Abstract
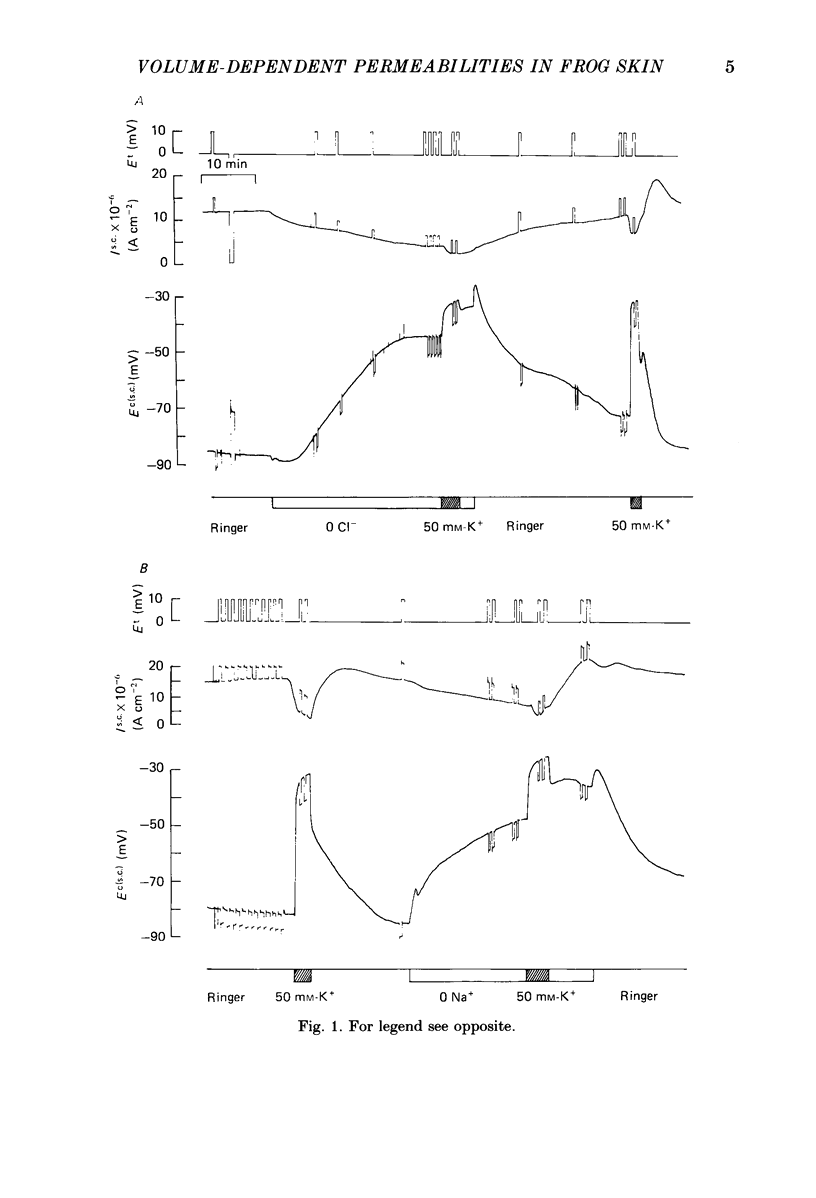
1. Membrane potential and conductances and short-circuit current were continuously measured with microelectrodes and conventional electrophysiological techniques in a stripped preparation of frog skin epithelium. The effects of the removal of chloride or sodium ions and the concentration or dilution of the serosal (inner) bathing solution were studied. 2. Chloride- or sodium-free solutions produced a cell depolarization of about 30 mV in parallel with a fall in the short-circuit current. Mucosal and serosal membrane conductances both decreased and the sodium permeability of the mucosal barrier was calculated to fall to about one-half its value in standard Ringer solution. The observed decrease in the short-circuit current is probably related to the combined effect of the decrease in sodium permeability and the decrease in the driving force across the mucosal membrane. 3. The removal of chloride or sodium ions reduced the depolarization caused by serosal perfusion with high-potassium solutions (50 mM-KCl). The ratio of the change in cell membrane potential under short-circuit conditions to the change in the potassium equilibrium potential (delta Ec(s.c.)/delta EK), was 0.59 in standard Ringer solution and 0.26 and 0.24 after the removal of chloride or sodium respectively. The depolarizing effect of barium-containing solutions (2 mM-BaCl2) was also markedly reduced in chloride- or sodium-free solutions, suggesting a decrease of the potassium selectivity of the serosal membrane in these conditions. 