium-force relationships as detected with aequorin in two different vascular smooth muscles of the ferret. J Physiol. 1985 Dec;369:269–282. doi: 10.1113/jphysiol.1985.sp015900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Interactions of physiological ligands with the Ca pump and Na/Ca exchange in squid axons. J Gen Physiol. 1984 Dec;84(6):895–914. doi: 10.1085/jgp.84.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Morel N., Godfraind T. Sodium/calcium exchange in smooth-muscle microsomal fractions. Biochem J. 1984 Mar 1;218(2):421–427. doi: 10.1042/bj2180421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J. Changes in sodium pump activity and vascular contraction. J Hypertens. 1985 Oct;3(5):429–436. [PubMed] [Google Scholar]
- Ozaki H., Karaki H., Urakawa N. Possible role of Na-Ca exchange mechanism in the contractions induced in guinea-pig aorta by potassium free solution and ouabain. Naunyn Schmiedebergs Arch Pharmacol. 1978 Oct;304(3):203–209. doi: 10.1007/BF00507959. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Urakawa N. Na-Ca exchange and tension development in guinea-pig aorta. Naunyn Schmiedebergs Arch Pharmacol. 1979 Nov;309(2):171–178. doi: 10.1007/BF00501226. [DOI] [PubMed] [Google Scholar]
- Petersen T. T., Mulvany M. J. Effect of sodium gradient on the rate of relaxation of rat mesenteric small arteries from potassium contractures. Blood Vessels. 1984;21(6):279–289. doi: 10.1159/000158530. [DOI] [PubMed] [Google Scholar]
- Pfitzer G., Hofmann F., DiSalvo J., Rüegg J. C. cGMP and cAMP inhibit tension development in skinned coronary arteries. Pflugers Arch. 1984 Jul;401(3):277–280. doi: 10.1007/BF00582596. [DOI] [PubMed] [Google Scholar]
- Raeymaekers L., Wuytack F., Casteels R. Na-Ca exchange in Taenia coli of the guinea-pig. Pflugers Arch. 1974 Mar 25;347(4):329–340. doi: 10.1007/BF00587173. [DOI] [PubMed] [Google Scholar]
- Rasgado-Flores H., Blaustein M. P. Na/Ca exchange in barnacle muscle cells has a stoichiometry of 3 Na+/1 Ca2+. Am J Physiol. 1987 May;252(5 Pt 1):C499–C504. doi: 10.1152/ajpcell.1987.252.5.C499. [DOI] [PubMed] [Google Scholar]
- Reuter H., Blaustein M. P., Haeusler G. Na-Ca exchange and tension development in arterial smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):87–94. doi: 10.1098/rstb.1973.0011. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Miller R. C. Role of sodium-calcium exchange and effects of calcium entry blockers on endothelial-mediated responses in rat isolated aorta. Mol Pharmacol. 1986 Jul;30(1):53–57. [PubMed] [Google Scholar]
- Sumimoto K., Kuriyama H. Mobilization of free Ca2+ measured during contraction-relaxation cycles in smooth muscle cells of the porcine coronary artery using quin2. Pflugers Arch. 1986 Feb;406(2):173–180. doi: 10.1007/BF00586679. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., Aaronson P., Loutzenhiser R. Sodium-calcium interactions in mammalian smooth muscle. Pharmacol Rev. 1978 Jun;30(2):167–208. [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]


