Abstract
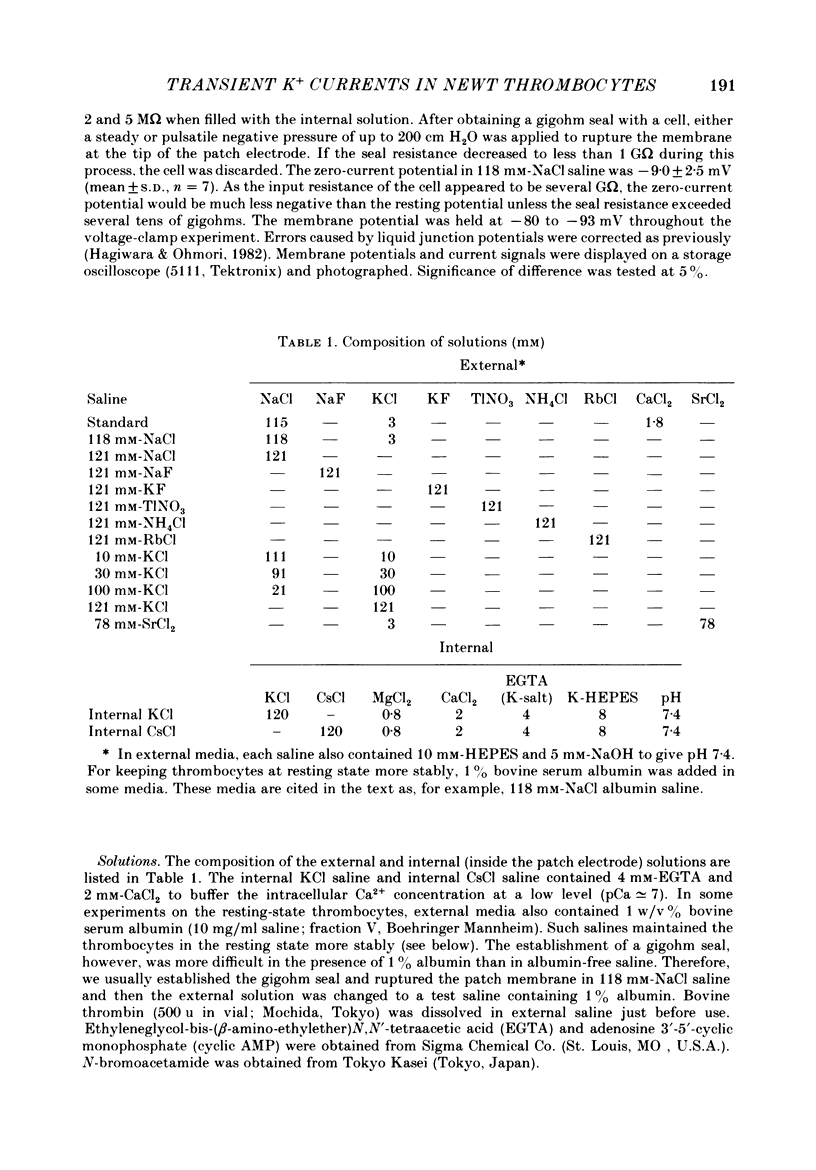
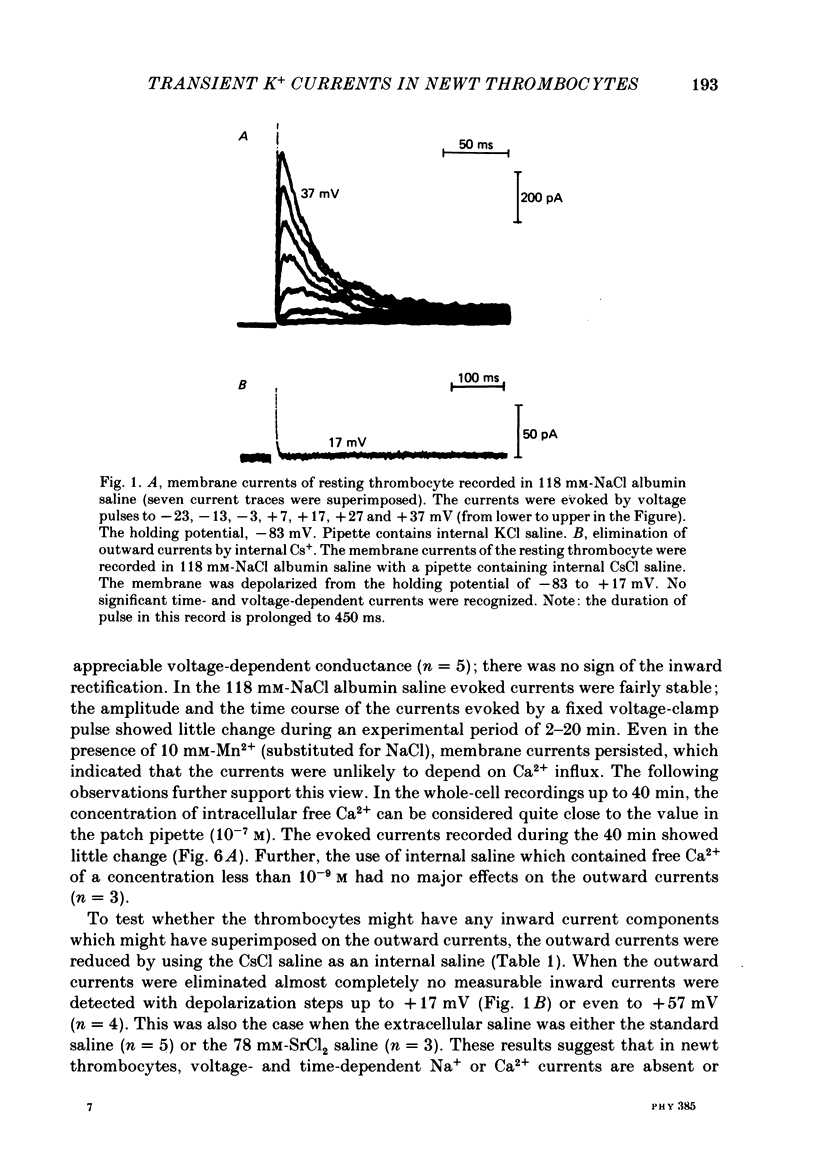
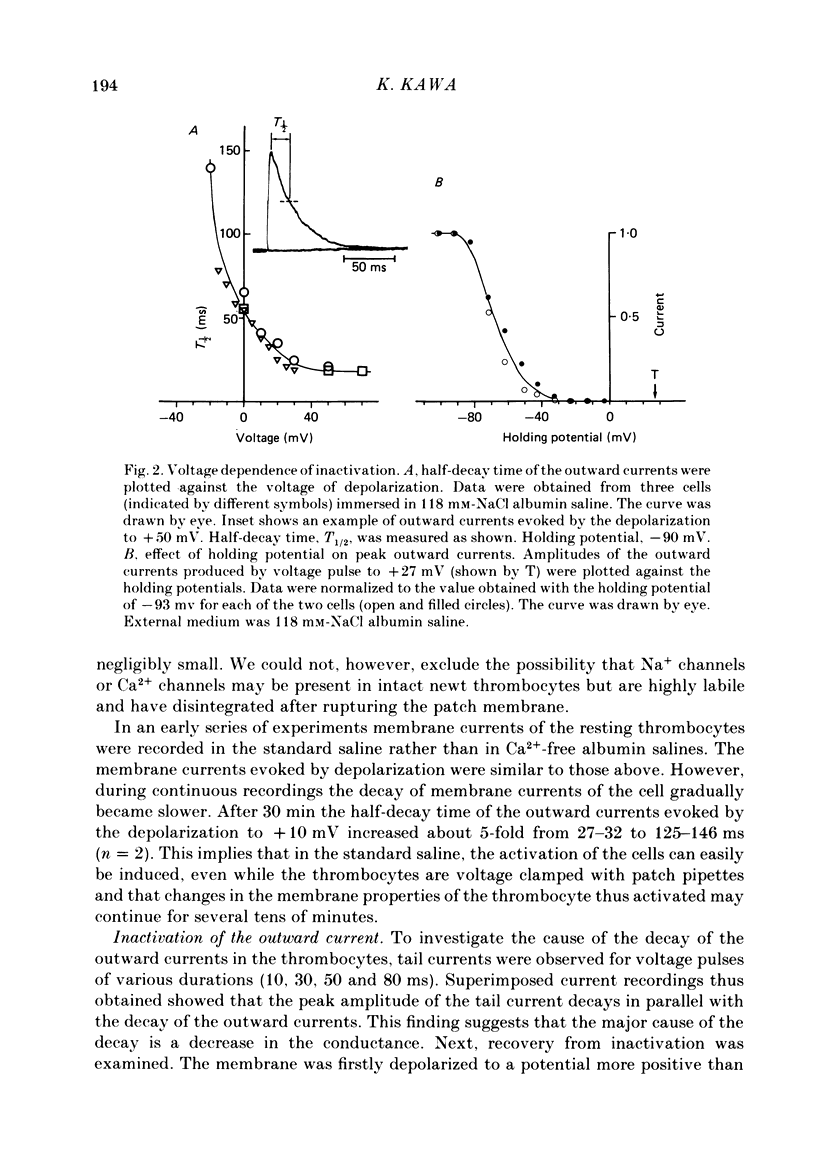
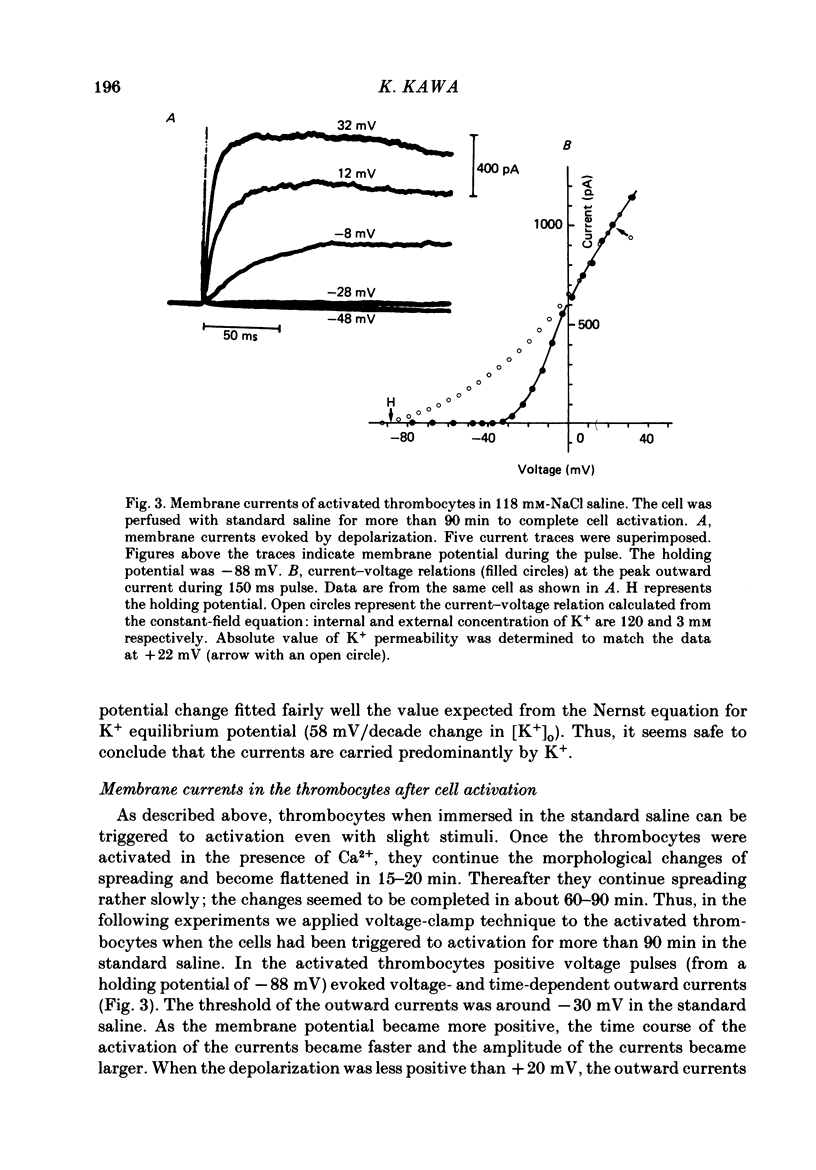
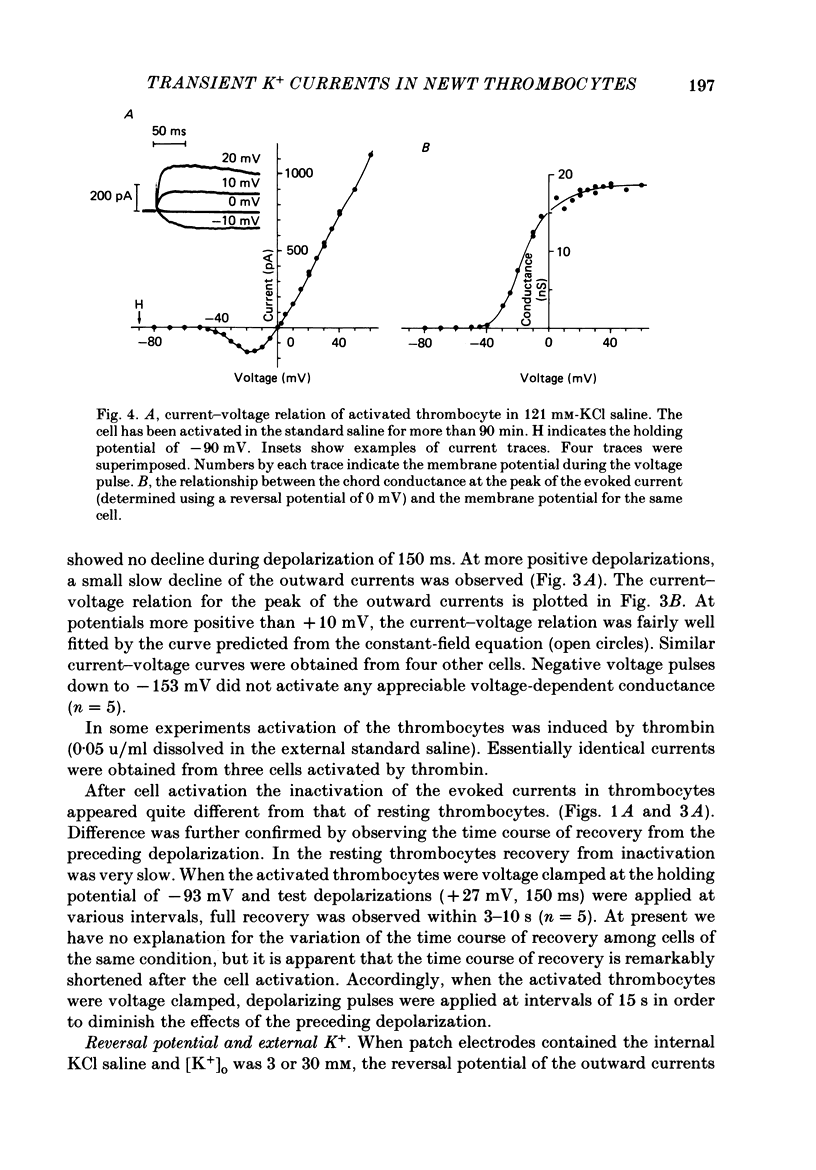
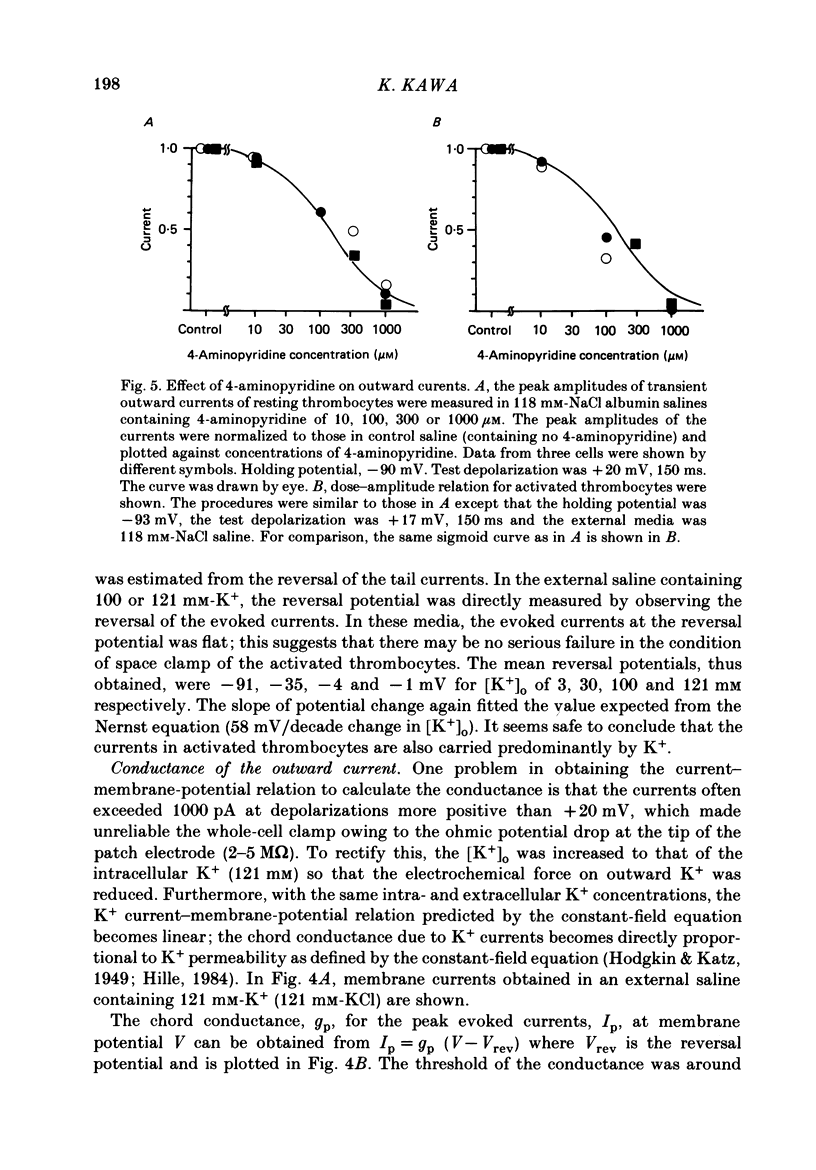
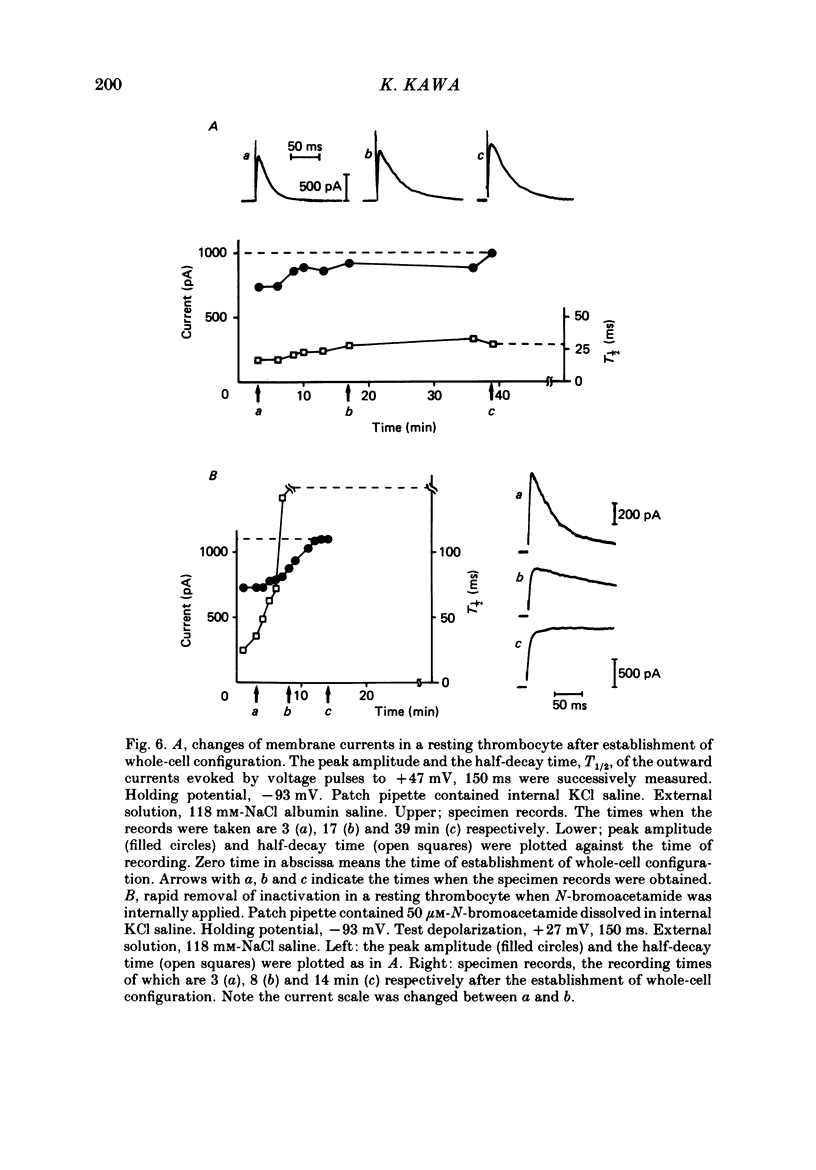
1. The electrical properties of the cell membrane of thrombocytes in the newt, Triturus pyrrhogaster, were studied using the whole-cell variation of the patch-electrode voltage-clamp technique. 2. In medium containing Ca2+ (1.8 mM), activated thrombocytes became round and then spread on the glass. Activation of thrombocytes was inhibited by the removal of external Ca2+ and addition of 1 w/v% albumin to the external media. 3. For thrombocytes kept in the resting state, depolarizations more positive than -30 mV evoked transient outward currents which decayed completely during the duration of the depolarization (150 ms). The half-decay time of the currents became smaller as the depolarizing pulse strengthened, reaching about 20 ms at +30 mV (20 degrees C). 