Abstract
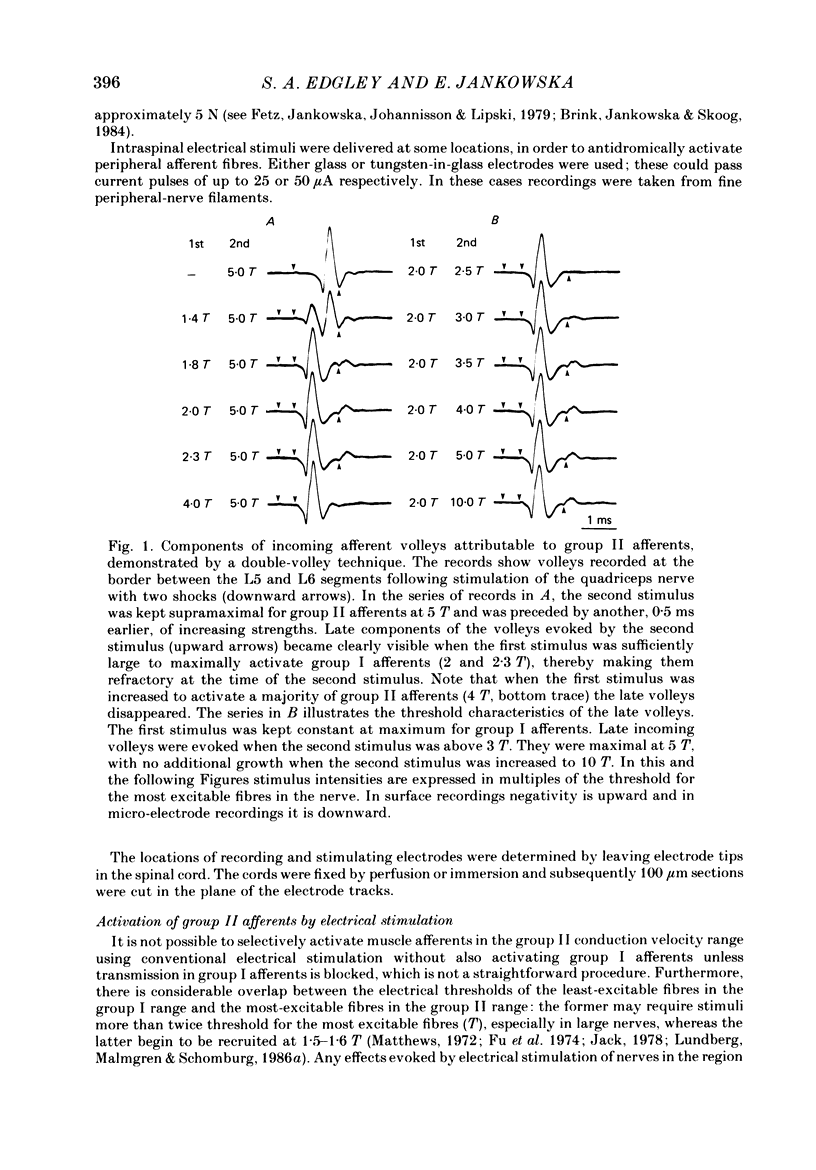
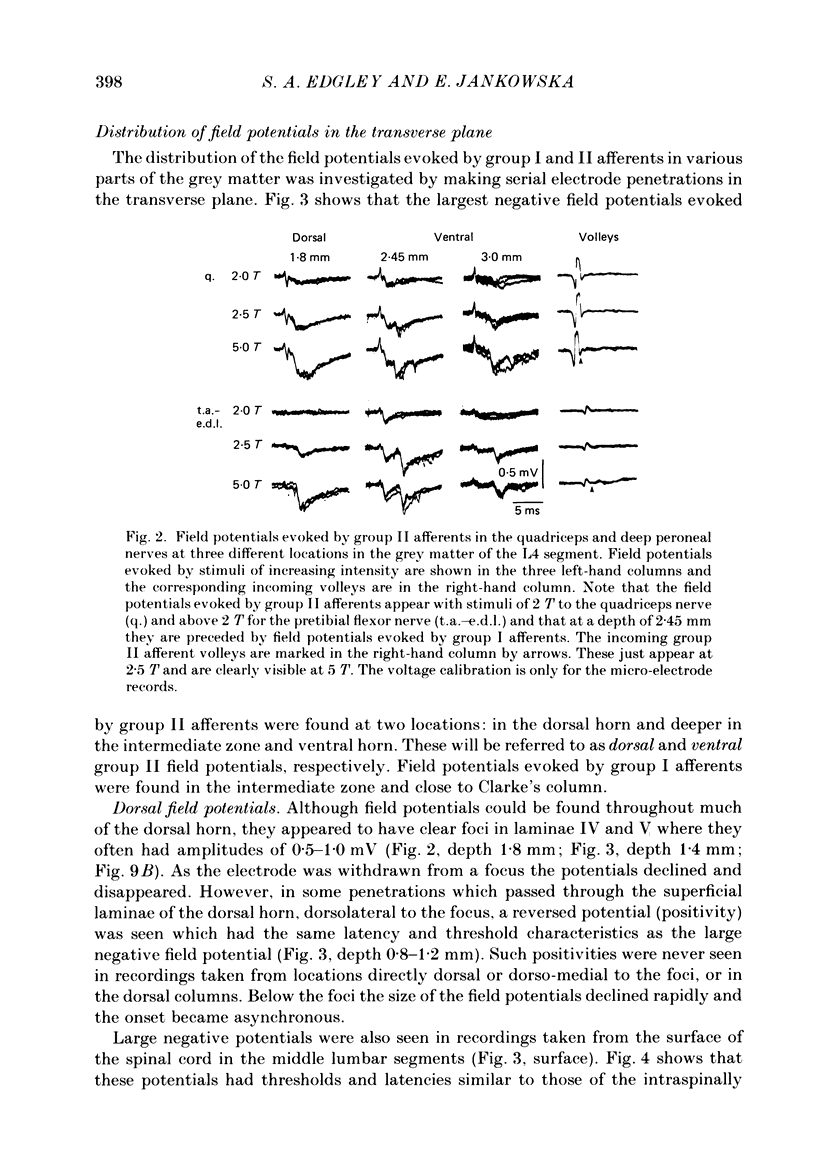
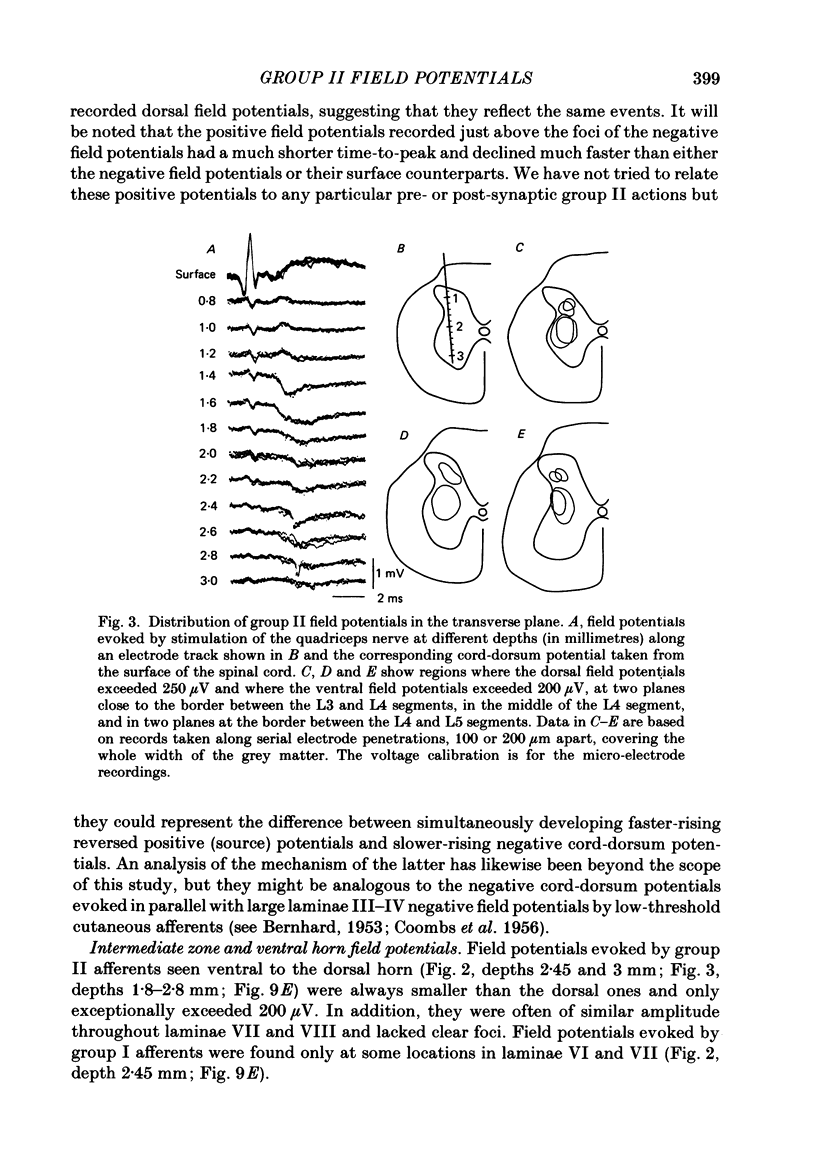
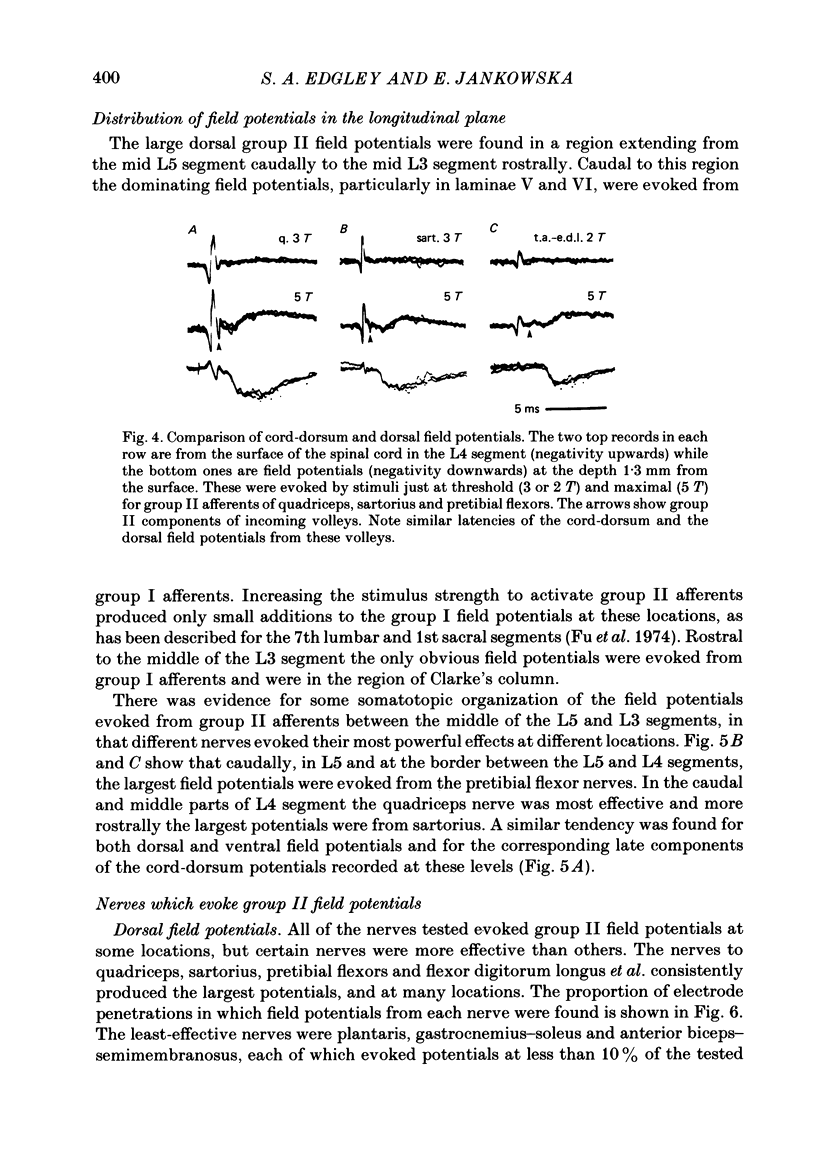
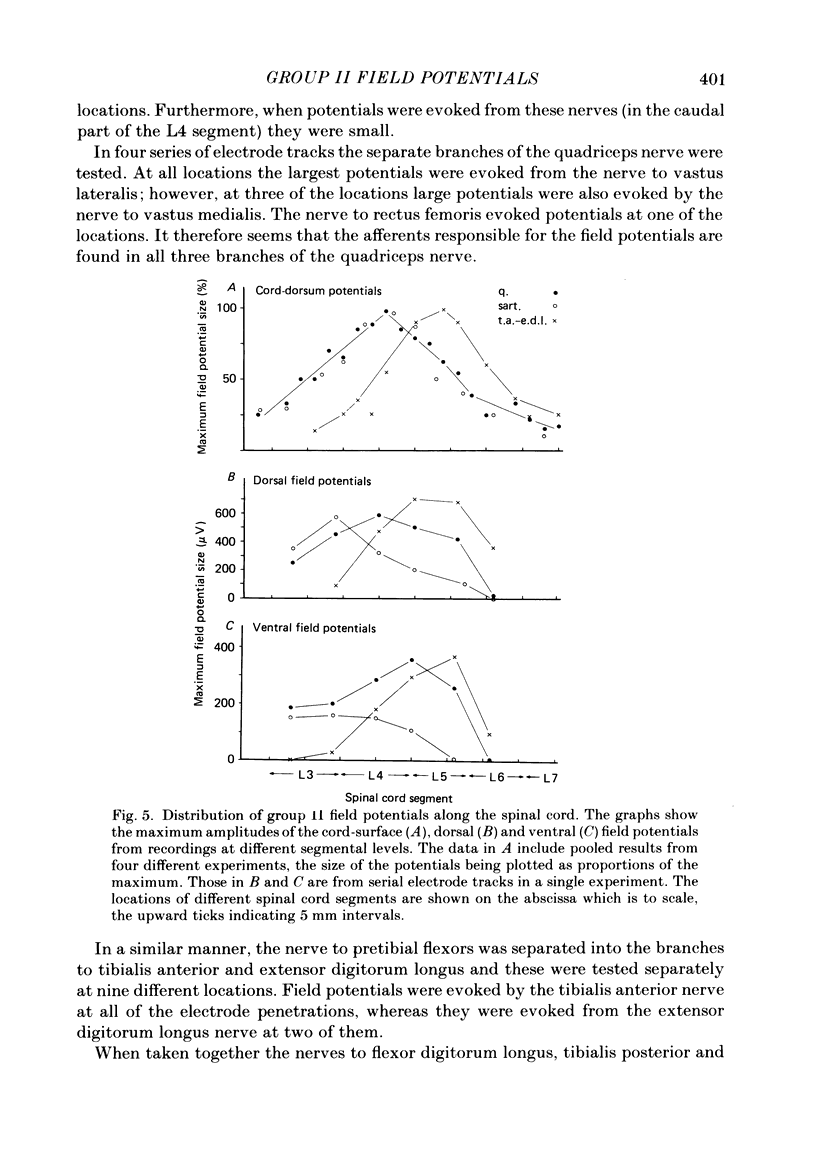
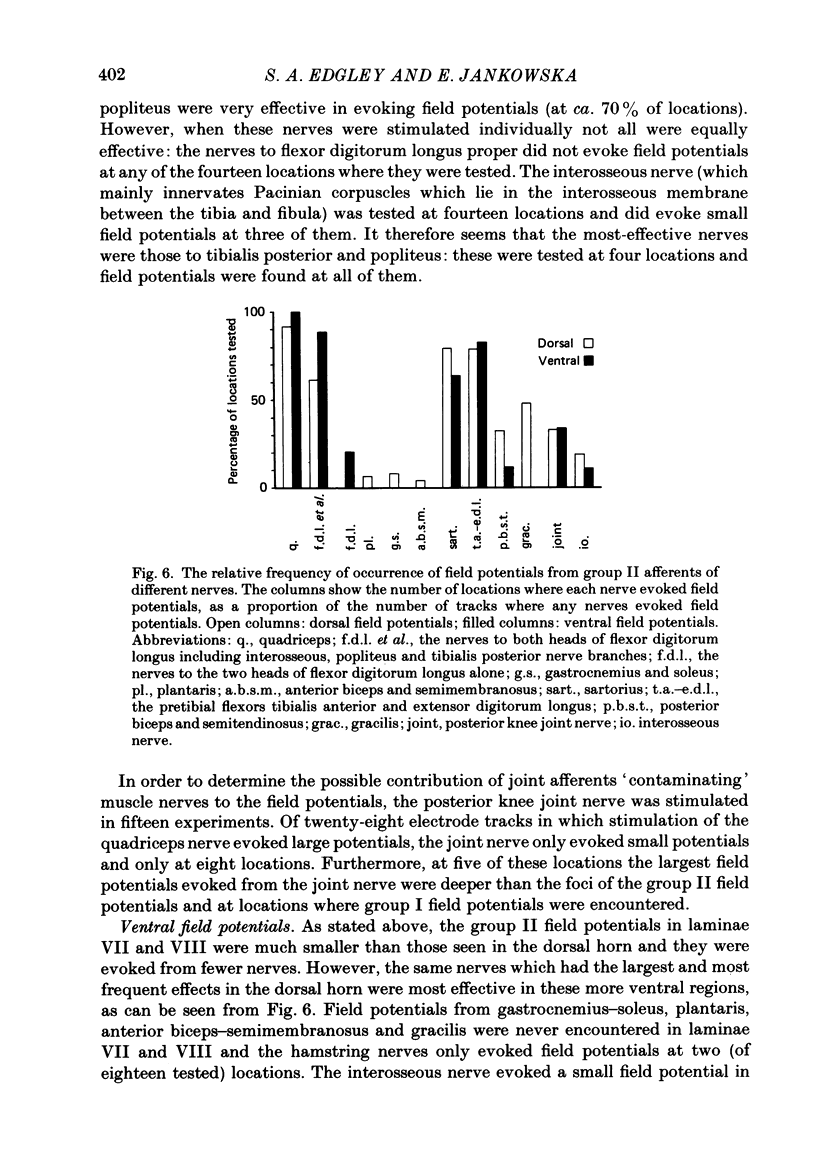
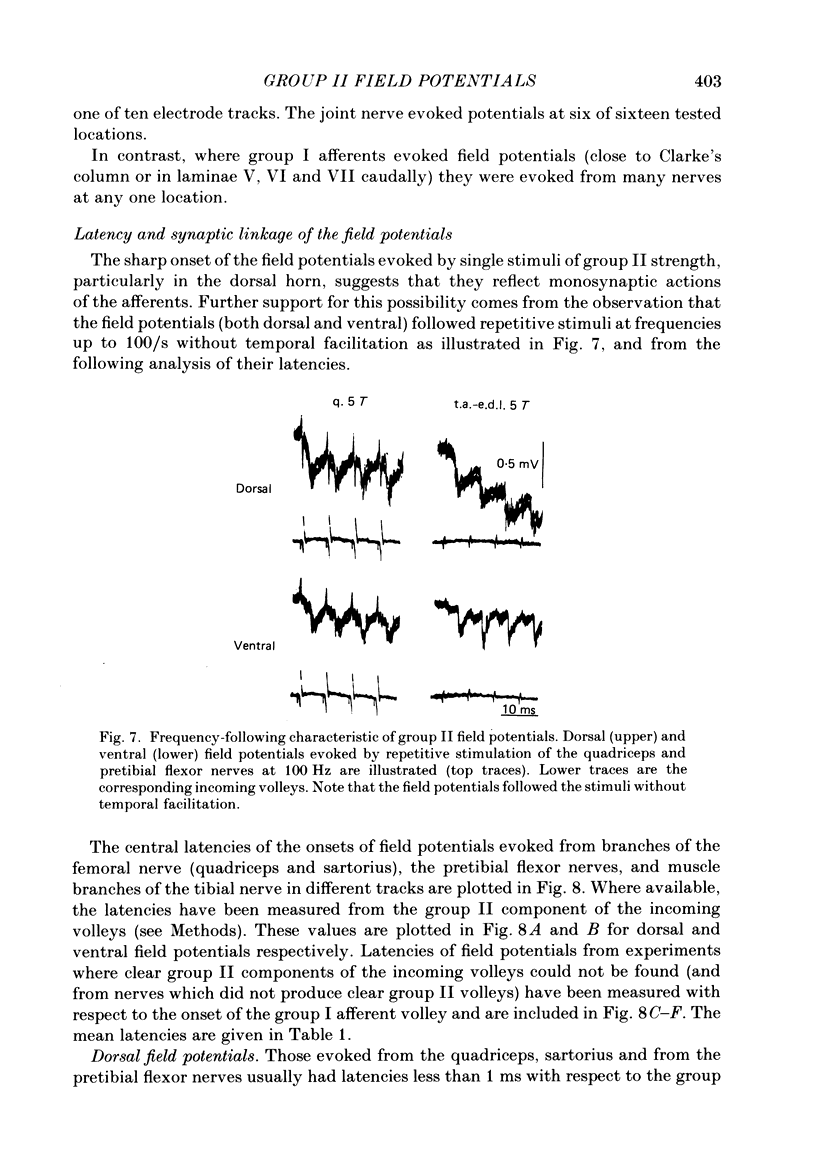
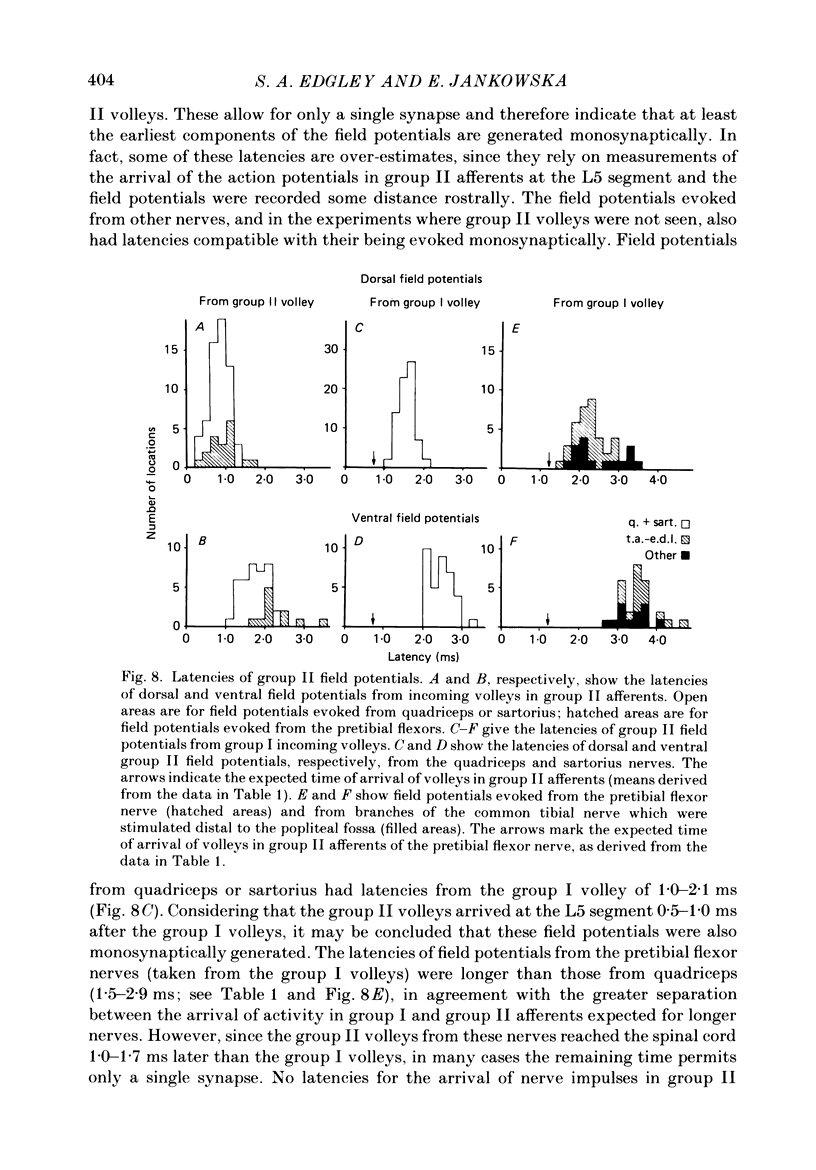
1. A powerful projection from group II muscle afferents of hind-limb muscles to the 3rd, 4th and 5th segments of the lumbar spinal cord has been demonstrated by focal synaptic field potential recording. 2. Field potentials were found at two locations: one in the dorsal horn (Rexed's laminae IV and V) and the other in the intermediate zone and ventral horn (Rexed's laminae VII and VIII). In the dorsal horn the field potentials were exceptionally large and were evoked only by group II afferents. At more ventral locations, they were smaller and were sometimes preceded by small field potentials evoked by group I afferents. 