Abstract
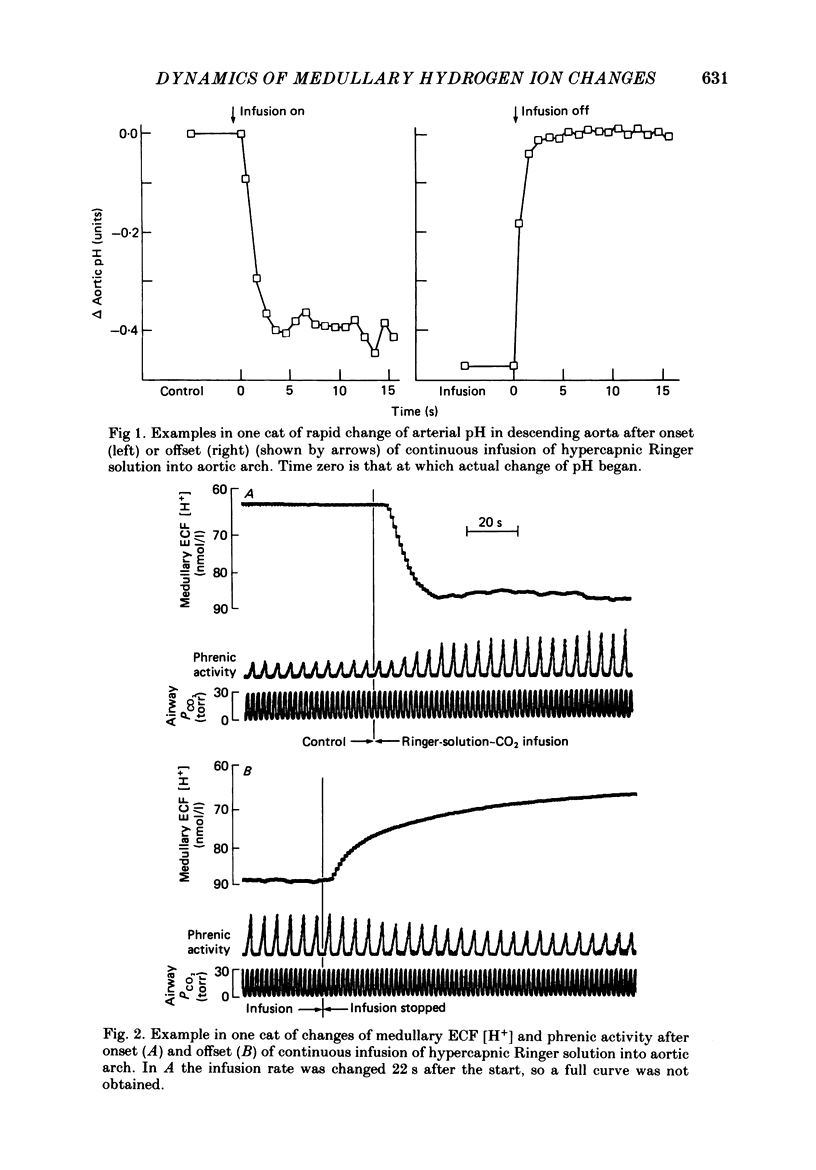
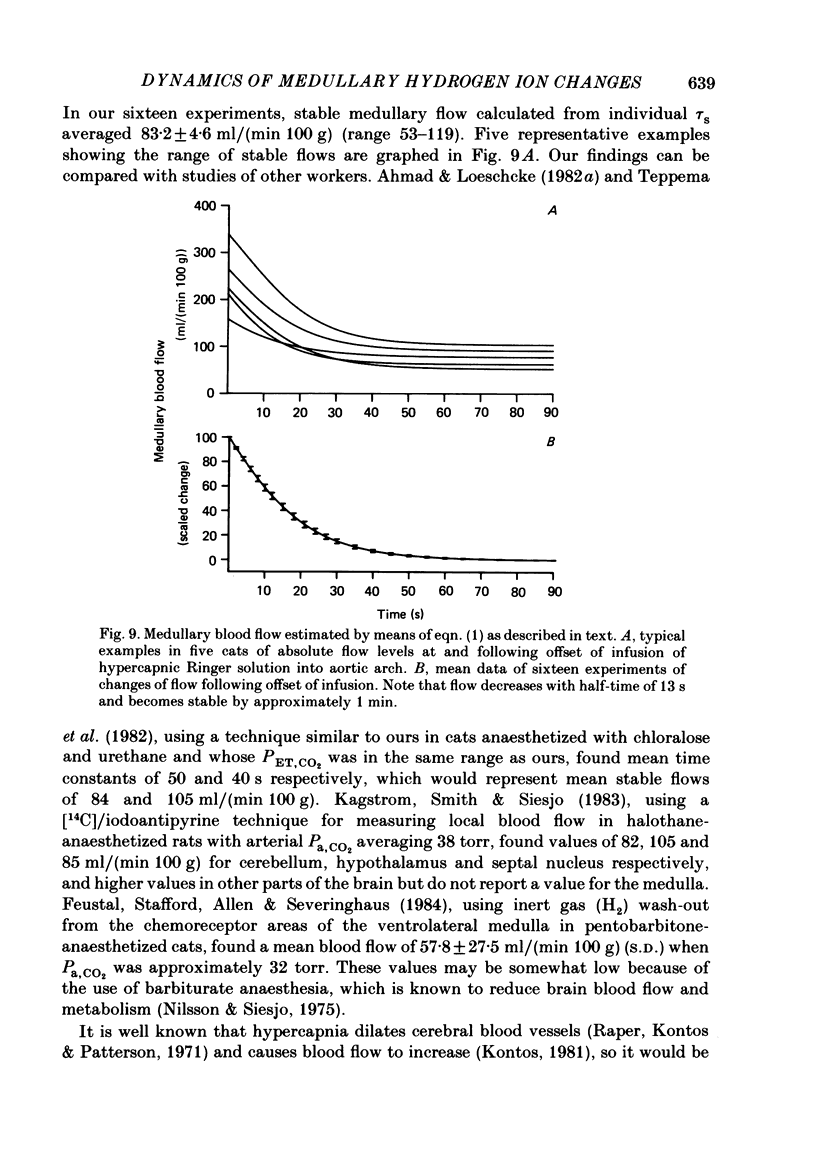
1. The dynamics of changes of medullary extracellular fluid (ECF) hydrogen ion concentration ([H+]) and respiration, measured as integrated phrenic nerve activity, were determined in anaesthetized, paralysed, vagotomized and glomectomized cats. ECF [H+] was measured directly by means of a small (2 mm diameter) glass pH electrode placed on the ventral surface of the medulla. The variables were measured continuously after a step change of arterial PCO2 produced by abruptly starting or stopping an infusion of hypercapnic fluid into the aortic arch. 2. Alteration of pH in the descending thoracic aorta at the onset or offset of infusion was complete within 1.5 s after the change began, indicating that it was nearly square wave in form. 3. In sixteen experiments, ECF [H+] began to fall within 2 s of offset of infusion, reflecting aortic-medullary circulation time. Thereafter, ECF [H+] decreased to a stable level over the next 5 min; the curve describing the decrease consisted of two exponential functions, one with a time constant (tau) of 9.5 +/- 0.6 s and a second with a tau of 53 +/- 3 s. 4. We interpret the findings at the offset of CO2 infusion in terms of CO2 wash-out from the medullary ECF. The slow function is associated with wash-out during stable medullary blood flow that develops after 1 min. The early fast function is associated with the decreasing medullary blood flow that occurs during the first minute after change from arterial hypercapnia to normocapnia. 5. We have estimated medullary blood flow using a mathematical model incorporating the two functions. The values obtained are consistent with those in the literature where other methods have been used. Changes of blood flow following the step change of CO2 are fairly rapid, half of the response occurring in 13 s. 6. The change of respiratory activity lags the change of stimulus expressed by [H+], throughout the recovery period and respiration requires up to 8 min to reach a stable level. We attribute this slow response to slow central neural respiratory dynamics, the respiratory after-discharge.