4. The outward currents are identified as K+ currents, since (1) their reversal potential depended on extracellular K+ concentration and (2) the outward currents were suppressed either by external application of 4-aminopyridine (1 mM) or by internal application of Cs+ (120 mM). The monovalent cation selectivities of the K+ channels were evaluated from the reversal potential as Tl (1.68) greater than K(1.0) greater than Rb (0.89) greater than NH4 (0.13) greater than Na(less than 0.03). 5. When the thrombocytes had been activated, depolarization again evoked K+ currents. The currents, however, showed negligible or small decay during the duration of the depolarization (150 ms). The rate of recovery from preceding depolarization was also reduced to about one-sixth. 6. The sensitivity to 4-aminopyridine and the selectivity of the K+ channels were not changed by cell activation. 7. We conclude that during activation of thrombocytes the inactivation of the K+ channels is almost eliminated. Removal of inactivation of the K+ channels was also induced in resting thrombocytes by intracellular application of 4-bromoacetamide (50 microM).
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984 Nov 30;226(4678):1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett W. F., Belville J. S., Lynch G. Calcium dependent serotonin release from blood platelets: a model system for neurosecretion. Neuroscience. 1979;4(8):1203–1208. doi: 10.1016/0306-4522(79)90203-3. [DOI] [PubMed] [Google Scholar]
- Burn P., Rotman A., Meyer R. K., Burger M. M. Diacylglycerol in large alpha-actinin/actin complexes and in the cytoskeleton of activated platelets. Nature. 1985 Apr 4;314(6010):469–472. doi: 10.1038/314469a0. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Chandy K. G., DeCoursey T. E., Gupta S. A voltage-gated potassium channel in human T lymphocytes. J Physiol. 1985 Jan;358:197–237. doi: 10.1113/jphysiol.1985.sp015548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G. Human platelet size, shape, and related functions in health and disease. Physiol Rev. 1982 Jan;62(1):185–261. doi: 10.1152/physrev.1982.62.1.185. [DOI] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S., Henkart M. Potassium current in clonal cytotoxic T lymphocytes from the mouse. J Physiol. 