3. At both locations field potentials could be evoked by stimulation of a number of hind-limb muscle nerves at strengths sufficient to activate group II afferents. However, some nerves consistently evoked more powerful effects than others and the largest potentials were from the nerves to quadriceps, sartorius and to the pretibial flexor muscles (tibialis anterior and extensor digitorum longus). Activation of articular afferents (from the knee joint nerve) or Pacinian corpuscle afferents (from the interosseous nerve) evoked small field potentials at some locations. 4. In the dorsal horn the latency of the field potentials was so short that they must have been generated monosynaptically. Field potentials in the ventral horn had longer latencies, by 0.5-1.0 ms, but they also appear to have been monosynaptically evoked by slowly conducting intraspinal collaterals. This conclusion is based primarily on the effects of intraspinal stimulation which was found to antidromically activate afferents with the appropriate latencies and thresholds. 5. Evidence is presented that the dorsal and ventral field potentials are generated by afferents whose receptors can be activated by small (less than 100 micron) muscle stretches.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E., Jankowska E., Skoog B. Convergence onto interneurons subserving primary afferent depolarization of group I afferents. J Neurophysiol. 1984 Mar;51(3):432–449. doi: 10.1152/jn.1984.51.3.432. [DOI] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., LANDGREN S. Spinal cord potentials generated by impulses in muscle and cutaneous afferent fibres. J Neurophysiol. 1956 Sep;19(5):452–467. doi: 10.1152/jn.1956.19.5.452. [DOI] [PubMed] [Google Scholar]
