Abstract
1. Hair cells were enzymatically isolated from identified regions of the turtle basilar papilla and studied with the patch-electrode technique. The experimental aim was to relate the resonance properties seen during current injection to the membrane currents measured in the same cell under whole-cell voltage clamp. 2. Solitary hair cells had resting potentials of about -50 mV, and produced a damped oscillation in membrane potential at the onset and termination of a small current step; the resonant frequency varied from 9 to 350 Hz between cells, and was correlated with the region of papilla from which a cell had been isolated. The inferred frequency map was consistent with the tonotopic arrangement described previously in the intact papilla. 3. Depolarizations from the resting potential under voltage clamp activated a large net outward current with a steep voltage dependence, and the steady-state current-voltage relationship was strongly rectified about the resting potential. Input resistances tended to be smaller in cells with higher resonant frequencies, although there was no concurrent variation in membrane area as inferred from the cell capacitance. 4. The kinetics of the outward current evoked by a small depolarizing step depended upon the resonant frequency, fo, of the hair cell, and were slower in low-frequency cells. On repolarization to the resting potential the current decayed exponentially with a time constant that changed from 150 ms in the lowest-frequency cell to less than 1 ms in the highest-frequency one. The time constant was approximately proportional to 1/f0(2). 5. Following repolarization to different membrane potentials, the tail current was found to reverse around -80 mV, indicating that the outward current was due mainly to K+. 