Full text
PDF




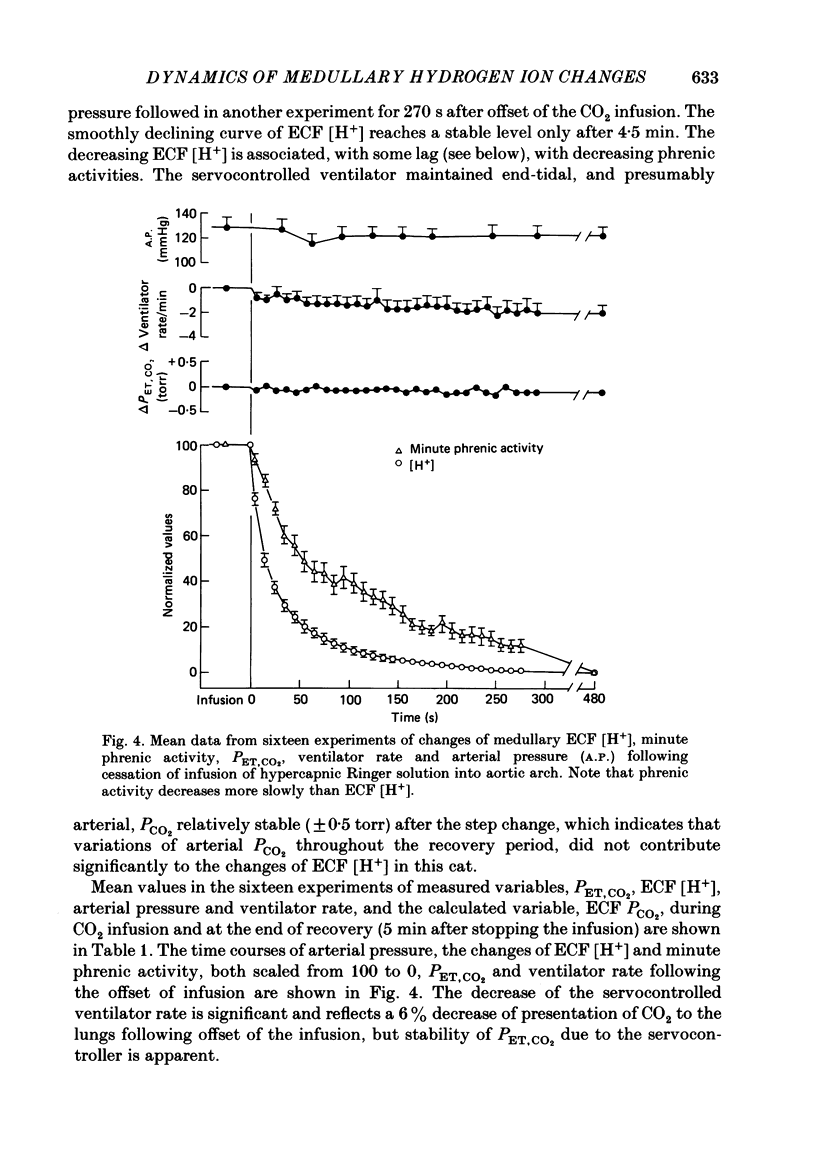








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Glasheen W. P., Severns M. L. Estimating medullary chemoreceptor blood flow from ventilatory-CO2 response transients: theory and data from anesthetized dogs. Ann Biomed Eng. 1984;12(1):1–13. doi: 10.1007/BF02410287. [DOI] [PubMed] [Google Scholar]
- Ahmad H. R., Loeschcke H. H. Fast bicarbonate-chloride exchange between brain cells and brain extracellular fluid in respiratory acidosis. Pflugers Arch. 1982 Dec;395(4):293–299. doi: 10.1007/BF00580792. [DOI] [PubMed] [Google Scholar]
- Ahmad H. R., Loeschcke H. H. Transient and steady state responses of pulmonary ventilation to the medullary extracellular pH after approximately rectangular changes in alveolar PCO2. Pflugers Arch. 1982 Dec;395(4):285–292. doi: 10.1007/BF00580791. [DOI] [PubMed] [Google Scholar]
- Cragg P., Patterson L., Purves M. J. The pH of brain extracellular fluid in the cat. J Physiol. 1977 Oct;272(1):137–166. doi: 10.1113/jphysiol.1977.sp012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr H. F., Cooper D. N., Milhorat T. H. Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp Eye Res. 1977;25 (Suppl):461–473. doi: 10.1016/s0014-4835(77)80041-9. [DOI] [PubMed] [Google Scholar]
- Dahlgren N., Ingvar M., Siesjö B. K. Effect of propranolol on local cerebral blood flow under normocapnic and hypercapnic conditions. J Cereb Blood Flow Metab. 1981;1(4):429–436. doi: 10.1038/jcbfm.1981.47. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J. Effects of pentobarbital on contractile responses of feline cerebral arteries. J Cereb Blood Flow Metab. 1981;1(4):437–440. doi: 10.1038/jcbfm.1981.48. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P. Central neural respiratory drive and afterdischarge. Respir Physiol. 1980 Apr;40(1):49–63. doi: 10.1016/0034-5687(80)90004-3. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Kiley J. P., Millhorn D. E. Respiratory effects of carbon dioxide-induced changes of medullary extracellular fluid pH in cats. J Physiol. 1984 Oct;355:177–189. doi: 10.1113/jphysiol.1984.sp015413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L., Kiley J. P., Millhorn D. E. Respiratory responses to medullary hydrogen ion changes in cats: different effects of respiratory and metabolic acidoses. J Physiol. 1985 Jan;358:285–297. doi: 10.1113/jphysiol.1985.sp015551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L. Relationship between phrenic nerve activity and ventilation. Am J Physiol. 1971 Aug;221(2):535–543. doi: 10.1152/ajplegacy.1971.221.2.535. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. Relationship between respiratory nerve and muscle activity and muscle force output. J Appl Physiol. 