4. Increasing the osmolality of the serosal bathing solution produced similar effects, i.e. cell depolarization, fall in the short-circuit current and membrane conductances and reduction of the depolarizing effect of high-potassium and barium solutions. On the contrary, dilution of the serosal bath produced the opposite effects, consistent with an increase in the serosal permeability to potassium. 5. The effects of chloride- or sodium-free solutions were reversed by the dilution of the serosal bath. Cells repolarized when exposed to low-osmolality solutions after being in the absence of serosal chloride or sodium. The repolarization ran in parallel with the restoration of the short-circuit current and the potassium selectivity of the serosal membrane. 6. The results show that the effects produced by the removal of sodium or chloride ions from the serosal bathing solution are most probably mediated by a reduction in cell volume. Cell volume changes would lead to changes in the serosal membrane selectivity to potassium and thus to changes in cell membrane potential and sodium transport.(ABSTRACT TRUNCATED AT 400 WORDS)
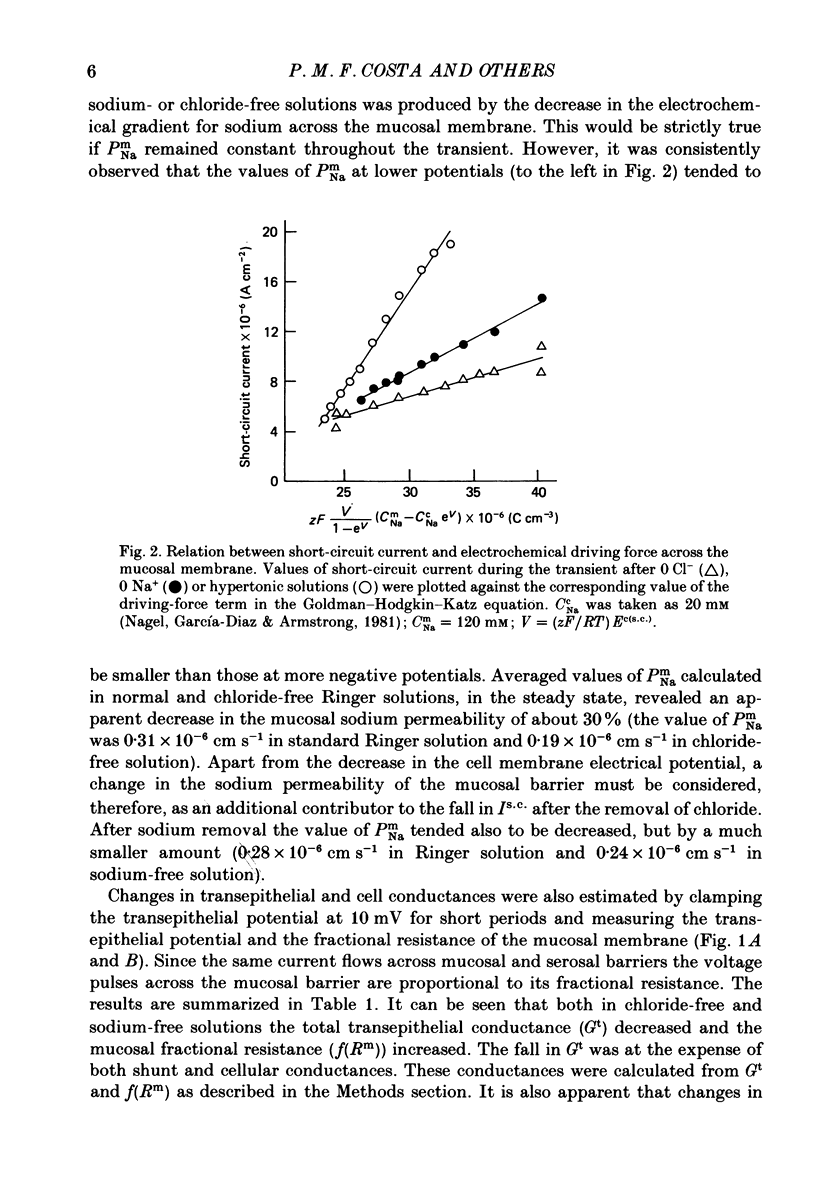
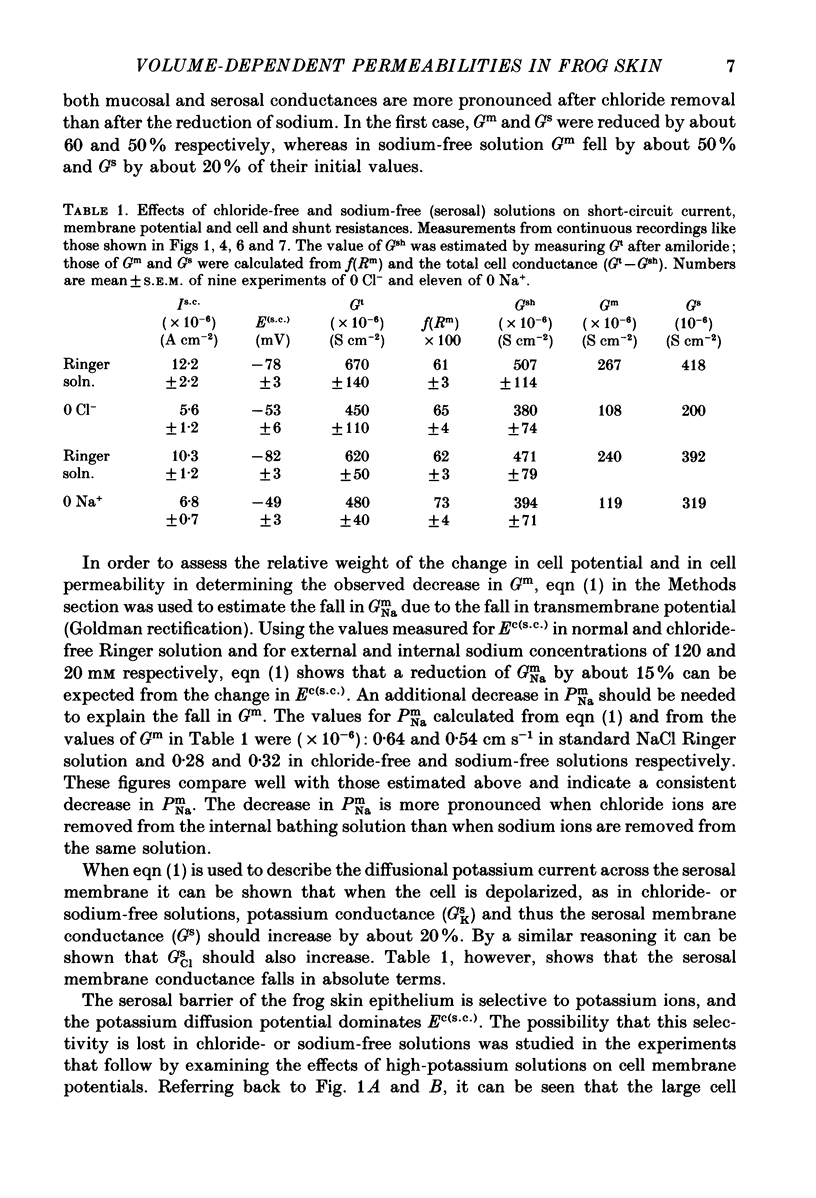
Full text
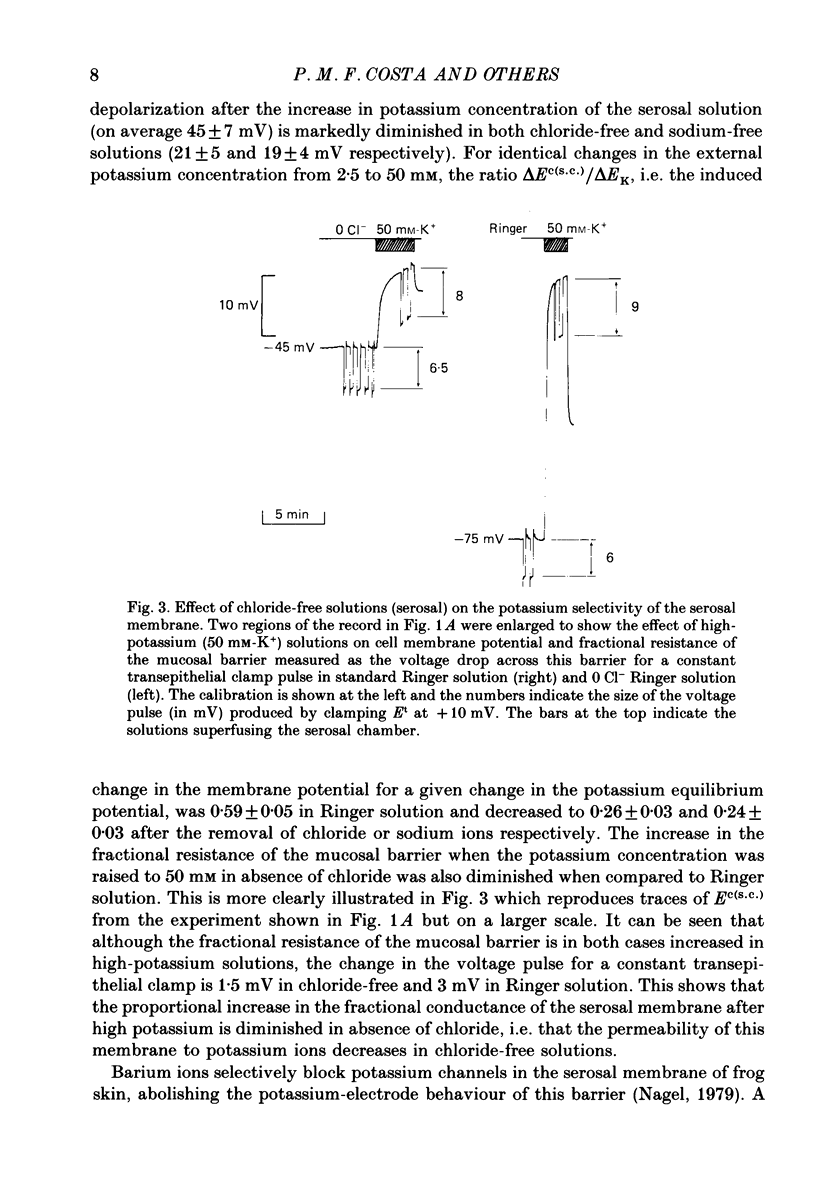
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adragna N. C., Tosteson D. C. Effect of volume changes on ouabain-insensitive net outward cation movements in human red cells. J Membr Biol. 1984;78(1):43–52. doi: 10.1007/BF01872531. [DOI] [PubMed] [Google Scholar]