1984 Jun;351:645–656. doi: 10.1113/jphysiol.1984.sp015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S., Saxton R. E. Variation of calcium current during the cell growth cycle in mouse hybridoma lines secreting immunoglobulins. J Physiol. 1984 Oct;355:313–321. doi: 10.1113/jphysiol.1984.sp015421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Woolum J. C., Cornwall M. C. Selectivity of the Ca2+-activated and light-dependent K+ channels for monovalent cations. Biophys J. 1982 Jun;38(3):319–322. doi: 10.1016/S0006-3495(82)84565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Kawa K. Calcium and potassium currents in spermatogenic cells dissociated from rat seminiferous tubules. J Physiol. 1984 Nov;356:135–149. doi: 10.1113/jphysiol.1984.sp015457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne W. C., Norman N. E., Schwartz D. B., Simons E. R. Changes in cytoplasmic pH and in membrane potential in thrombin-stimulated human platelets. Eur J Biochem. 1981 Nov;120(2):295–302. doi: 10.1111/j.1432-1033.1981.tb05703.x. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K., Strumwasser F. A voltage-clamp analysis of currents underlying cyclic AMP-induced membrane modulation in isolated peptidergic neurons of Aplysia. J Neurophysiol. 1984 Aug;52(2):340–349. doi: 10.1152/jn.1984.52.2.340. [DOI] [PubMed] [Google Scholar]