1975 Oct;39(4):567–574. doi: 10.1152/jappl.1975.39.4.567. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. The importance of timing on the respiratory effects of intermittent carotid body chemoreceptor stimulation. J Physiol. 1972 Apr;222(2):319–333. doi: 10.1113/jphysiol.1972.sp009799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feustel P. J., Stafford M. J., Allen J. S., Severinghaus J. W. Ventrolateral medullary surface blood flow determined by hydrogen clearance. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jan;56(1):150–154. doi: 10.1152/jappl.1984.56.1.150. [DOI] [PubMed] [Google Scholar]
- Gelfand R., Lambertsen C. J. Dynamic respiratory response to abrupt change of inspired CO2 at normal and high PO2. J Appl Physiol. 1973 Dec;35(6):903–913. doi: 10.1152/jappl.1973.35.6.903. [DOI] [PubMed] [Google Scholar]
- KETY S. S. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol Rev. 1951 Mar;3(1):1–41. [PubMed] [Google Scholar]
- Kiley J. P., Eldridge F. L., Millhorn D. E. The roles of medullary extracellular and cerebrospinal fluid pH in control of respiration. Respir Physiol. 1985 Feb;59(2):117–130. doi: 10.1016/0034-5687(85)90001-5. [DOI] [PubMed] [Google Scholar]
- Kontos H. A. Regulation of the cerebral circulation. Annu Rev Physiol. 1981;43:397–407. doi: 10.1146/annurev.ph.43.030181.002145. [DOI] [PubMed] [Google Scholar]
- Kågström E., Smith M. L., Siesjö B. K. Cerebral circulatory responses to hypercapnia and hypoxia in the recovery period following complete and incomplete cerebral ischemia in the rat. Acta Physiol Scand. 1983 Jul;118(3):281–291. doi: 10.1111/j.1748-1716.1983.tb07272.x. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L., Kiley J. P. Oscillations of medullary extracellular fluid pH caused by breathing. Respir Physiol. 1984 Feb;55(2):193–203. doi: 10.1016/0034-5687(84)90022-7. [DOI] [PubMed] [Google Scholar]
- Nilsson L., Siesjö B. K. The effect of phenobarbitone anaesthesia on blood flow and oxygen consumption in the rat brain. Acta Anaesthesiol Scand Suppl. 1975;57:18–24. doi: 10.1111/j.1399-6576.1975.tb05408.x. [DOI] [PubMed] [Google Scholar]
- Nye P. C., Hanson M. A., Torrance R. W. The effect on breathing of abruptly reducing the discharge of central chemoreceptors. Respir Physiol. 1983 Jan;51(1):109–118. doi: 10.1016/0034-5687(83)90105-6. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Farhi L. E. Cerebral gas exchange: perfusion and diffusion limitations. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jun;46(6):1164–1168. doi: 10.1152/jappl.1979.46.6.1164. [DOI] [PubMed] [Google Scholar]
