Abstract
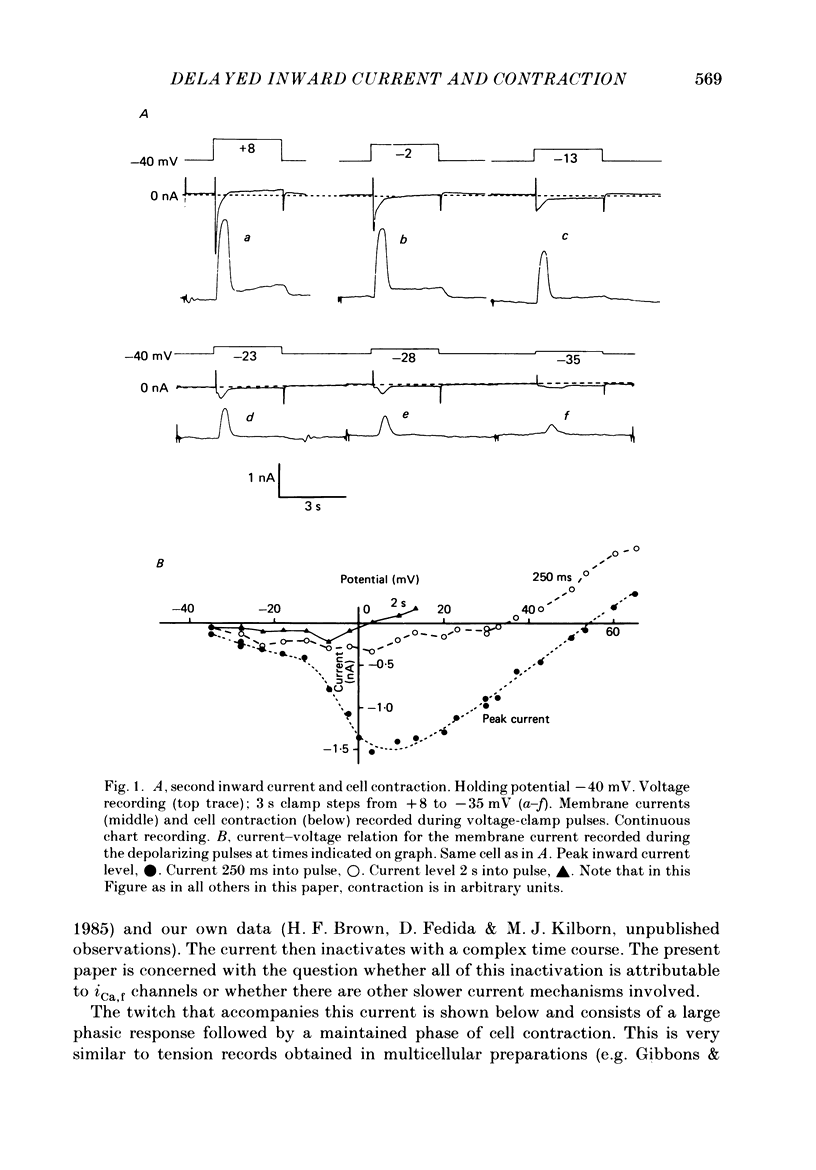
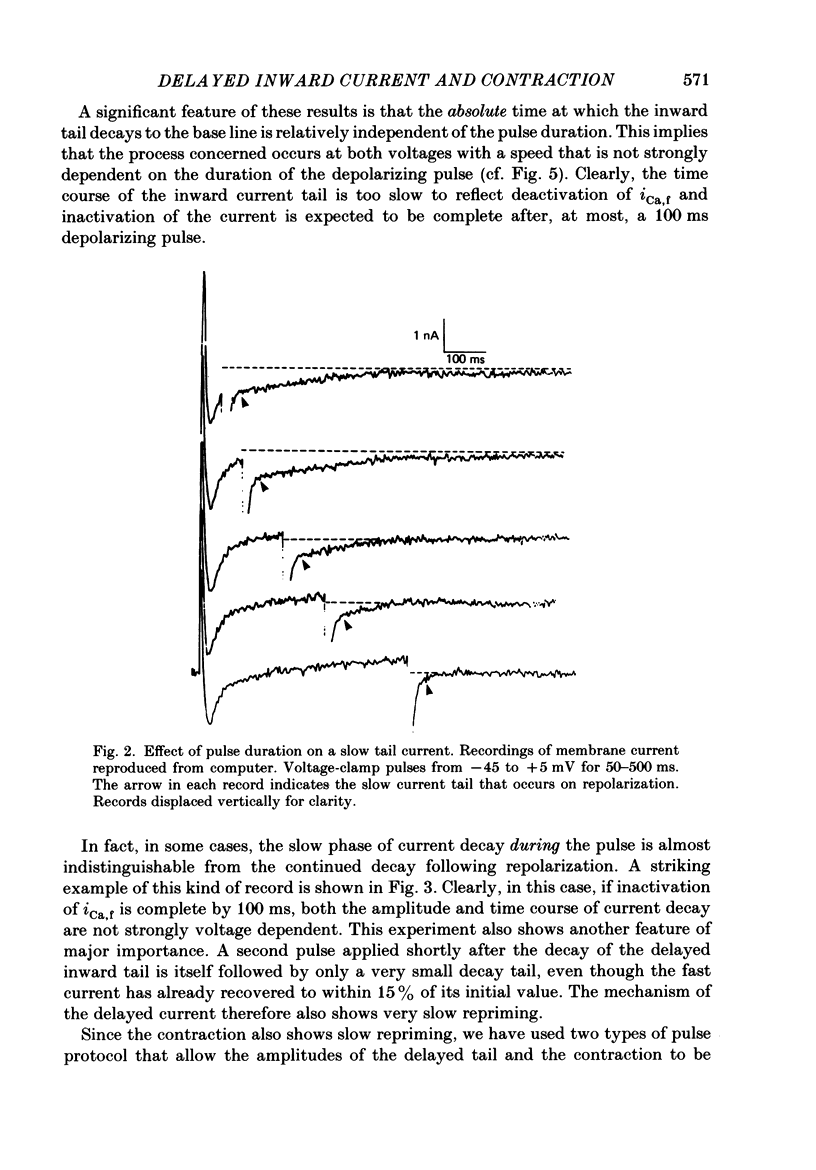
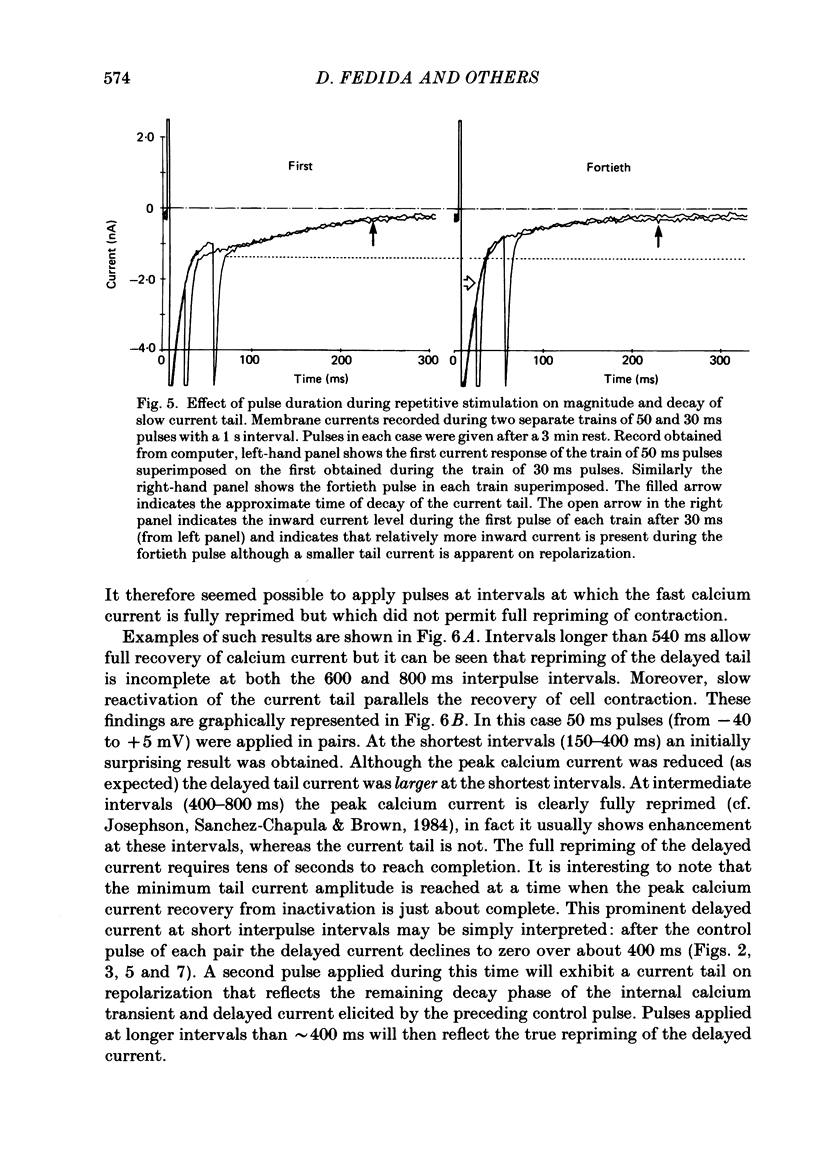
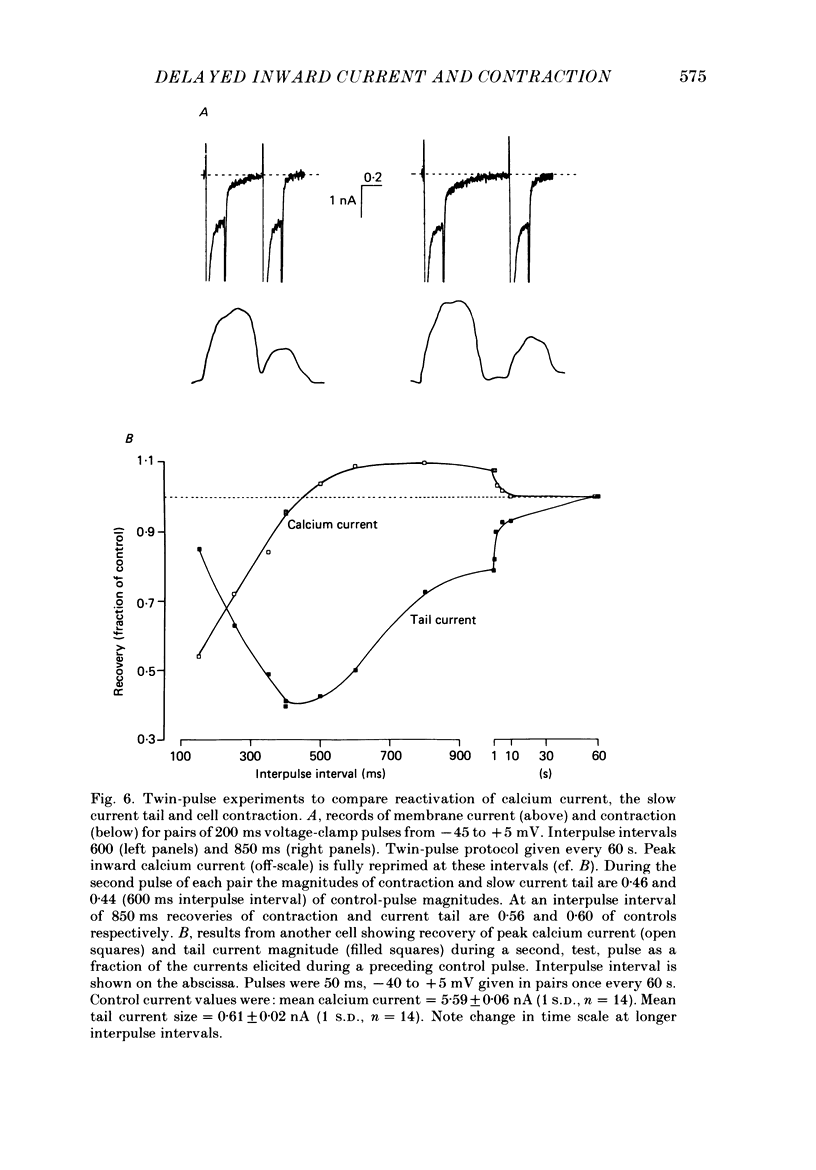
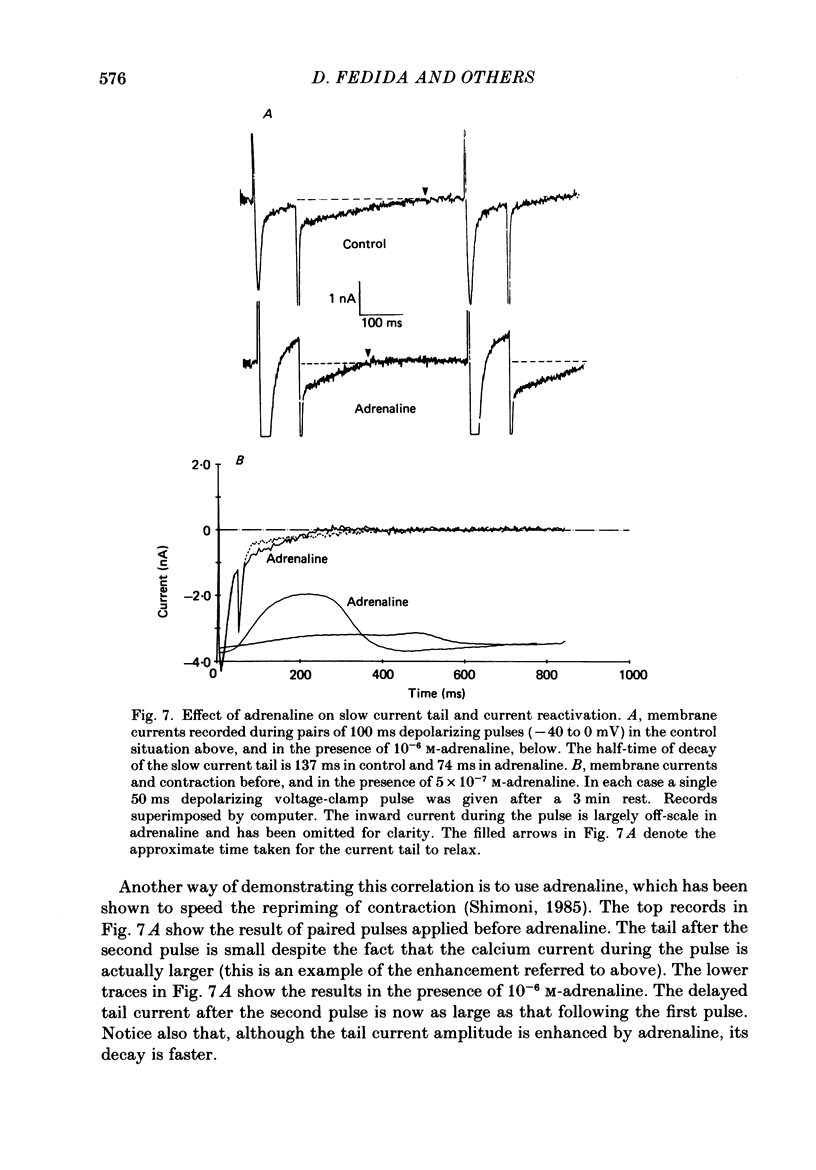
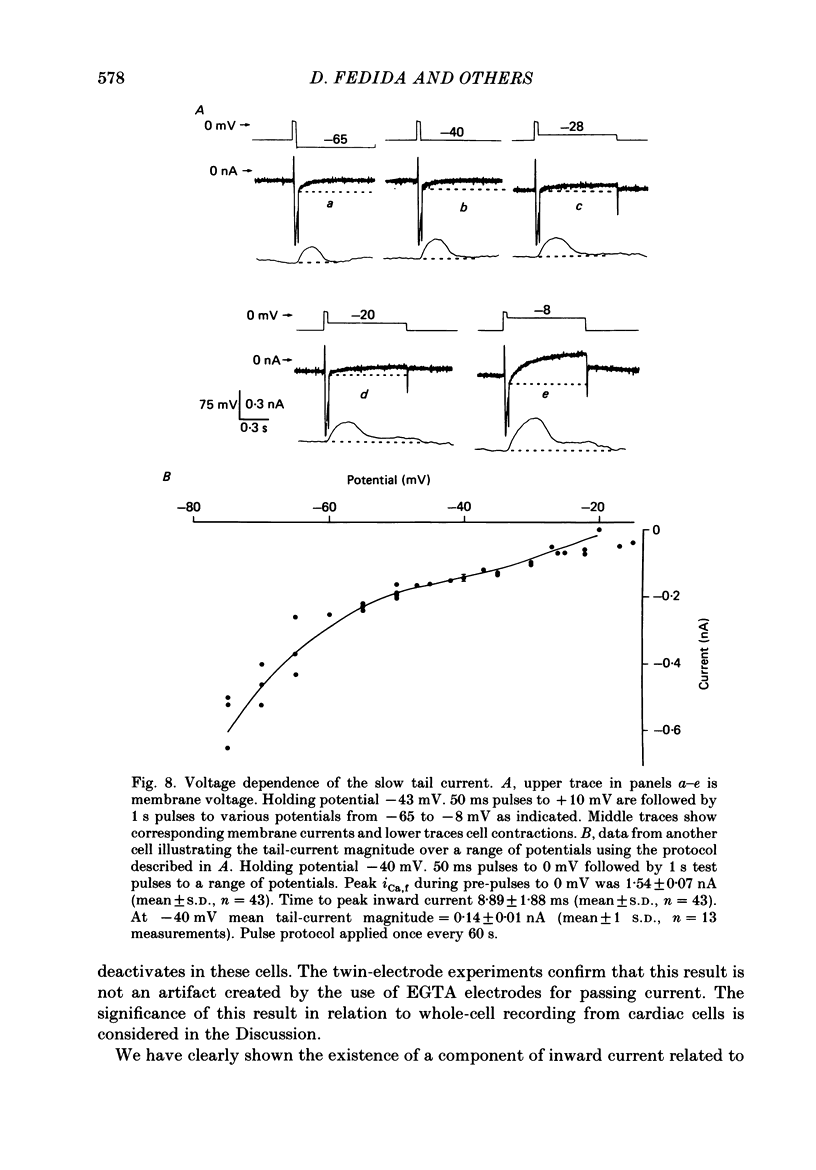
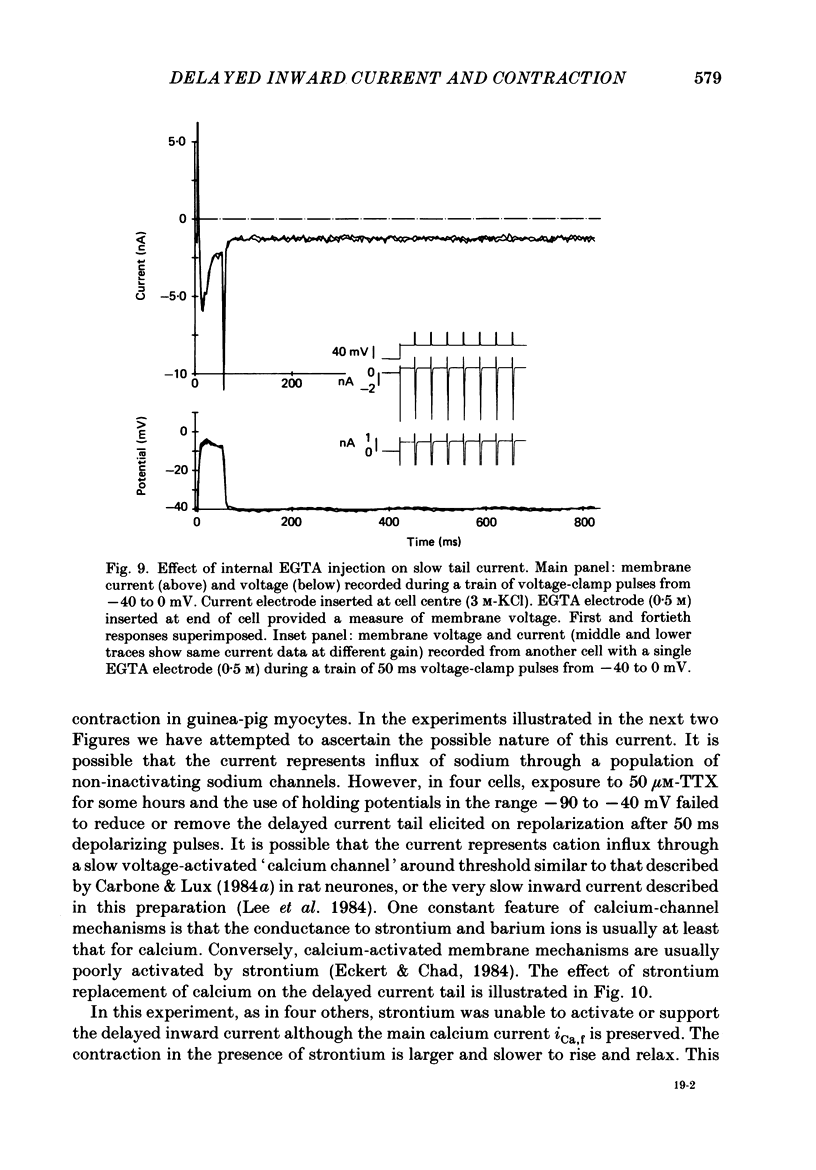
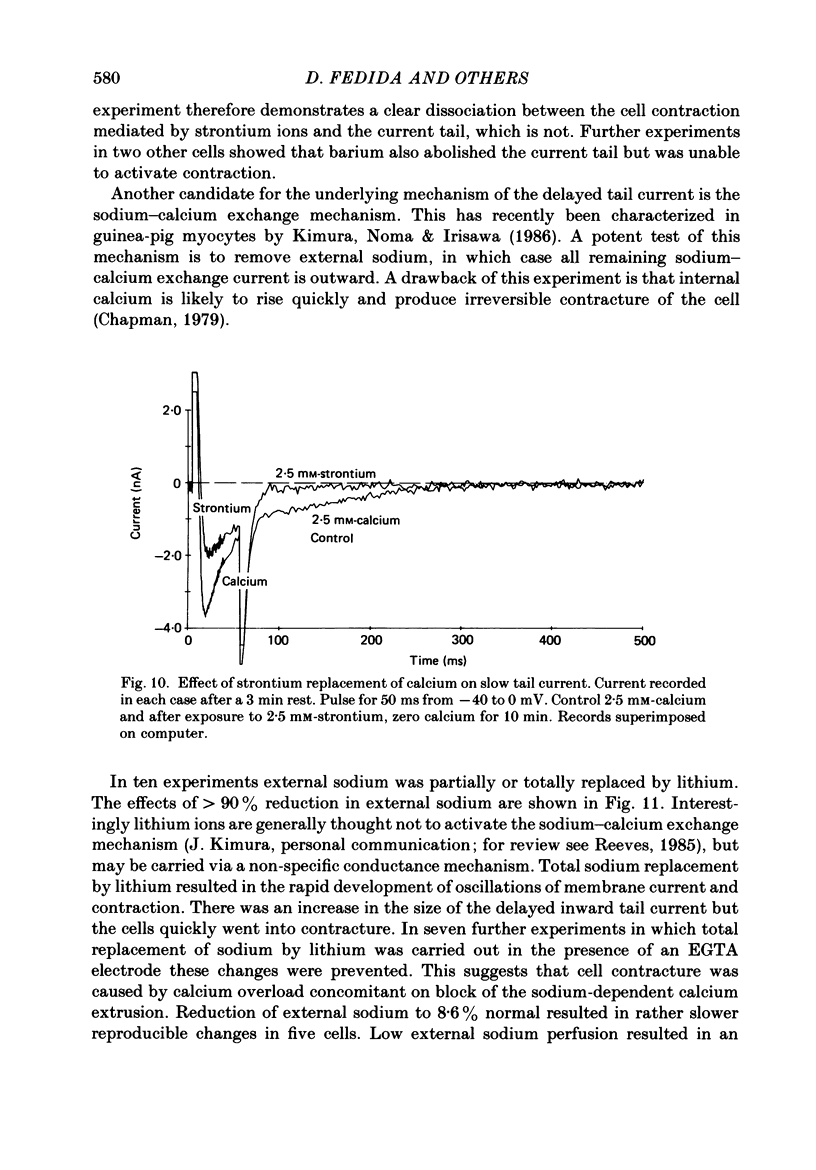
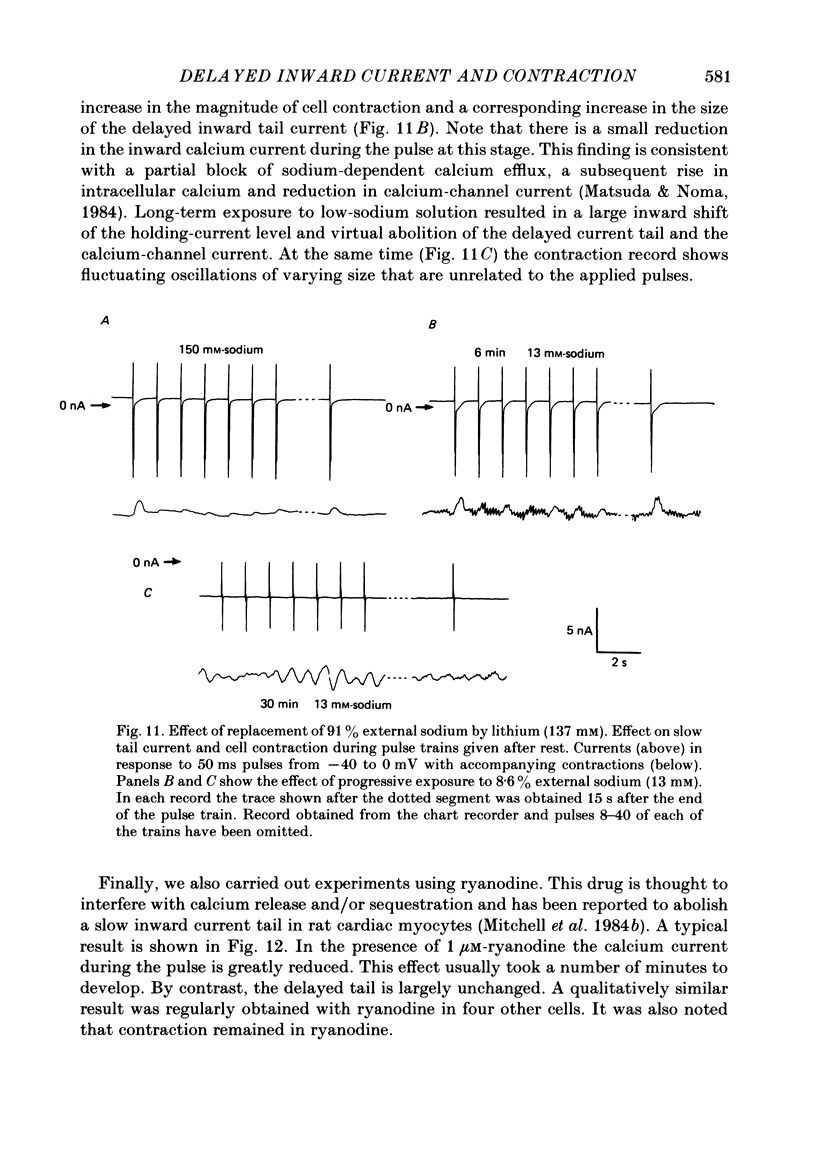
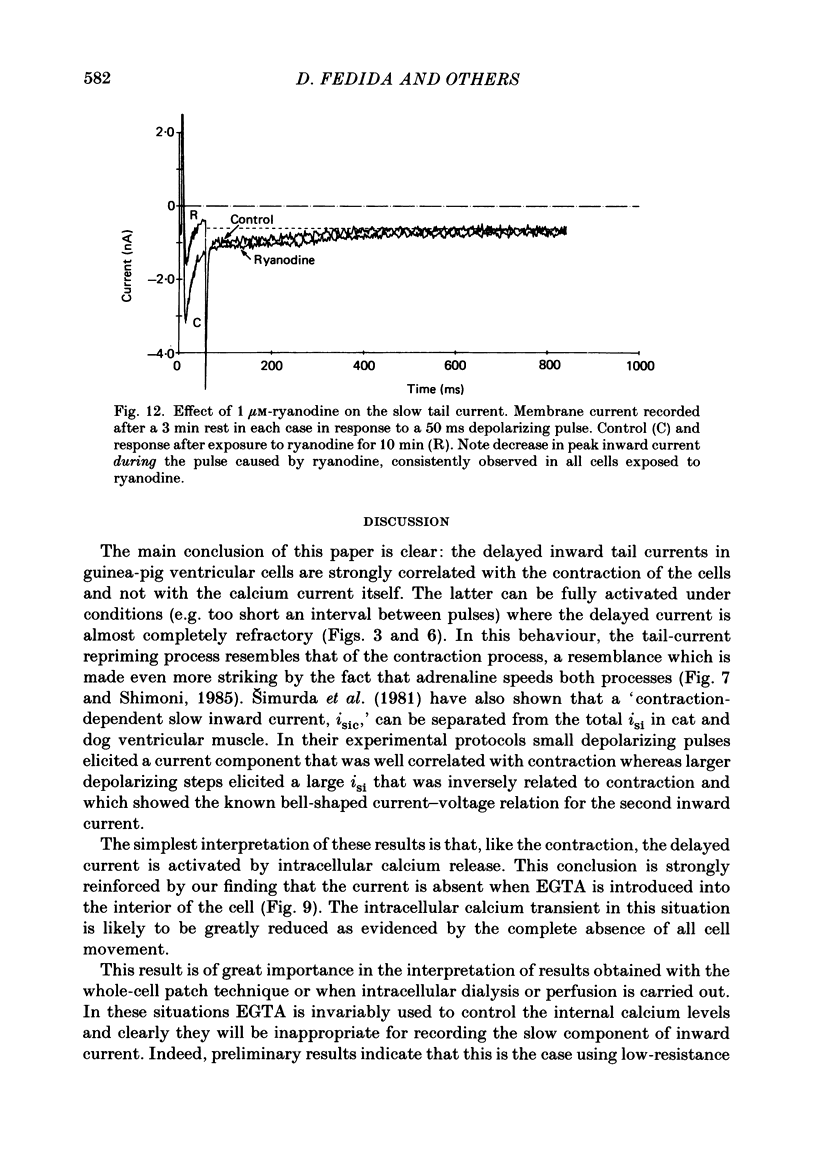
1. A component of inward current has been identified in isolated guinea-pig ventricular cells that is closely correlated with the contraction of the cell and not with the rapidly activated calcium current. This is a delayed current most clearly seen as a current 'tail' after 50-200 ms depolarizing pulses. At 22 degrees C the delayed current has a maximum amplitude of approximately 0.5 nA at -40 mV (consistently 10-20% of the peak amplitude of the calcium current) and decays with a half time of approximately 150 ms. 2. Paired-pulse protocols show that at pulse intervals (300-400 ms) at which the calcium current is nearly fully reprimed, the delayed component is very small. It recovers over a time course of several seconds, as does the contraction. Adrenaline speeds the decay of the delayed current (approximately 50%) and similarly accelerates cell relaxation. Adrenaline also shortens the recovery time of both the contraction and the delayed current. 