6. The outward current was abolished by extracellular application of 25 mM-tetraethylammonium chloride (TEA), or on exchange of Cs+ for K+ in the intracellular medium filling the recording electrode, each experiment supporting the contention that K+ is the major current carrier. Such treatments also removed the oscillations in membrane potential evoked by imposed current steps. 7. Addition of TEA or intracellular perfusion with Cs+ also revealed a fast inward current with an ionic sensitivity consistent with its being carried by Ca2+. Like the K+ current, the Ca2+ current was activated by small depolarization from the resting potential, and over this voltage range it was about five to ten times smaller than the K+ current. Its activation was more rapid than the fastest outward currents in high-frequency cells.(ABSTRACT TRUNCATED AT 400 WORDS)
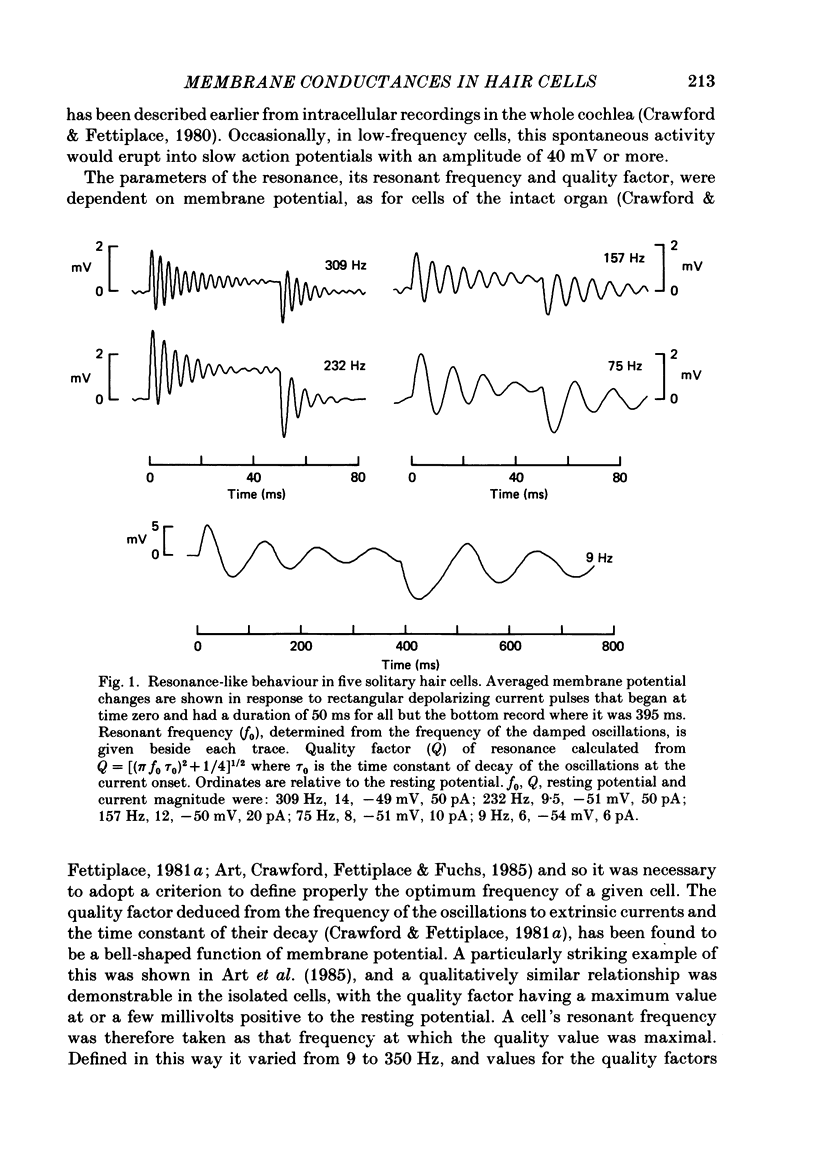