- Pontén U., Siesjö B. K. Gradients of CO2 tension in the brain. Acta Physiol Scand. 1966 Jun;67(2):129–140. doi: 10.1111/j.1748-1716.1966.tb03294.x. [DOI] [PubMed] [Google Scholar]
- SHAPIRO E., WASSERMAN A. J., PATTERSON J. L., Jr HUMAN CEREBROVASCULAR RESPONSE TIME TO ELEVATION OF ARTERIAL CARBON DIOXIDE TENSION. Arch Neurol. 1965 Aug;13:130–138. doi: 10.1001/archneur.1965.00470020020003. [DOI] [PubMed] [Google Scholar]
- Severinghaus J. W., Lassen N. Step hypocapnia to separate arterial from tissue PCO2 in the regulation of cerebral blood flow. Circ Res. 1967 Feb;20(2):272–278. doi: 10.1161/01.res.20.2.272. [DOI] [PubMed] [Google Scholar]
- Shams H. Differential effects of CO2 and H+ as central stimuli of respiration in the cat. J Appl Physiol (1985) 1985 Feb;58(2):357–364. doi: 10.1152/jappl.1985.58.2.357. [DOI] [PubMed] [Google Scholar]
- Shapiro W., Wasserman A. J., Patterson J. L., Jr Mechanism and pattern of human cerebrovascular regulation after rapid changes in blood CO2 tension. J Clin Invest. 1966 Jun;45(6):913–922. doi: 10.1172/JCI105406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjö B. K. Cerebral metabolic rate in hypercarbia--a controversy. Anesthesiology. 1980 Jun;52(6):461–465. doi: 10.1097/00000542-198006000-00001. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Mercer R. R., Eldridge F. L. Servo control of end-tidal CO2 in paralyzed animals. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):133–136. doi: 10.1152/jappl.1978.45.1.133. [DOI] [PubMed] [Google Scholar]
- Teppema L. J., Vis A., Evers J. A., Folgering H. T. Dynamics of brain extracellular fluid pH and phrenic nerve activity in cats after end-tidal CO2 forcing. Respir Physiol. 1982 Dec;50(3):359–380. doi: 10.1016/0034-5687(82)90029-9. [DOI] [PubMed] [Google Scholar]
- Tuteur P., Reivich M., Goldberg H. I., Cooper E. S., West J. W., McHenry L. C., Cherniack N. Transient responses of cerebral blood flow and ventilation to changes in PaCO2 in normal subjects and patients with cerebrovascular disease. Stroke. 1976 Nov-Dec;7(6):584–590. doi: 10.1161/01.str.7.6.584. [DOI] [PubMed] [Google Scholar]
- Vis A., Folgering H. The dynamic effect of PETCO, on vertebral bloodflow in cats. Respir Physiol. 1980 Nov;42(2):131–143. doi: 10.1016/0034-5687(80)90110-3. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Traystman R. J., Rapela C. E. Transient analysis of the canine cerebrovascular response to carbon dioxide. Circ Res. 1985 Apr;56(4):596–605. doi: 10.1161/01.res.56.4.596. [DOI] [PubMed] [Google Scholar]
- Young W. H2 clearance measurement of blood flow: a review of technique and polarographic principles. Stroke. 1980 Sep-Oct;11(5):552–564. doi: 10.1161/01.str.11.5.552. [DOI] [PubMed] [Google Scholar]