- Bakker-Grunwald T. Effect of anions of potassium self-exchange in ascites tumor cells. Biochim Biophys Acta. 1978 Nov 2;513(2):292–295. doi: 10.1016/0005-2736(78)90181-5. [DOI] [PubMed] [Google Scholar]
- Erlij D., Smith M. W. Sodium uptake by frog skin and its modification by inhibitors of transepithelial sodium transport. J Physiol. 1973 Jan;228(1):221–239. doi: 10.1113/jphysiol.1973.sp010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira H. G., Ferreira K. T. Epithelial transport parameters: an analysis of experimental strategies. Proc R Soc Lond B Biol Sci. 1983 Jun 22;218(1212):309–329. doi: 10.1098/rspb.1983.0041. [DOI] [PubMed] [Google Scholar]
- Ferreira K. T. Anionic dependence of sodium transport in the frog skin. Biochim Biophys Acta. 1968 Jun 11;150(4):587–598. doi: 10.1016/0005-2736(68)90048-5. [DOI] [PubMed] [Google Scholar]
- Ferreira K. T., Ferreira H. G. The regulation of volume and ion composition in frog skin. Biochim Biophys Acta. 1981 Aug 20;646(2):193–202. doi: 10.1016/0005-2736(81)90325-4. [DOI] [PubMed] [Google Scholar]
- Ferreira K. T., Swensson W. M. The use of 60Co-EDTA as an extracellular marker in frog skin. Biochim Biophys Acta. 1979 Mar 23;552(1):178–182. doi: 10.1016/0005-2736(79)90256-6. [DOI] [PubMed] [Google Scholar]
- Frömter E. The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol. 1972;8(3):259–301. doi: 10.1007/BF01868106. [DOI] [PubMed] [Google Scholar]