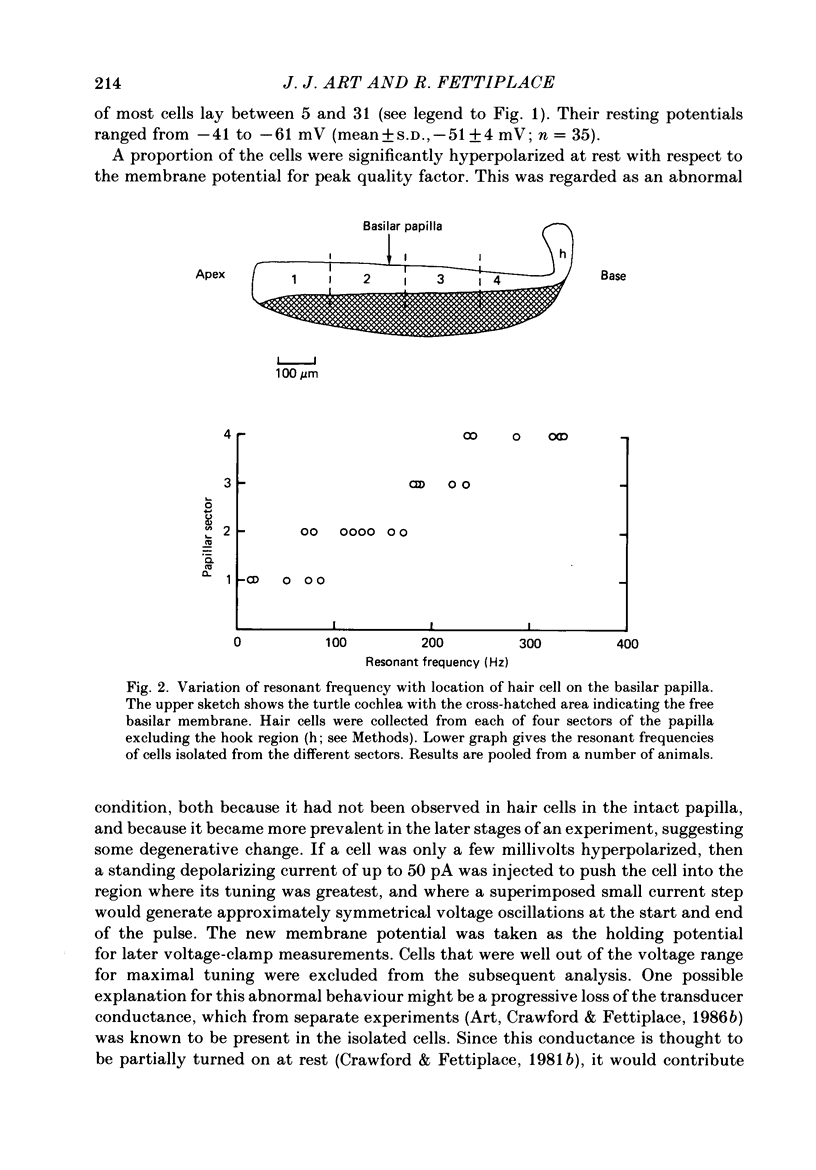
Full text
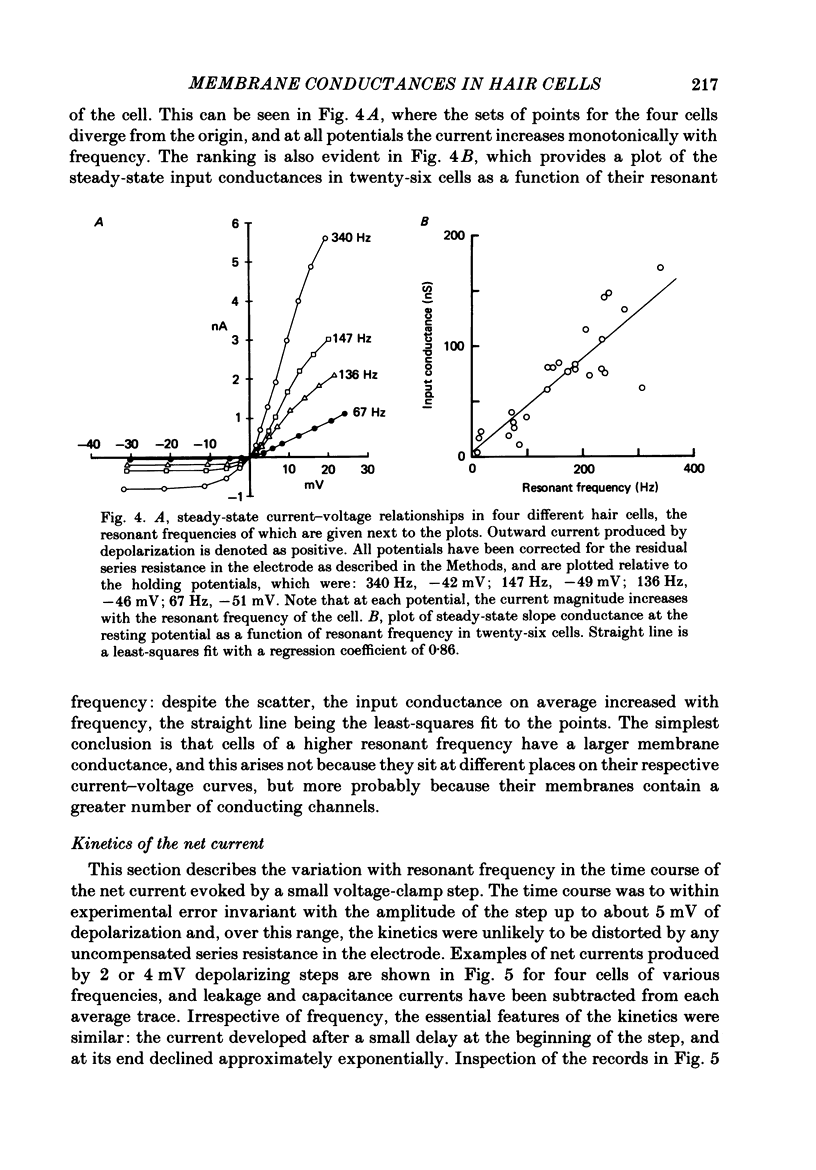
PDF





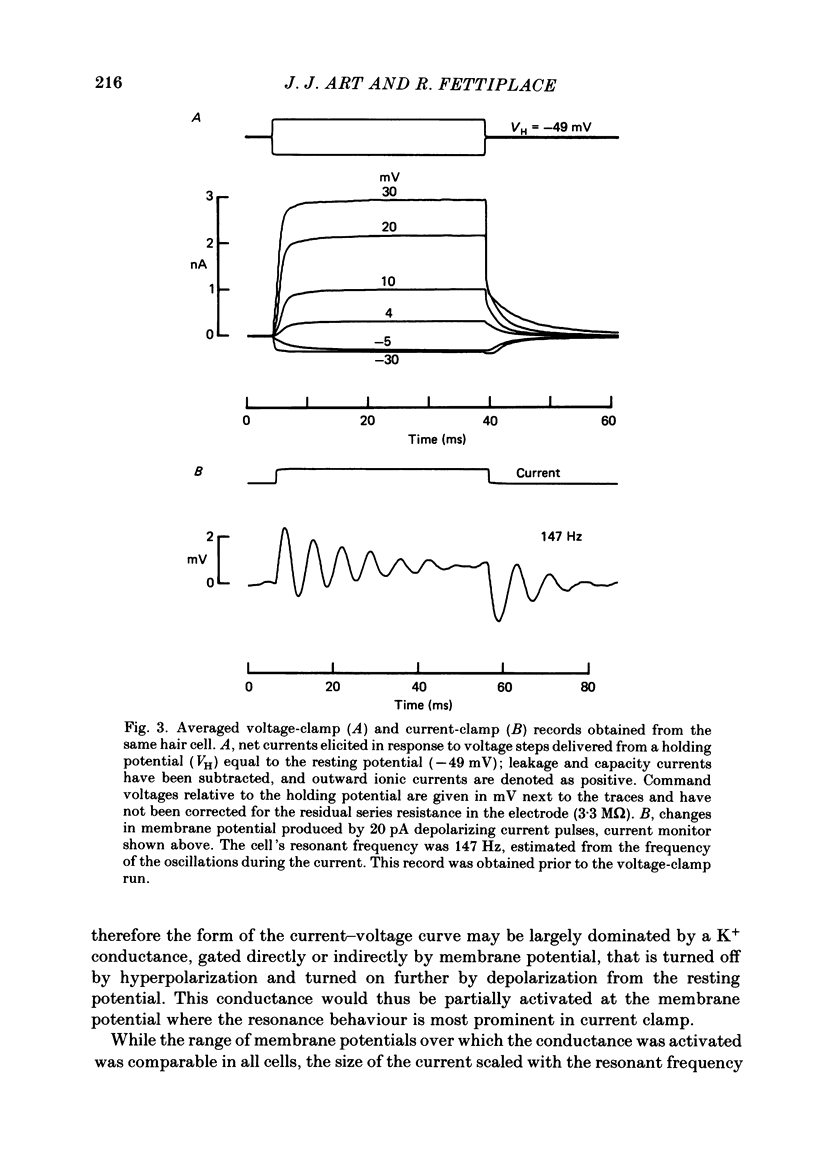
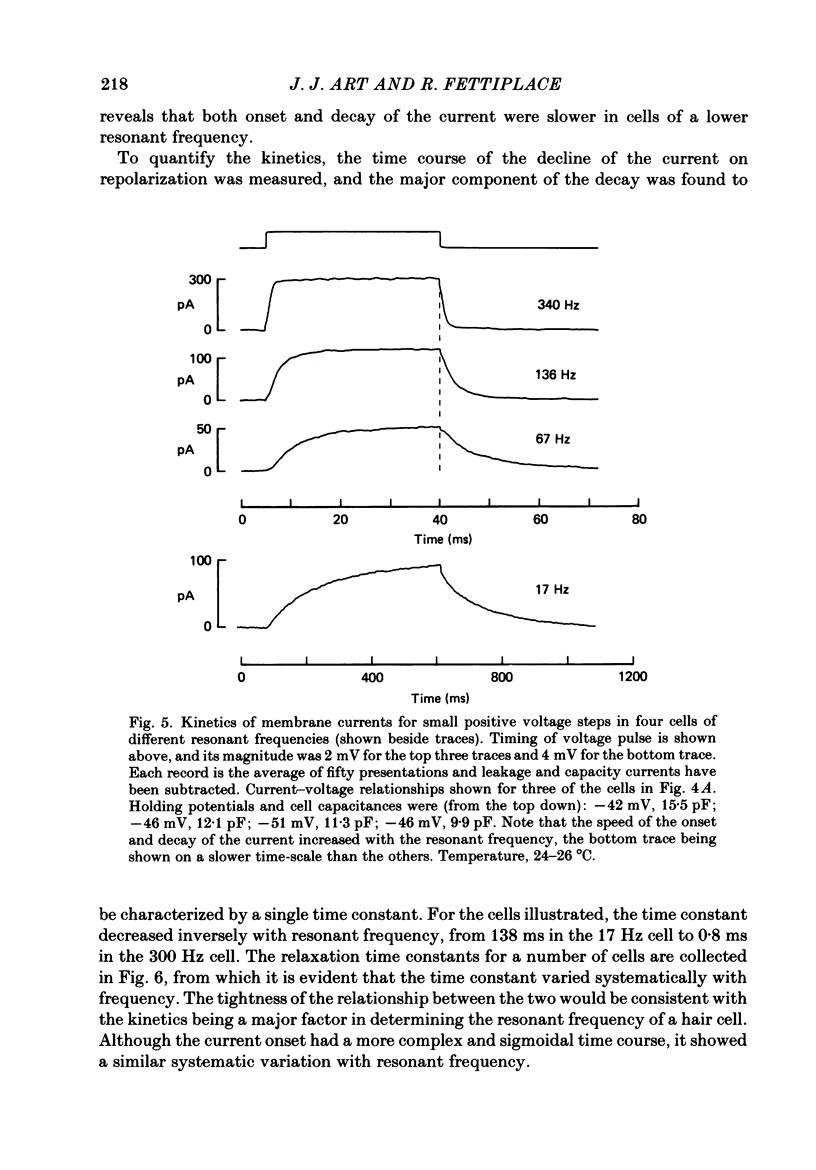
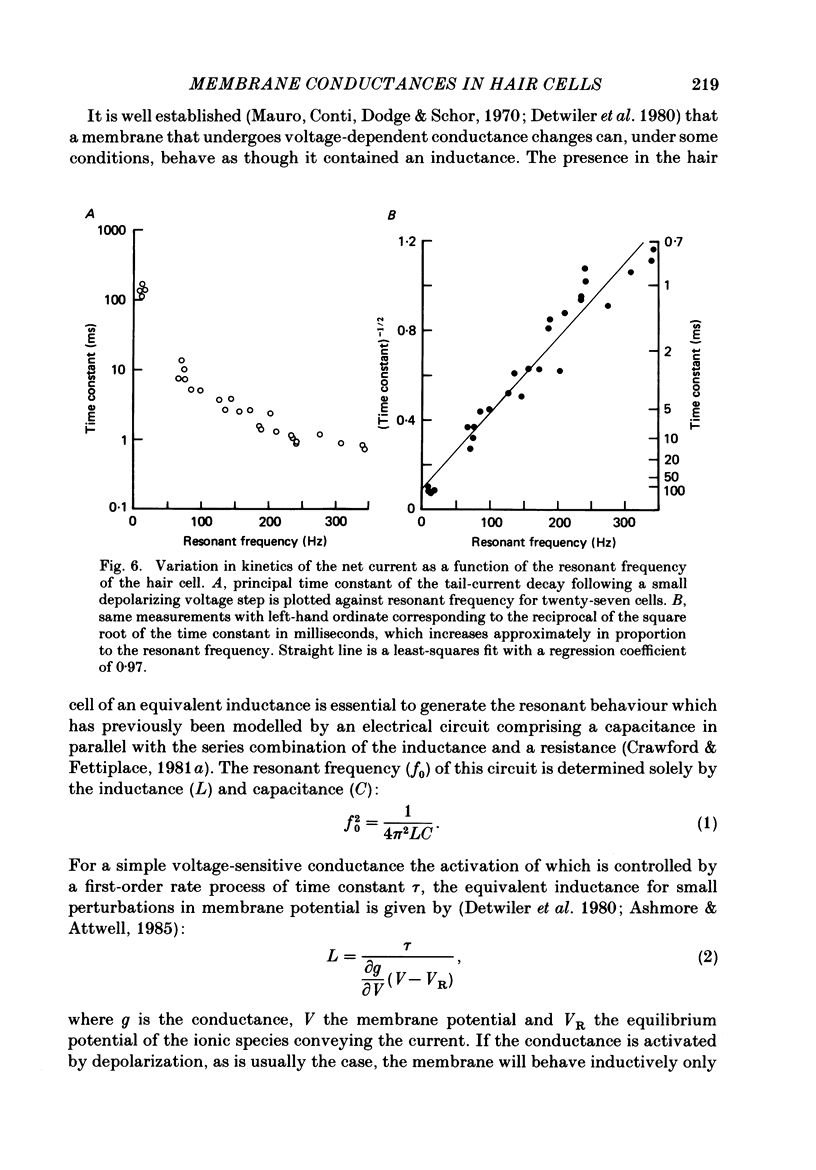

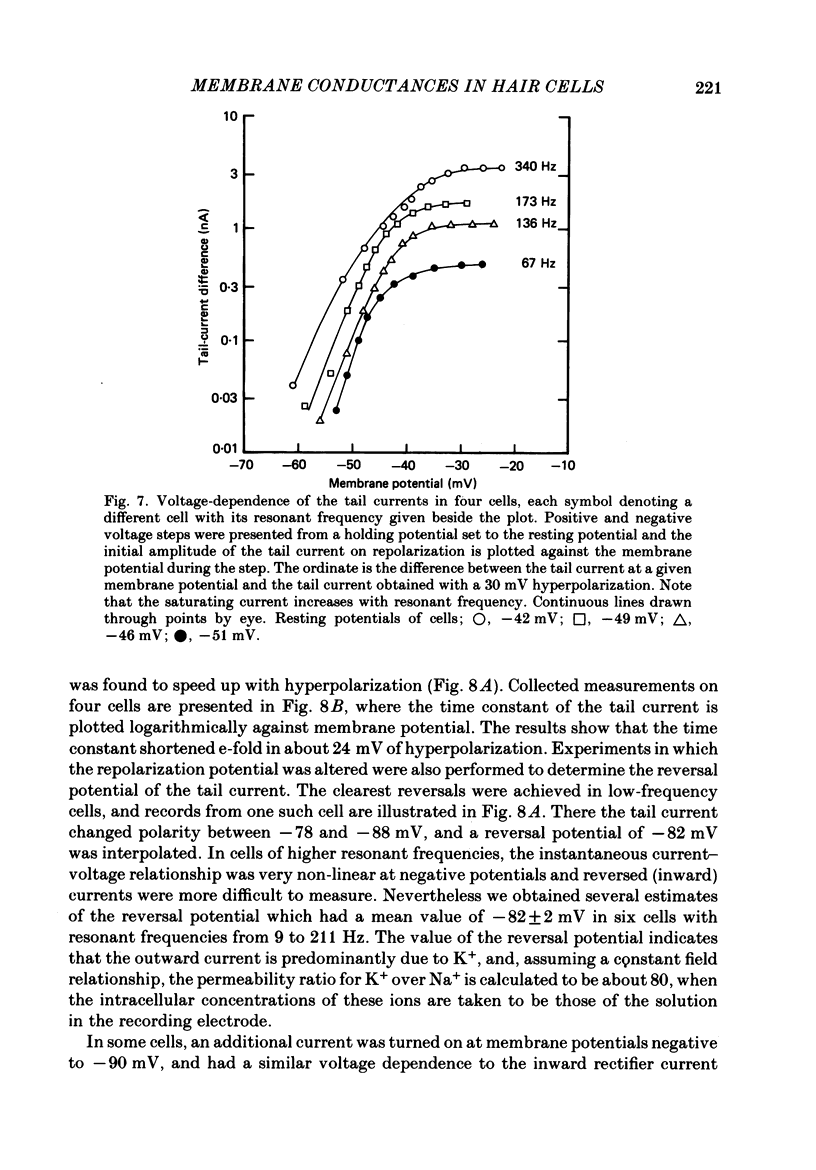
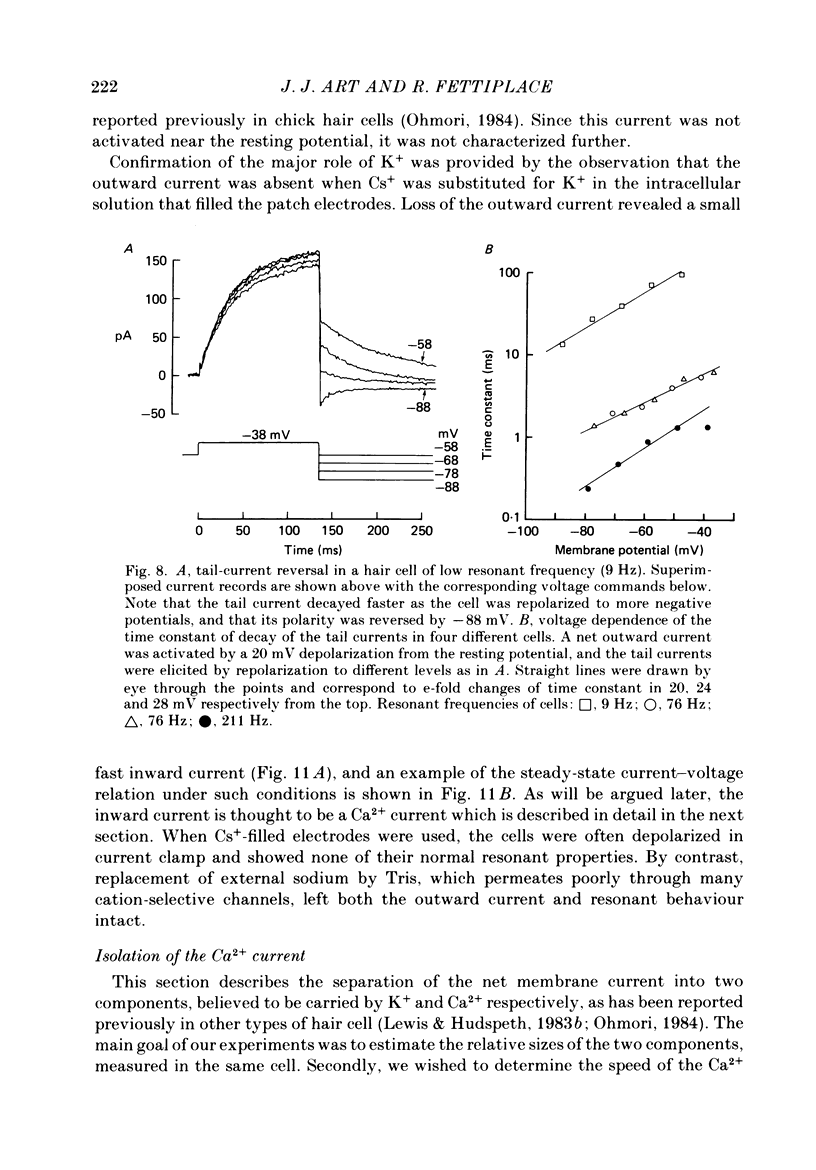
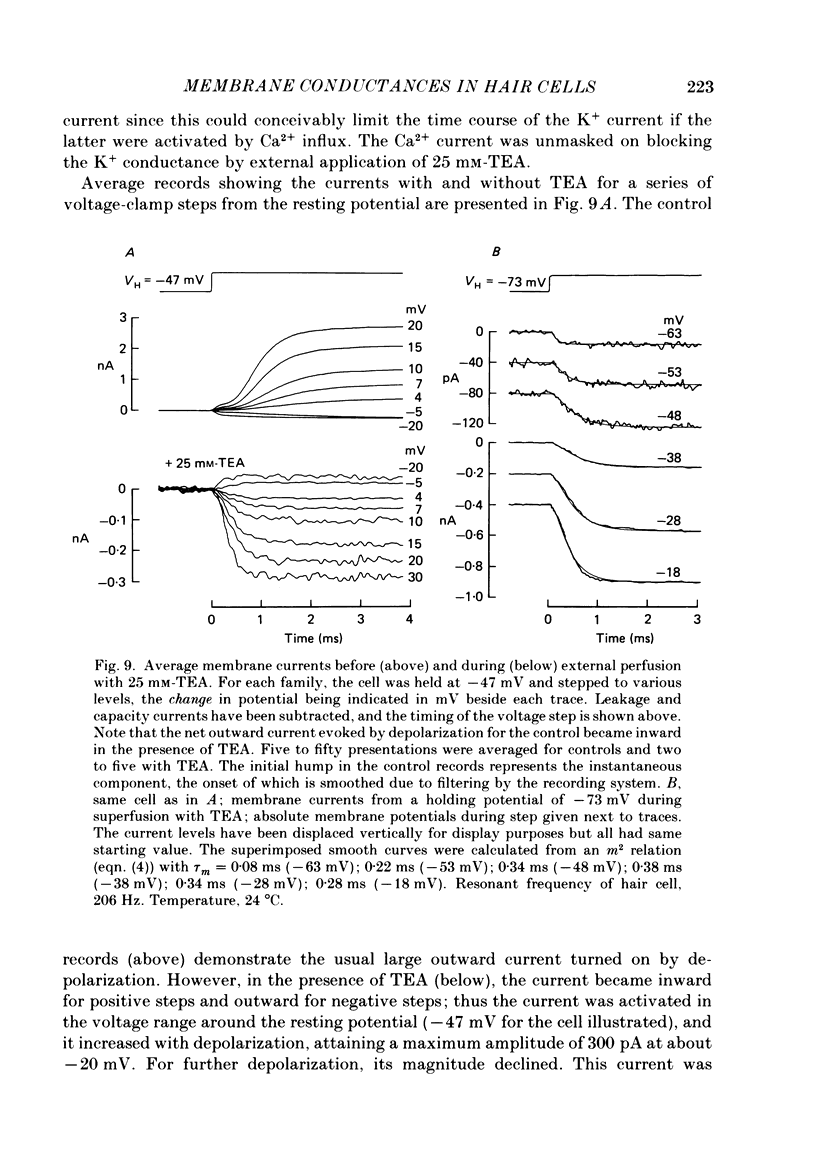
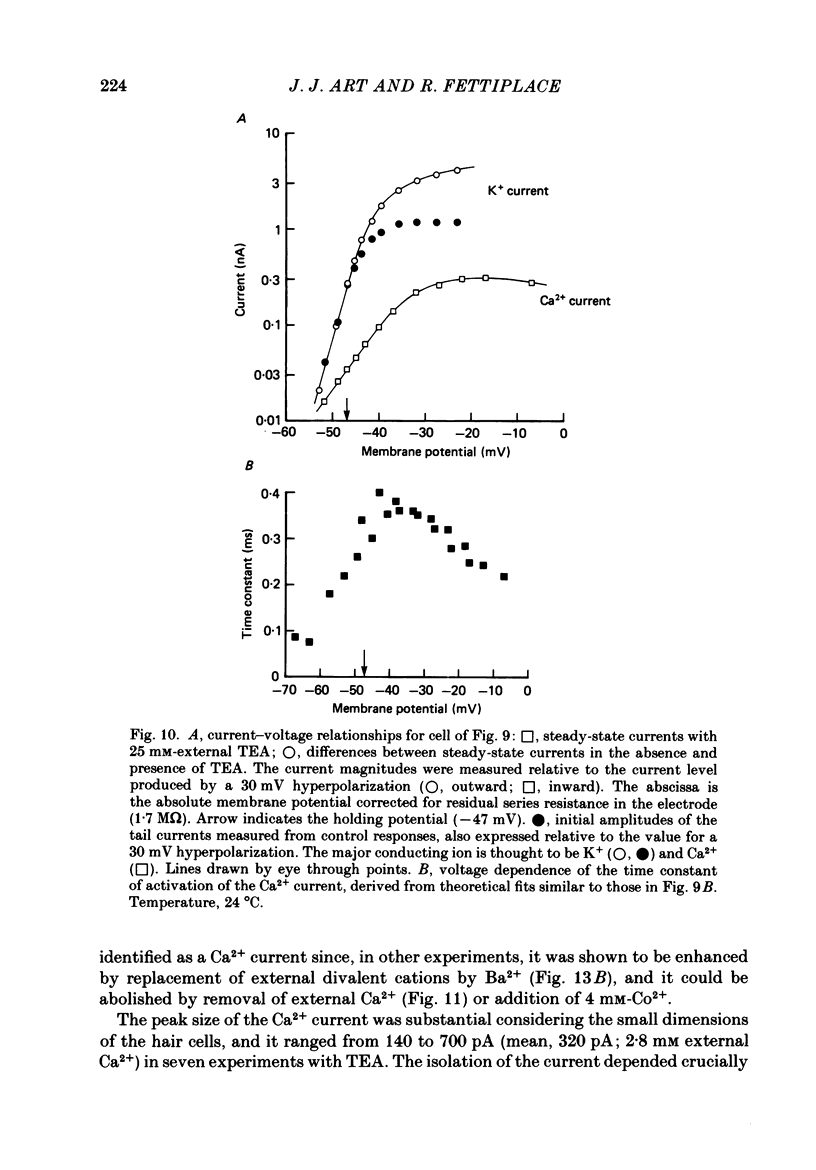

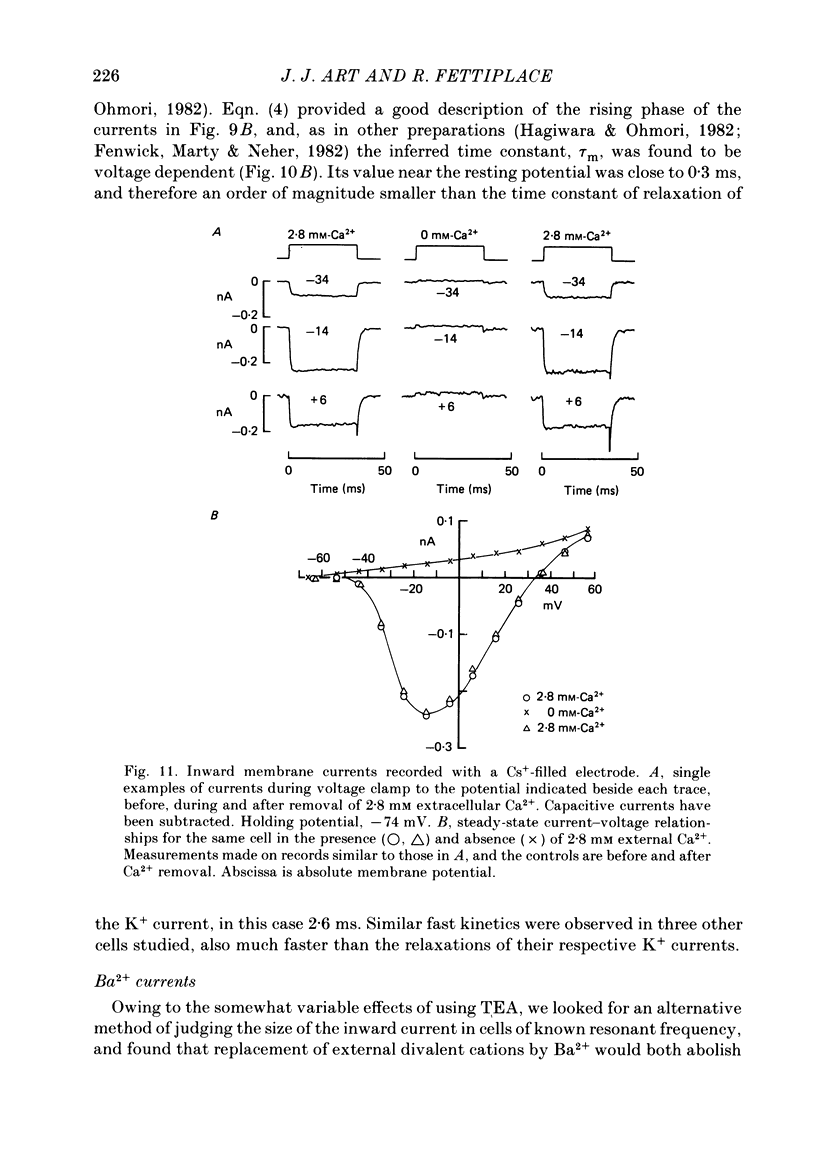
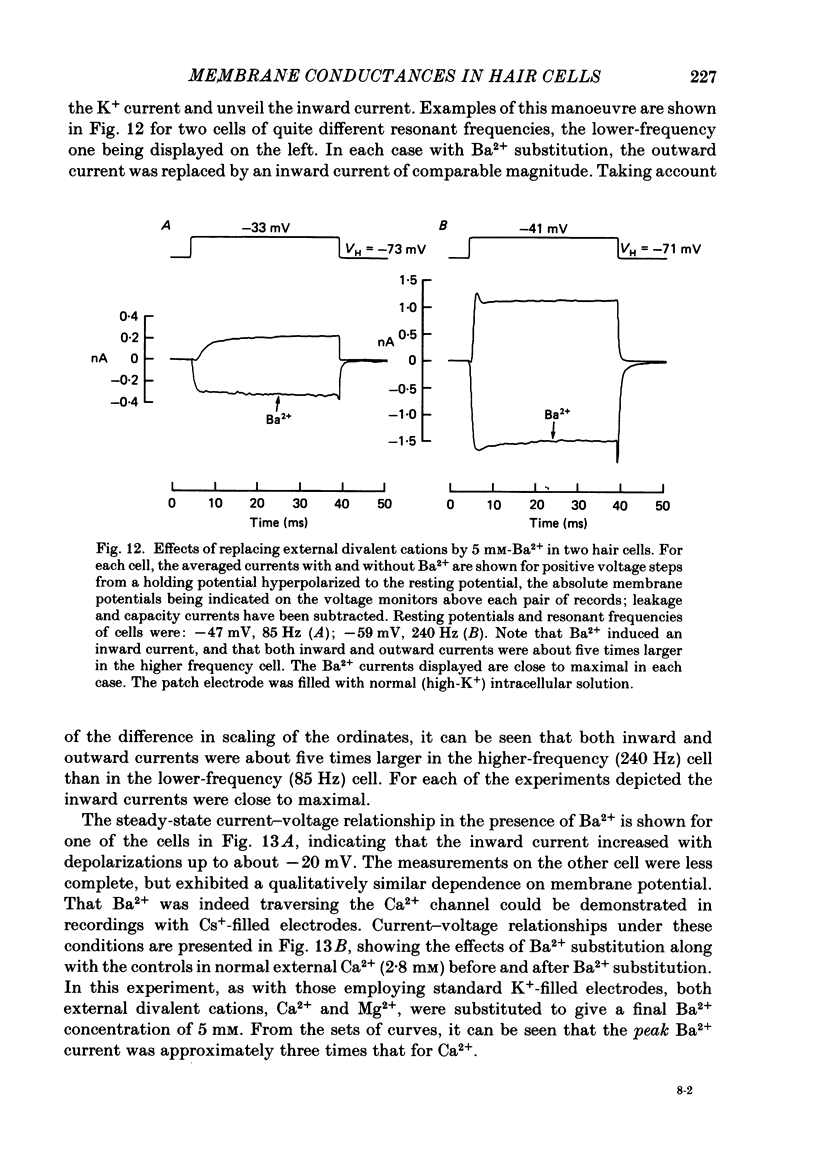
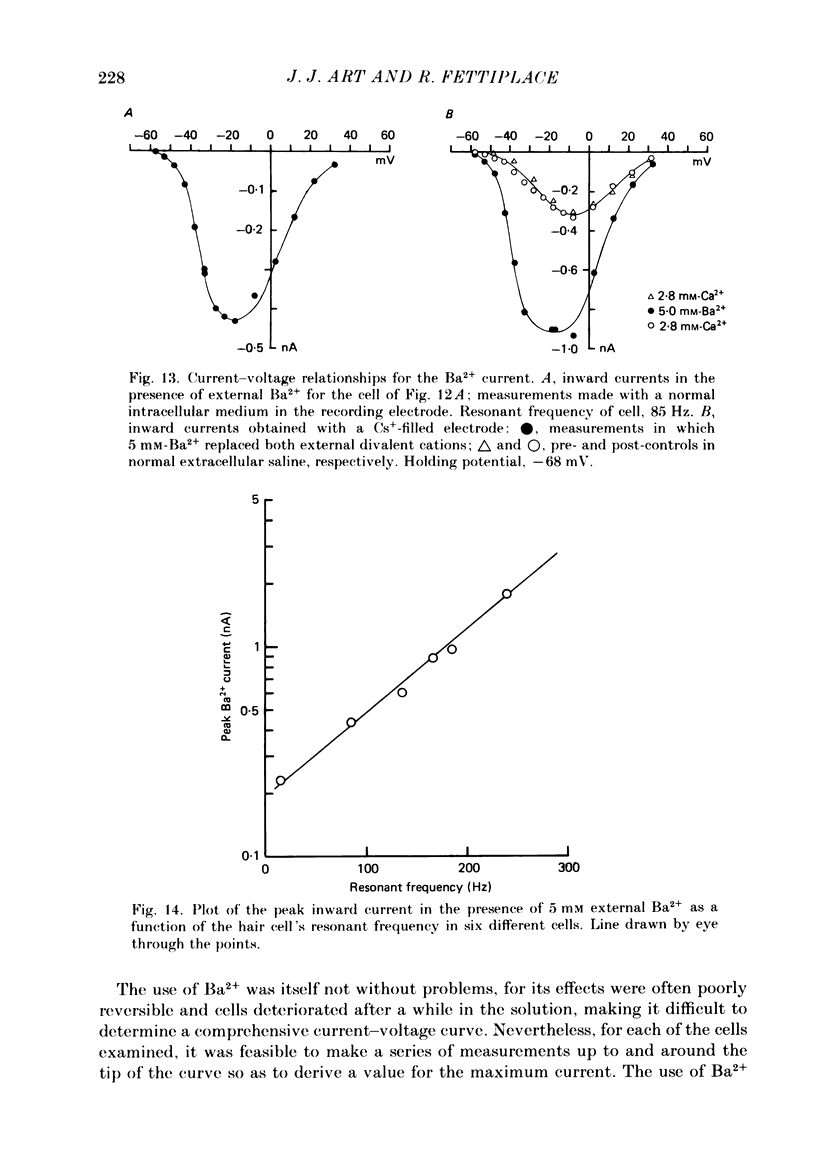
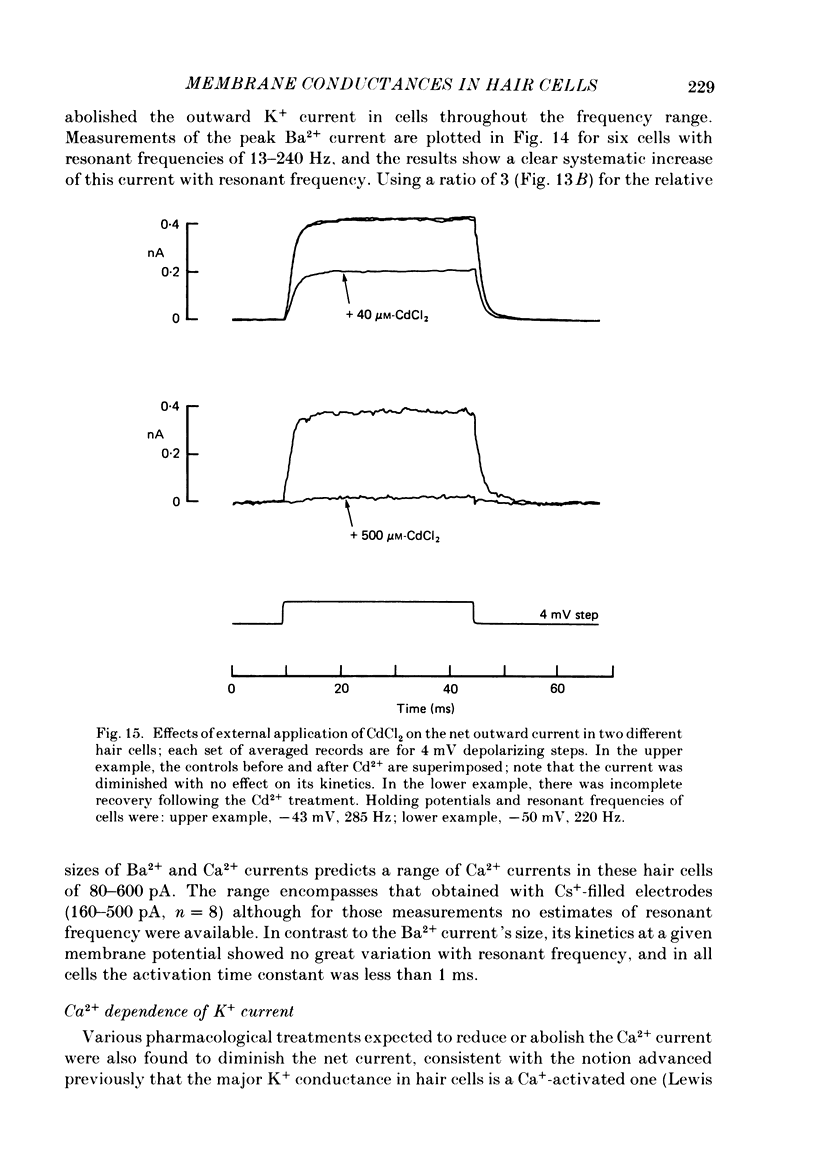

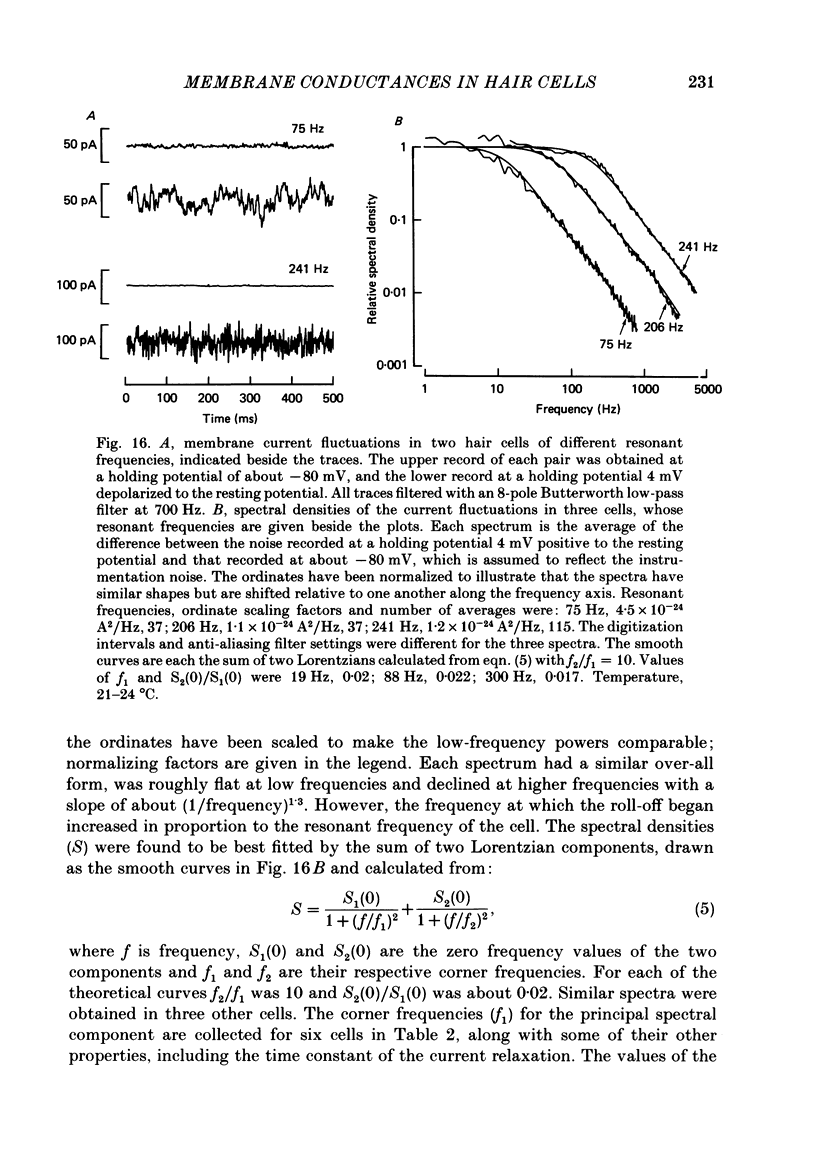
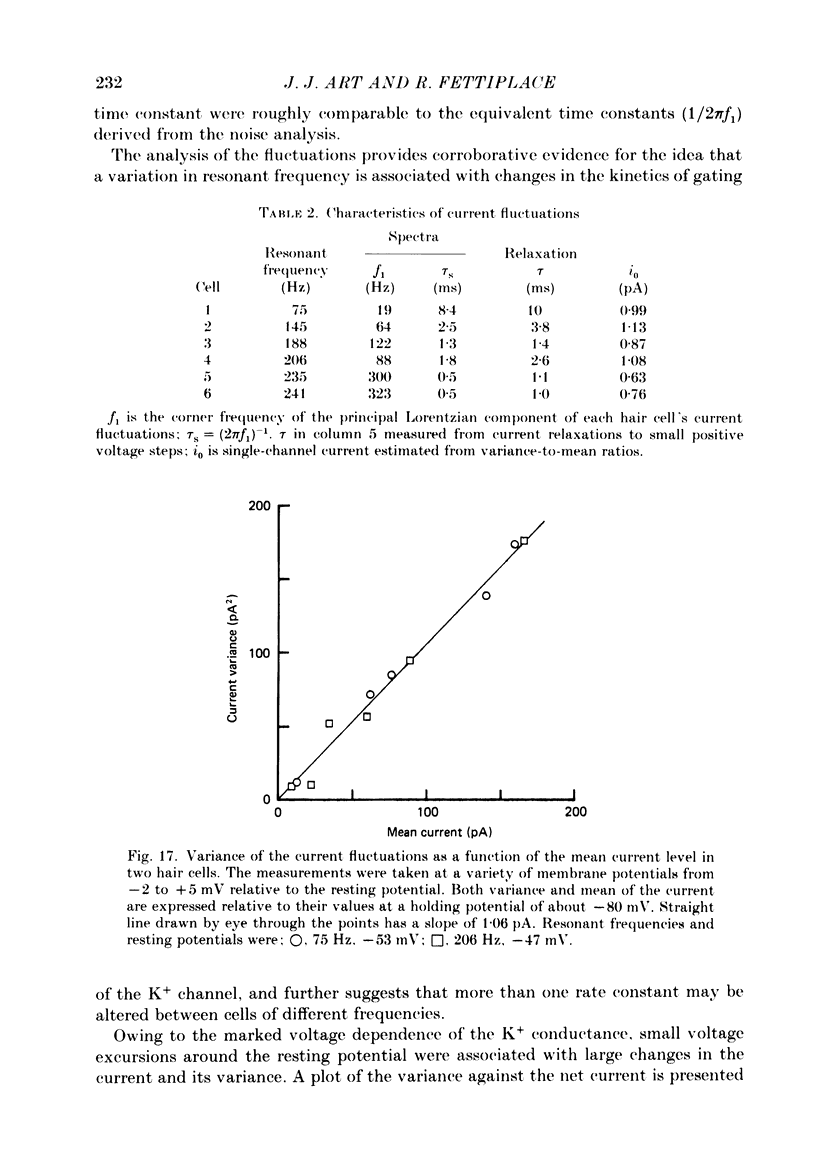

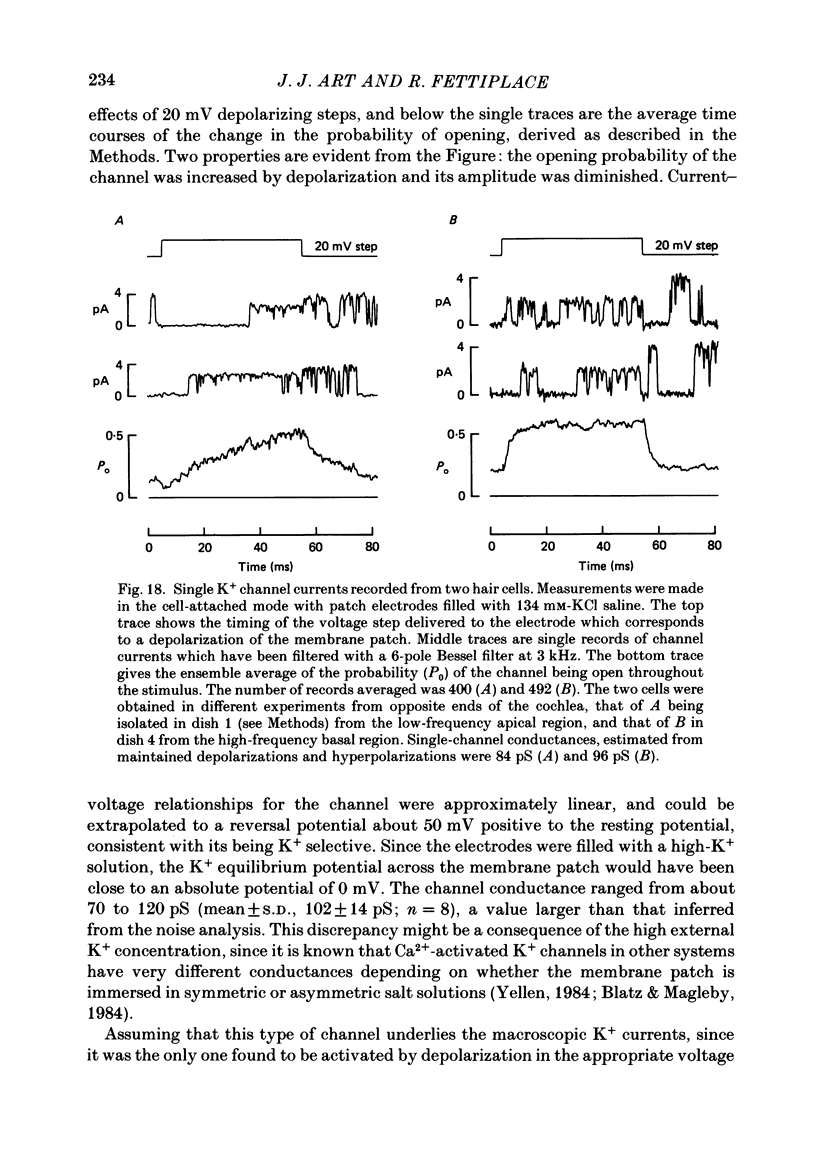

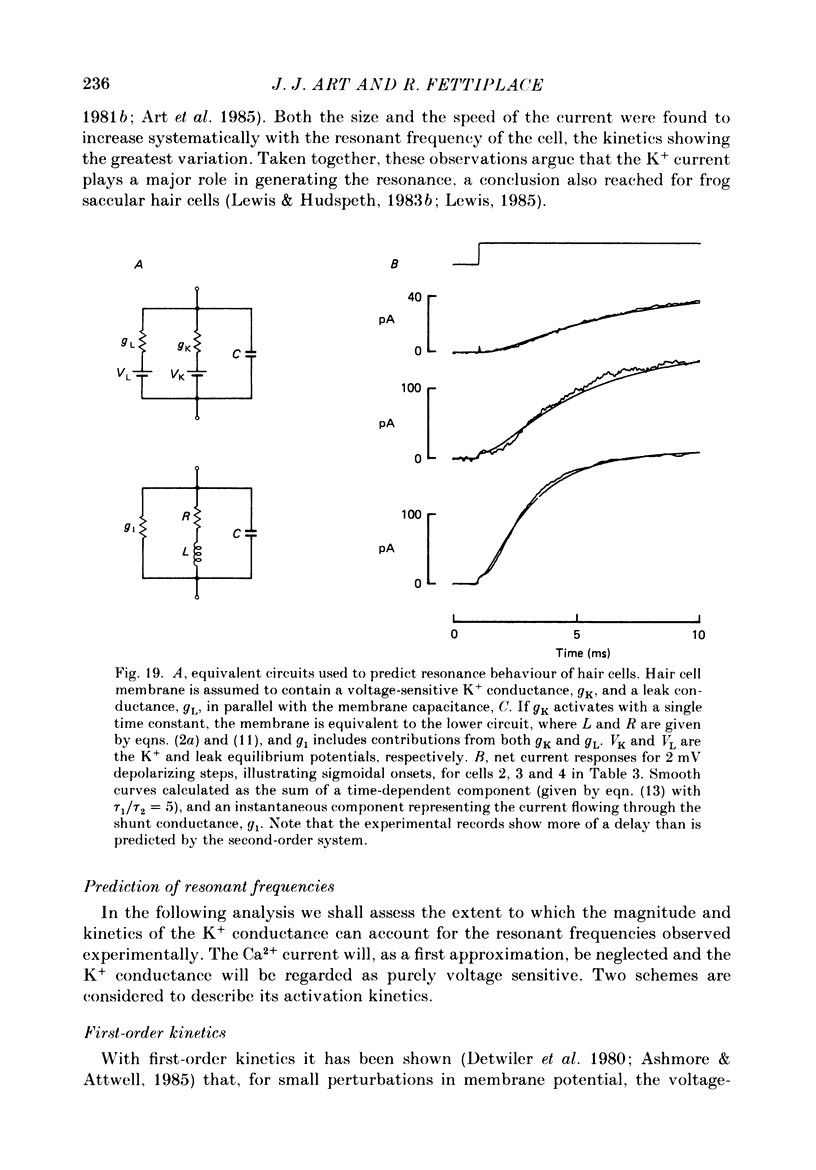


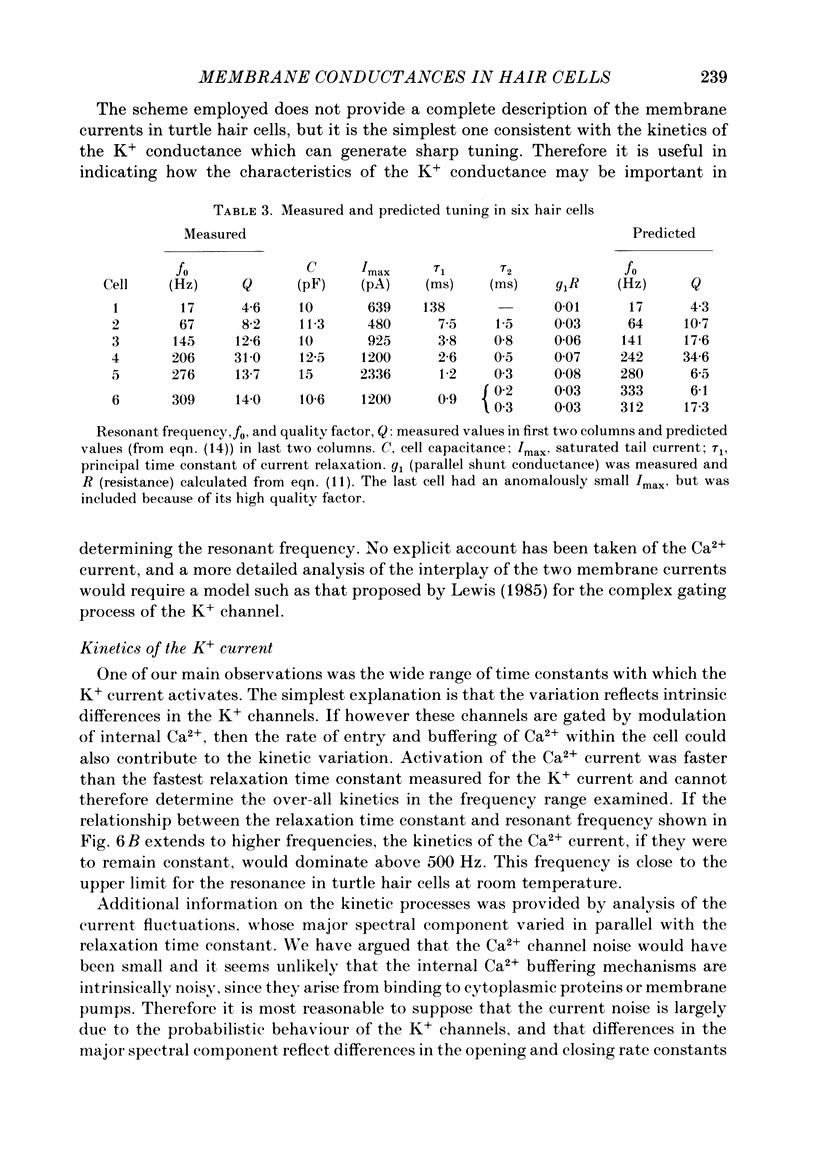



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Art J. J., Crawford A. C., Fettiplace R. Electrical resonance and membrane currents in turtle cochlear hair cells. Hear Res. 1986;22:31–36. doi: 10.1016/0378-5955(86)90073-0. [DOI] [PubMed] [Google Scholar]