- Cleland C. L., Rymer W. Z., Edwards F. R. Force-sensitive interneurons in the spinal cord of the cat. Science. 1982 Aug 13;217(4560):652–655. doi: 10.1126/science.7089586. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., LANDGREN S., WINSBURY G. J. Spinal cord potentials generated by volleys in the large muscle afferents. J Physiol. 1954 Sep 28;125(3):590–606. doi: 10.1113/jphysiol.1954.sp005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., OSCARSSON O., WILLIS W. D. Synaptic action of group I and II afferent fibres of muscle on the cells of the dorsal spinocerebellar tract. J Physiol. 1961 Oct;158:517–543. doi: 10.1113/jphysiol.1961.sp006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide E., Fedina L., Jansen J., Lundberg A., Vyklický L. Properties of Clarke's column neurones. Acta Physiol Scand. 1969 Sep-Oct;77(1):125–144. doi: 10.1111/j.1748-1716.1969.tb04558.x. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R., Tripathi A. Closely coupled excitation of gamma-motoneurones by group III Muscle afferents with low mechanical threshold in the cat. J Physiol. 1982 Oct;331:481–498. doi: 10.1113/jphysiol.1982.sp014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Jankowska E., Johannisson T., Lipski J. Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. J Physiol. 1979 Aug;293:173–195. doi: 10.1113/jphysiol.1979.sp012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman R. D., Kenshalo D. R., Jr, Schmidt R. F., Willis W. D. Field potentials and excitation of primate spinothalamic neurones in response to volleys in muscle afferents. J Physiol. 1979 Jan;286:197–213. doi: 10.1113/jphysiol.1979.sp012614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. A., Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. J Anat. 1967 Jun;101(Pt 3):505–532. [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Santini M., Schomburg E. D. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiol Scand. 1974 Jul;91(3):298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Fu T. C., Schomburg E. D. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiol Scand. 1974 Jul;91(3):314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. Characteristics of responses from receptors from the flexor longus digitorum muscle and the adjoining interosseous region of the cat. J Physiol. 1960 Aug;153:74–87. doi: 10.1113/jphysiol.1960.sp006519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund U., Lundgren O. Reactions within consecutive vascular sections of the small intestine of the cat during prolonged hypotension. Acta Physiol Scand. 1972 Feb;84(2):151–163. doi: 10.1111/j.1748-1716.1972.tb05166.x. [DOI] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985 Apr;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the lumbosacral cord. Exp Brain Res. 1966;1(4):338–358. doi: 10.1007/BF00237706. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from muscle spindle secondary endings of intercostal and triceps surae muscles in the cat. J Physiol. 1975 Feb;245(2):64P–66P. [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Comments on reflex actions evoked by electrical stimulation of group II muscle afferents. Brain Res. 1977 Feb 25;122(3):551–555. doi: 10.1016/0006-8993(77)90466-8. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Fetz E., Henneman E. Postsynatpic population potentials recorded from ventral roots perfused with isotonic sucrose: connections of groups Ia and II spindle afferent fibers with large populations of motoneurons. J Neurophysiol. 1979 Jul;42(4):1146–1164. doi: 10.1152/jn.1979.42.4.1146. [DOI] [PubMed] [Google Scholar]
- MacLennan C. R. The behaviour of receptors of extramuscular and muscular origin with afferent fibres contributing to the group I and the group II of the cat tibialis anterior muscle nerve. J Physiol. 1972 Apr;222(1):90P–91P. [PubMed] [Google Scholar]
- McIntyre A. K., Proske U., Tracey D. J. Afferent fibres from muscle receptors in the posterior nerve of the cat's knee joint. Exp Brain Res. 1978 Nov 15;33(3-4):415–424. doi: 10.1007/BF00235563. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Fleshman J. W., Sypert G. W. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. J Neurophysiol. 1980 Oct;44(4):713–725. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- Stacey M. J. Free nerve endings in skeletal muscle of the cat. J Anat. 1969 Sep;105(Pt 2):231–254. [PMC free article] [PubMed] [Google Scholar]
- Stauffer E. K., Watt D. G., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. J Neurophysiol. 1976 Nov;39(6):1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]
- Stuart D. G., Mosher C. G., Gerlach R. L., Reinking R. M. Selective activation of Ia afferents by transient muscle stretch. Exp Brain Res. 1970 Jun 25;10(5):477–487. doi: 10.1007/BF00234264. [DOI] [PubMed] [Google Scholar]