- Giraldez F., Ferreira K. T. Intracellular chloride activity and membrane potential in stripped frog skin (Rana temporaria). Biochim Biophys Acta. 1984 Feb 15;769(3):625–628. doi: 10.1016/0005-2736(84)90062-2. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Dupre A., Rothstein A. Volume regulation by human lymphocytes. Role of calcium. J Gen Physiol. 1982 May;79(5):849–868. doi: 10.1085/jgp.79.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman S. I., Fisher R. S. Microelectrode studies of the active Na transport pathway of frog skin. J Gen Physiol. 1977 May;69(5):571–604. doi: 10.1085/jgp.69.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregenow F. M. Osmoregulatory salt transporting mechanisms: control of cell volume in anisotonic media. Annu Rev Physiol. 1981;43:493–505. doi: 10.1146/annurev.ph.43.030181.002425. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Ferreira H. G., Moura T. The behaviour of transporting epithelial cells. I. Computer analysis of a basic model. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1162):53–83. doi: 10.1098/rspb.1979.0091. [DOI] [PubMed] [Google Scholar]
- London R., Cohen B., Guggino W. B., Giebisch G. Regulation of intracellular chloride activity during perfusion with hypertonic solutions in the Necturus proximal tubule. J Membr Biol. 1983;75(3):253–258. doi: 10.1007/BF01871956. [DOI] [PubMed] [Google Scholar]
- MACROBBIE E. A., USSING H. H. Osmotic behaviour of the epithelial cells of frog skin. Acta Physiol Scand. 1961 Nov-Dec;53:348–365. doi: 10.1111/j.1748-1716.1961.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Nagel W. Effects of antidiuretic hormone upon electrical potential and resistance of apical and basolateral membranes of frog skin. J Membr Biol. 1978 Sep 18;42(2):99–122. doi: 10.1007/BF01885366. [DOI] [PubMed] [Google Scholar]