- Art J. J., Crawford A. C., Fettiplace R., Fuchs P. A. Efferent modulation of hair cell tuning in the cochlea of the turtle. J Physiol. 1985 Mar;360:397–421. doi: 10.1113/jphysiol.1985.sp015624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Stanfield P. R. Calcium inactivation in skeletal muscle fibres of the stick insect, Carausius morosus. J Physiol. 1982 Sep;330:349–372. doi: 10.1113/jphysiol.1982.sp014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F. Frequency tuning in a frog vestibular organ. Nature. 1983 Aug 11;304(5926):536–538. doi: 10.1038/304536a0. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Ion conductance and selectivity of single calcium-activated potassium channels in cultured rat muscle. J Gen Physiol. 1984 Jul;84(1):1–23. doi: 10.1085/jgp.84.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979 Oct 25;281(5733):675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol. 1981 Mar;312:377–412. doi: 10.1113/jphysiol.1981.sp013634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. Non-linearities in the responses of turtle hair cells. J Physiol. 1981 Jun;315:317–338. doi: 10.1113/jphysiol.1981.sp013750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J Physiol. 1980 Sep;306:79–125. doi: 10.1113/jphysiol.1980.sp013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol. 1985 Jul;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. Temporal and spatial characteristics of the voltage response of rods in the retina of the snapping turtle. J Physiol. 1980 Mar;300:213–250. doi: 10.1113/jphysiol.1980.sp013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A., Thomas M. V. Ionic requirements for membrane oscillations and their dependence on the calcium concentration in a molluscan pace-maker neurone. J Physiol. 