3. During long trains of repetitive pulses, the delayed current amplitude follows that of the contraction 'staircase'. The half-time of the decay of the current 'tail' also matches that of contraction and suggests that both may reflect the time course of the underlying intracellular calcium transient. 4. The half-time of decay of the delayed current is only moderately voltage dependent over the potential range -80 to 0 mV. The amplitude of the delayed current normally reaches a minimum around -20 mV and increases at more negative potentials. 5. The voltage dependence and kinetics of decay of the current show that it should flow and decay largely during the action potential plateau and repolarization rather than during diastole. 6. Diffusion of high concentrations of EGTA into cells abolishes the delayed current and cell contraction. Under these conditions the fast calcium current is increased and its inactivation delayed. 7. When calcium is replaced by strontium, the delayed current amplitude is greatly reduced even though the contraction is larger and slower. 8. The results are consistent with the hypothesis that the delayed inward current is activated by the intracellular calcium transient. It may be carried by the sodium-calcium exchange process and/or by calcium-activated non-specific channels (especially when interal calcium is elevated by reduction of external sodium). 9. In the presence of 1 microM-ryanodine, the calcium current is greatly reduced, whereas the delayed current is not significantly altered.
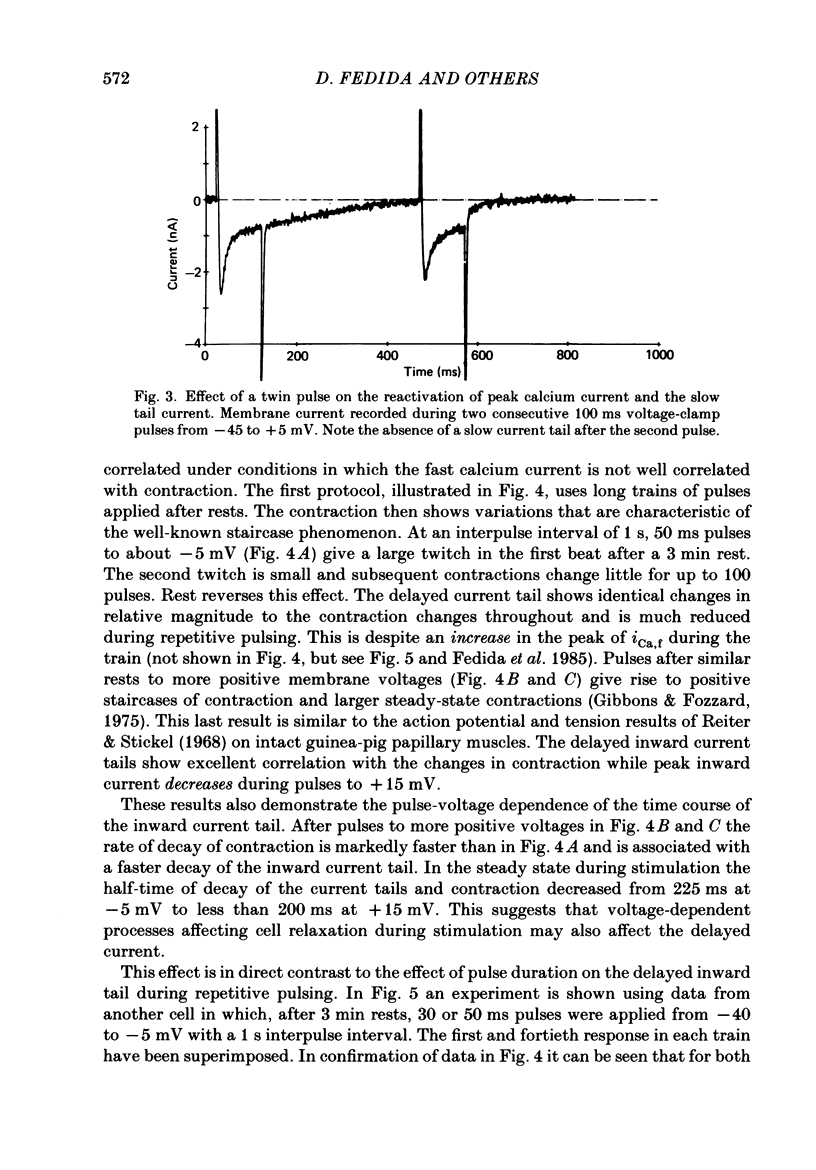
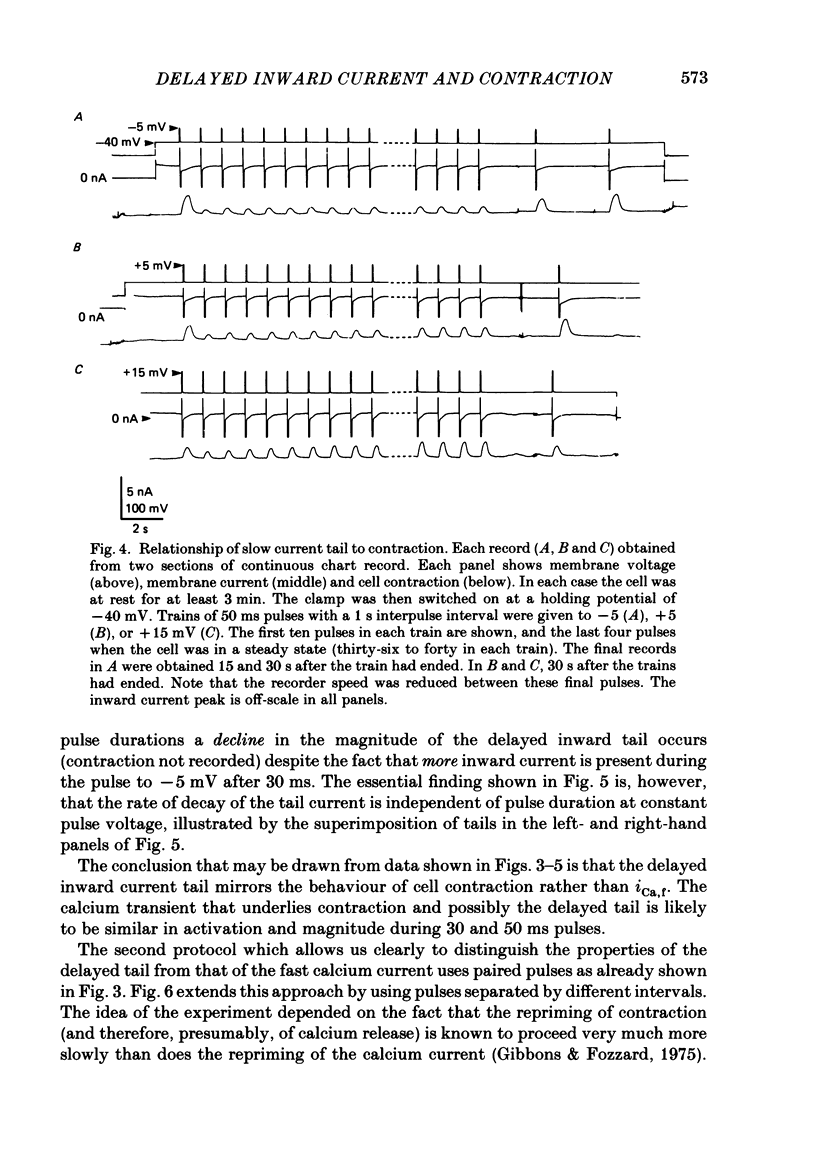
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Matteson D. R. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985 Jan 4;227(4682):65–67. doi: 10.1126/science.2578071. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. The relation between membrane potential, membrane currents and activation of contraction in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):211–229. doi: 10.1113/jphysiol.1970.sp009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M. Ca influx and sarcoplasmic reticulum Ca release in cardiac muscle activation during postrest recovery. Am J Physiol. 1985 Mar;248(3 Pt 2):H366–H381. doi: 10.1152/ajpheart.1985.248.3.H366. [DOI] [PubMed] [Google Scholar]
- Brown H. F., Kimura J., Noble D., Noble S. J., Taupignon A. The slow inward current, isi, in the rabbit sino-atrial node investigated by voltage clamp and computer simulation. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):305–328. doi: 10.1098/rspb.1984.0066. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984 Sep;46(3):413–418. doi: 10.1016/S0006-3495(84)84037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Cavalié A., McDonald T. F., Pelzer D., Trautwein W. Temperature-induced transitory and steady-state changes in the calcium current of guinea pig ventricular myocytes. Pflugers Arch. 1985 Oct;405(3):294–296. doi: 10.1007/BF00582574. [DOI] [PubMed] [Google Scholar]
- Chapman R. A. Excitation-contraction coupling in cardiac muscle. Prog Biophys Mol Biol. 1979;35(1):1–52. doi: 10.1016/0079-6107(80)90002-4. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W. Evidence for two voltage-dependent calcium currents in the membrane of the ciliate Stylonychia. J Physiol. 1984 Oct;355:137–159. doi: 10.1113/jphysiol.1984.sp015411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A non-inactivating inward current recorded during small depolarizing voltage steps in snail pacemaker neurons. Brain Res. 1975 Jan 17;83(3):486–489. doi: 10.1016/0006-8993(75)90840-9. [DOI] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A voltage-sensitive persistent calcium conductance in neuronal somata of Helix. J Physiol. 1976 Jan;254(1):129–151. doi: 10.1113/jphysiol.1976.sp011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Noble D. Caffeine and tetracaine abolish the slow inward calcium current in sheep cardiac Purkinje fibres [proceedings]. J Physiol. 