- Nagel W., Garcia-Diaz J. F., Armstrong W. M. Intracellular ionic activities in frog skin. J Membr Biol. 1981;61(2):127–134. doi: 10.1007/BF02007639. [DOI] [PubMed] [Google Scholar]
- Nagel W. Inhibition of potassium conductance by barium in frog skin epithelium. Biochim Biophys Acta. 1979 Apr 4;552(2):346–357. doi: 10.1016/0005-2736(79)90289-x. [DOI] [PubMed] [Google Scholar]
- Nagel W. The intracellular electrical potential profile of the frog skin epithelium. Pflugers Arch. 1976 Sep 30;365(2-3):135–143. doi: 10.1007/BF01067010. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Mack E., Rothstein A. Ionic events during the volume response of human peripheral blood lymphocytes to hypotonic media. I. Distinctions between volume-activated Cl- and K+ conductance pathways. J Gen Physiol. 1984 Apr;83(4):497–512. doi: 10.1085/jgp.83.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by "flush-through". Am J Physiol. 1981 Dec;241(6):F579–F590. doi: 10.1152/ajprenal.1981.241.6.F579. [DOI] [PubMed] [Google Scholar]
- USSING H. H. RELATIONSHIP BETWEEN OSMOTIC REACTIONS AND ACTIVE SODIUM TRANSPORT IN THE FROG SKIN EPITHELIUM. Acta Physiol Scand. 1965 Jan-Feb;63:141–155. doi: 10.1111/j.1748-1716.1965.tb04052.x. [DOI] [PubMed] [Google Scholar]
- Ussing H. H. Volume regulation of frog skin epithelium. Acta Physiol Scand. 1982 Mar;114(3):363–369. doi: 10.1111/j.1748-1716.1982.tb06996.x. [DOI] [PubMed] [Google Scholar]