- Kien M., Belamarich F. A., Shepro D. Effect of adenosine and related compounds on thrombocyte and platelet aggregation. Am J Physiol. 1971 Mar;220(3):604–608. doi: 10.1152/ajplegacy.1971.220.3.604. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., Montecucco C., Rink T. J. Platelet membrane potential assessed with a fluorescent probe, dipropylthiadicarbocyanine: has this potential a role in triggering aggregation? [proceedings]. J Physiol. 1979 Apr;289:34P–35P. [PubMed] [Google Scholar]
- Matteson D. R., Deutsch C. K channels in T lymphocytes: a patch clamp study using monoclonal antibody adhesion. Nature. 1984 Feb 2;307(5950):468–471. doi: 10.1038/307468a0. [DOI] [PubMed] [Google Scholar]
- Motamed M., Michal F., Born G. V. Increase in sialic acids removable by neuraminidase during the shape change of platelets. Biochem J. 1976 Sep 15;158(3):655–657. doi: 10.1042/bj1580655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. Factors influencing platelet function: adhesion, release, and aggregation. Pharmacol Rev. 1970 Jun;22(2):97–187. [PubMed] [Google Scholar]
- Nachmias V. T. Cytoskeleton of human platelets at rest and after spreading. J Cell Biol. 1980 Sep;86(3):795–802. doi: 10.1083/jcb.86.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias V. T., Sullender J. S., Fallon J. R. Effects of local anesthetics on human platelets: filopodial suppression and endogenous proteolysis. Blood. 1979 Jan;53(1):63–72. [PubMed] [Google Scholar]
- Oxford G. S., Wu C. H., Narahashi T. Removal of sodium channel inactivation in squid giant axons by n-bromoacetamide. J Gen Physiol. 1978 Mar;71(3):227–247. doi: 10.1085/jgp.71.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J., Horn R. Effect of N-bromoacetamide on single sodium channel currents in excised membrane patches. J Gen Physiol. 1982 Mar;79(3):333–351. doi: 10.1085/jgp.79.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipili E. Platelet membrane potential: simultaneous measurement of diSC3(5) fluorescence and optical density. Thromb Haemost. 1985 Oct 30;54(3):645–649. [PubMed] [Google Scholar]