1979 Aug;293:76P–77P. [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The control of tonic tension by membrane potential and intracellular sodium activity in the sheep cardiac Purkinje fibre. J Physiol. 1983 Feb;335:723–743. doi: 10.1113/jphysiol.1983.sp014560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Relaxing and inotropic effects of cyclic AMP on skinned cardiac cells. Nature. 1975 Feb 13;253(5492):556–558. doi: 10.1038/253556b0. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Krasne S. Two calcium currents in Neanthes arenaceodentatus egg cell membranes. J Physiol. 1984 Nov;356:491–505. doi: 10.1113/jphysiol.1984.sp015479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. Slow inward current and contraction of sheep cardiac Purkinje fibers. J Gen Physiol. 1975 Mar;65(3):367–384. doi: 10.1085/jgp.65.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W. Extracellular calcium transients and action potential configuration changes related to post-stimulatory potentiation in rabbit atrium. J Gen Physiol. 1986 May;87(5):675–706. doi: 10.1085/jgp.87.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D. W. Extracellular calcium transients at single excitations in rabbit atrium measured with tetramethylmurexide. J Gen Physiol. 1986 May;87(5):707–735. doi: 10.1085/jgp.87.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Giles W. Ionic currents in single isolated bullfrog atrial cells. J Gen Physiol. 