1982 Jun;327:185–217. doi: 10.1113/jphysiol.1982.sp014227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hermann A., Hartung K. Noise and relaxation measurements of the Ca2+ activated K+ current in Helix neurones. Pflugers Arch. 1982 May;393(3):254–261. doi: 10.1007/BF00584079. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Hudspeth A. J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983 Aug 11;304(5926):538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Lim D. J. Cochlear anatomy related to cochlear micromechanics. A review. J Acoust Soc Am. 1980 May;67(5):1686–1695. doi: 10.1121/1.384295. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A., Conti F., Dodge F., Schor R. Subthreshold behavior and phenomenological impedance of the squid giant axon. J Gen Physiol. 1970 Apr;55(4):497–523. doi: 10.1085/jgp.55.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. R. Scanning electron microscope studies of the papilla basilaris of some turtles and snakes. Am J Anat. 1978 Mar;151(3):409–435. doi: 10.1002/aja.1001510306. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Studies of ionic currents in the isolated vestibular hair cell of the chick. J Physiol. 1984 May;350:561–581. doi: 10.1113/jphysiol.1984.sp015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Saunders J. C. Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J Cell Biol. 1983 Mar;96(3):807–821. doi: 10.1083/jcb.96.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Tetraethylammonium contains an impurity which alkalizes cytoplasm and reduce calcium buffering in neurons. Brain Res. 1981 Mar 16;208(2):473–478. doi: 10.1016/0006-8993(81)90580-1. [DOI] [PubMed] [Google Scholar]