- Reuter H., Stevens C. F. Ion conductance and ion selectivity of potassium channels in snail neurones. J Membr Biol. 1980 Dec 15;57(2):103–118. doi: 10.1007/BF01868997. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Smith S. W., Tsien R. Y. Cytoplasmic free Ca2+ in human platelets: Ca2+ thresholds and Ca-independent activation for shape-change and secretion. FEBS Lett. 1982 Nov 1;148(1):21–26. doi: 10.1016/0014-5793(82)81234-9. [DOI] [PubMed] [Google Scholar]
- Sneddon J. M. Blood platelets as a model for monoamine-containing neurones. Prog Neurobiol. 1973;1(2):151–198. doi: 10.1016/0301-0082(73)90019-1. [DOI] [PubMed] [Google Scholar]
- Stokes E. E., Firkin B. G. Studies of the peripheral blood of the Port Jackson shark (Heterodontus portusjacksoni) with particular reference to the thrombocyte. Br J Haematol. 1971 Apr;20(4):427–435. doi: 10.1111/j.1365-2141.1971.tb07054.x. [DOI] [PubMed] [Google Scholar]
- Strong J. A. Modulation of potassium current kinetics in bag cell neurons of Aplysia by an activator of adenylate cyclase. J Neurosci. 1984 Nov;4(11):2772–2783. doi: 10.1523/JNEUROSCI.04-11-02772.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