1983 Feb;81(2):153–194. doi: 10.1085/jgp.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. A comparison of calcium currents in rat and guinea pig single ventricular cells. Circ Res. 1984 Feb;54(2):144–156. doi: 10.1161/01.res.54.2.144. [DOI] [PubMed] [Google Scholar]
- Kass R. S. An optical monitor of tension for small cardiac preparations. Biophys J. 1981 Apr;34(1):165–170. doi: 10.1016/S0006-3495(81)84843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Scheuer T. Slow inactivation of calcium channels in the cardiac Purkinje fiber. J Mol Cell Cardiol. 1982 Oct;14(10):615–618. doi: 10.1016/0022-2828(82)90148-1. [DOI] [PubMed] [Google Scholar]
- Kimura J., Noma A., Irisawa H. Na-Ca exchange current in mammalian heart cells. Nature. 1986 Feb 13;319(6054):596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Irisawa H. Effects of various intracellular Ca ion concentrations on the calcium current of guinea-pig single ventricular cells. Jpn J Physiol. 1984;34(4):599–611. doi: 10.2170/jjphysiol.34.599. [DOI] [PubMed] [Google Scholar]
- Kono T. Roles of collagenases and other proteolytic enzymes in the dispersal of animal tissues. Biochim Biophys Acta. 1969 Apr 22;178(2):397–400. doi: 10.1016/0005-2744(69)90410-0. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Spindler A. J., Eisner D. A. Thick slurry bevelling: a new technique for bevelling extremely fine microelectrodes and micropipettes. Pflugers Arch. 1979 Sep;381(3):287–288. doi: 10.1007/BF00583261. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Noble D., Lee E., Spindler A. J. A new calcium current underlying the plateau of the cardiac action potential. Proc R Soc Lond B Biol Sci. 1984 Nov 22;223(1230):35–48. doi: 10.1098/rspb.1984.0081. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. High selectivity of calcium channels in single dialysed heart cells of the guinea-pig. J Physiol. 1984 Sep;354:253–272. doi: 10.1113/jphysiol.1984.sp015374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Reversal of current through calcium channels in dialysed single heart cells. Nature. 1982 Jun 10;297(5866):498–501. doi: 10.1038/297498a0. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechmann S., Pott L. Identification of Na-Ca exchange current in single cardiac myocytes. Nature. 1986 Feb 13;319(6054):597–599. doi: 10.1038/319597a0. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Characteristics of the second inward current in cells isolated from rat ventricular muscle. Proc R Soc Lond B Biol Sci. 1983 Oct 22;219(1217):447–469. doi: 10.1098/rspb.1983.0084. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. The effects of ryanodine, EGTA and low-sodium on action potentials in rat and guinea-pig ventricular myocytes: evidence for two inward currents during the plateau. Br J Pharmacol. 1984 Mar;81(3):543–550. doi: 10.1111/j.1476-5381.1984.tb10107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Powell T., Twist V. W. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976 Sep 7;72(1):327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Reiter M., Stickel F. J. Der Einfluss der Kontraktionsfrequenz auf das Aktionspotential des Meerschweinchem-Papillarmuskels. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;260(4):342–365. [PubMed] [Google Scholar]
- Reuter H. A variety of calcium channels. Nature. 1985 Aug 1;316(6027):391–391. doi: 10.1038/316391a0. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J Physiol. 1967 Sep;192(2):479–492. doi: 10.1113/jphysiol.1967.sp008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten V. J., ter Keurs H. E. The slow repolarization phase of the action potential in rat heart. J Physiol. 1985 Mar;360:13–25. doi: 10.1113/jphysiol.1985.sp015601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simurda J., Simurdová M., Bravený P., Sumbera J. Activity-dependent changes of slow inward current in ventricular heart muscle. Pflugers Arch. 1981 Oct;391(4):277–283. doi: 10.1007/BF00581507. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]


